Study on Adsorption Properties of Calcined Mg–Al Hydrotalcite for Sulfate Ion and Chloride Ion in Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Materials of Preparing Mg–Al Hydrotalcite
2.1.2. Prepare Raw Materials of Target Ion Aqueous Solution
2.1.3. Raw Materials of Cement Paste Test Pieces
2.2. Methods
2.2.1. XRD and TG–DSC Analysis
2.2.2. SEM Analysis
2.2.3. FTIR Analysis
2.2.4. AQ Analysis
2.2.5. Deformation Performance Test of Cement Paste Specimen
2.2.6. Compressive Strength of Cement Paste
3. Preparation Method of Magnesium Aluminum Hydrotalcite
4. Results
4.1. Characterization of LDHs and LDO
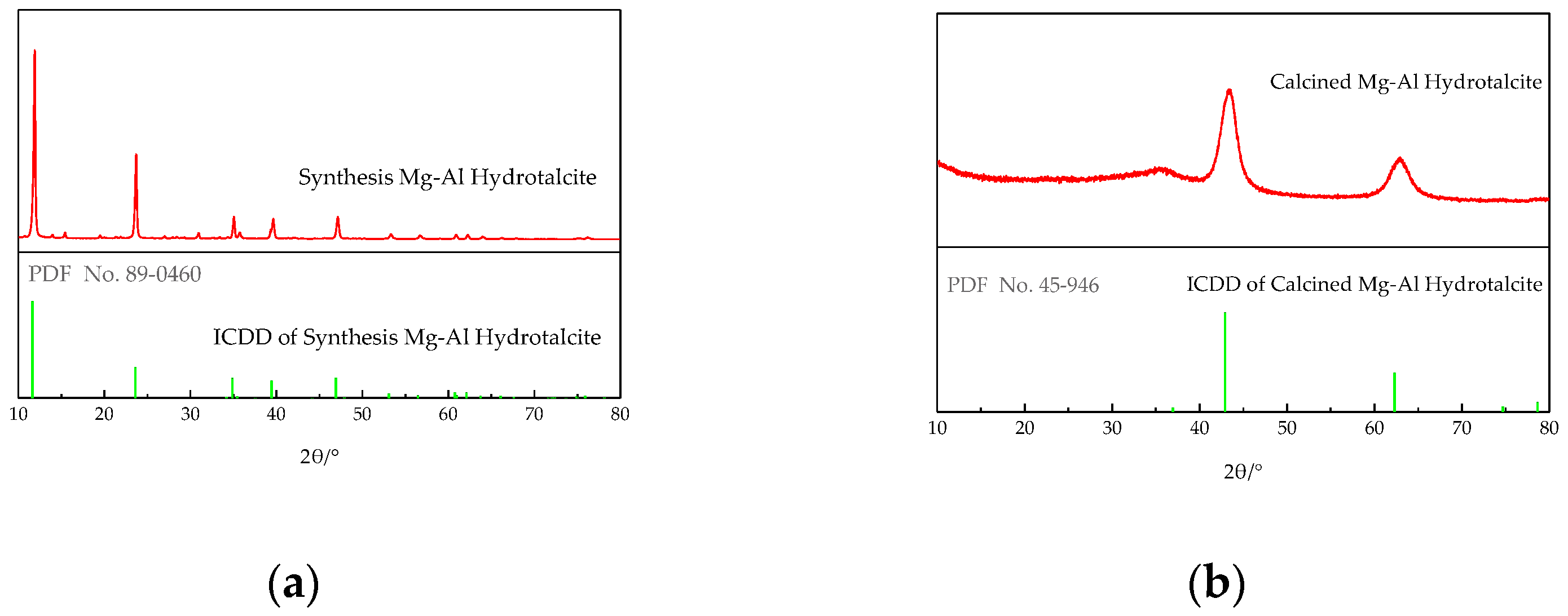
4.1.1. XRD Analysis of LDHs and LDO
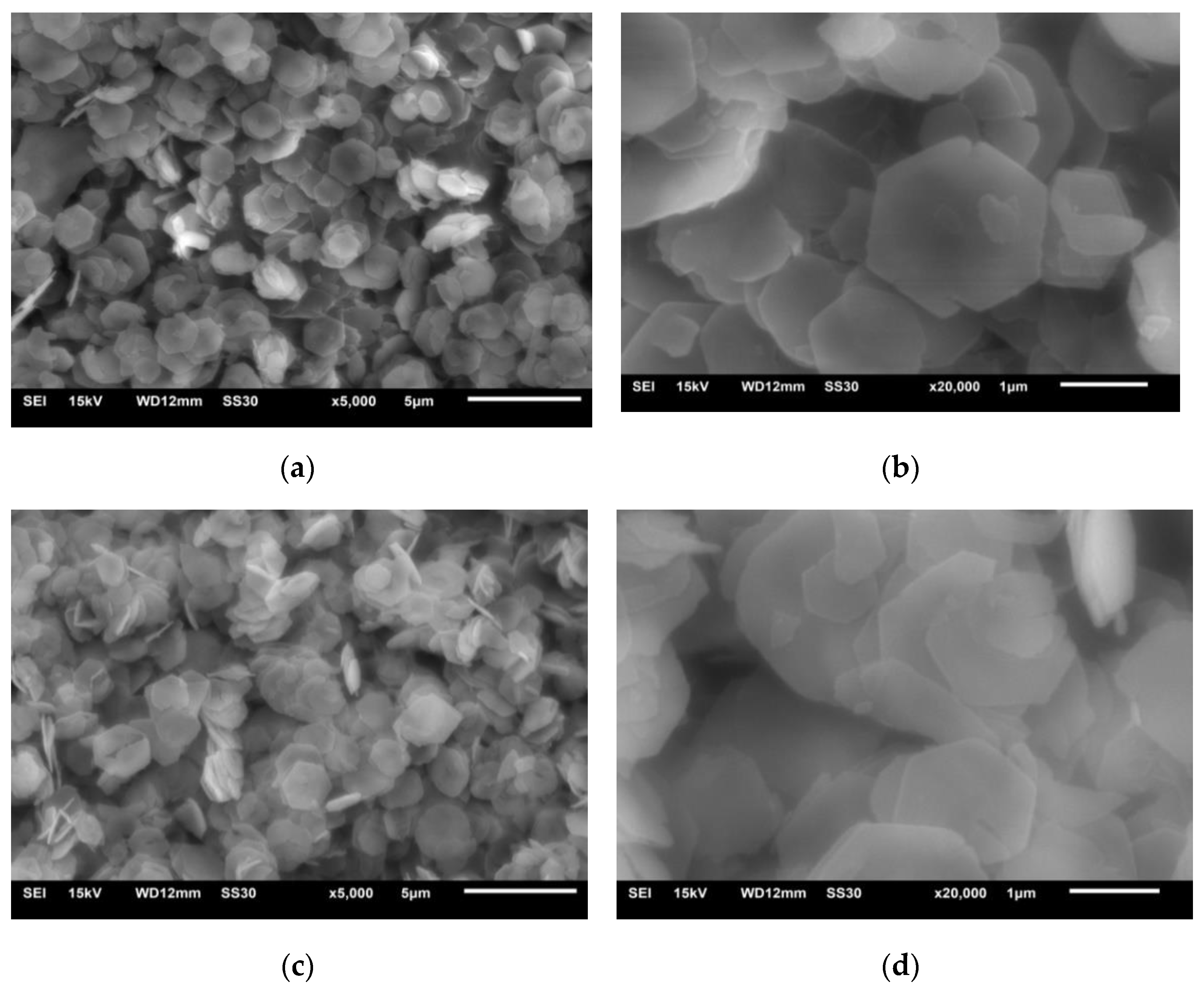
4.1.2. The SEM Analysis of LDHs and LDO
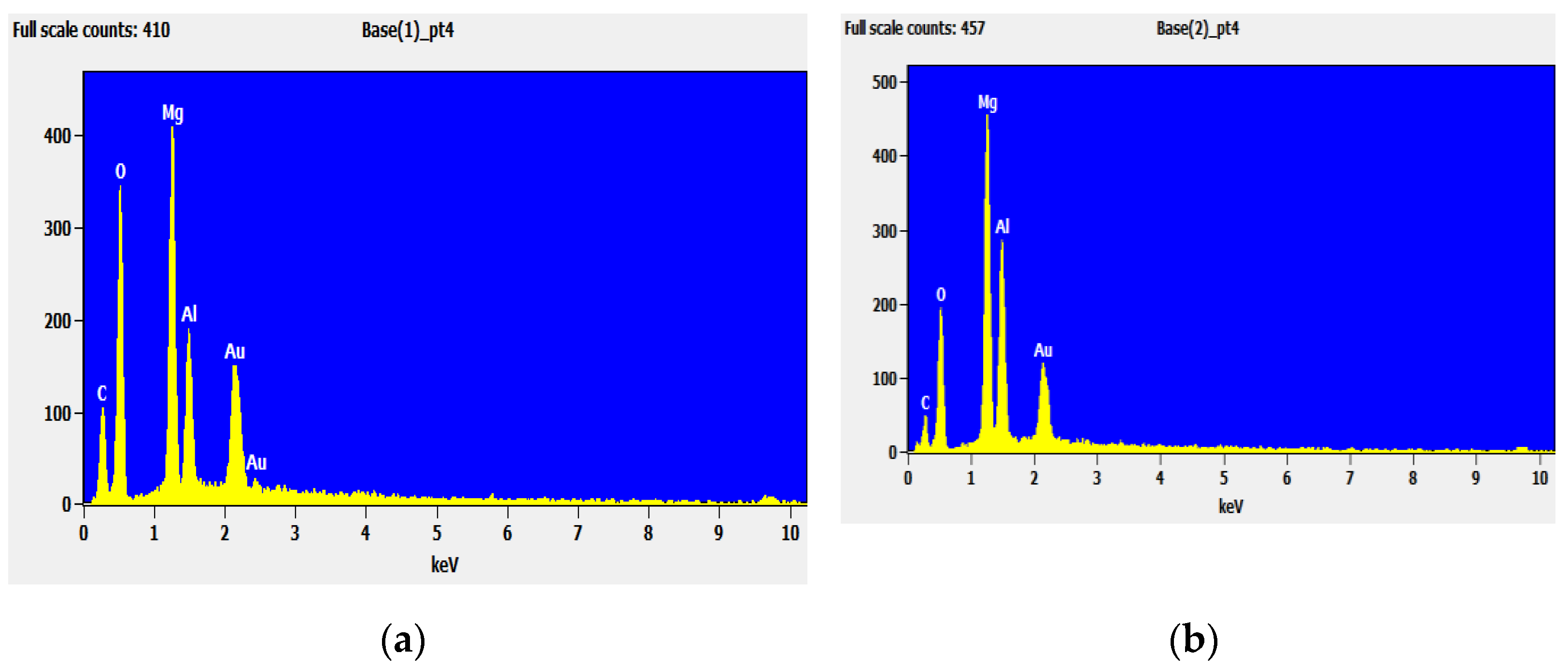
4.1.3. The EDS Analysis of LDHs and LDO
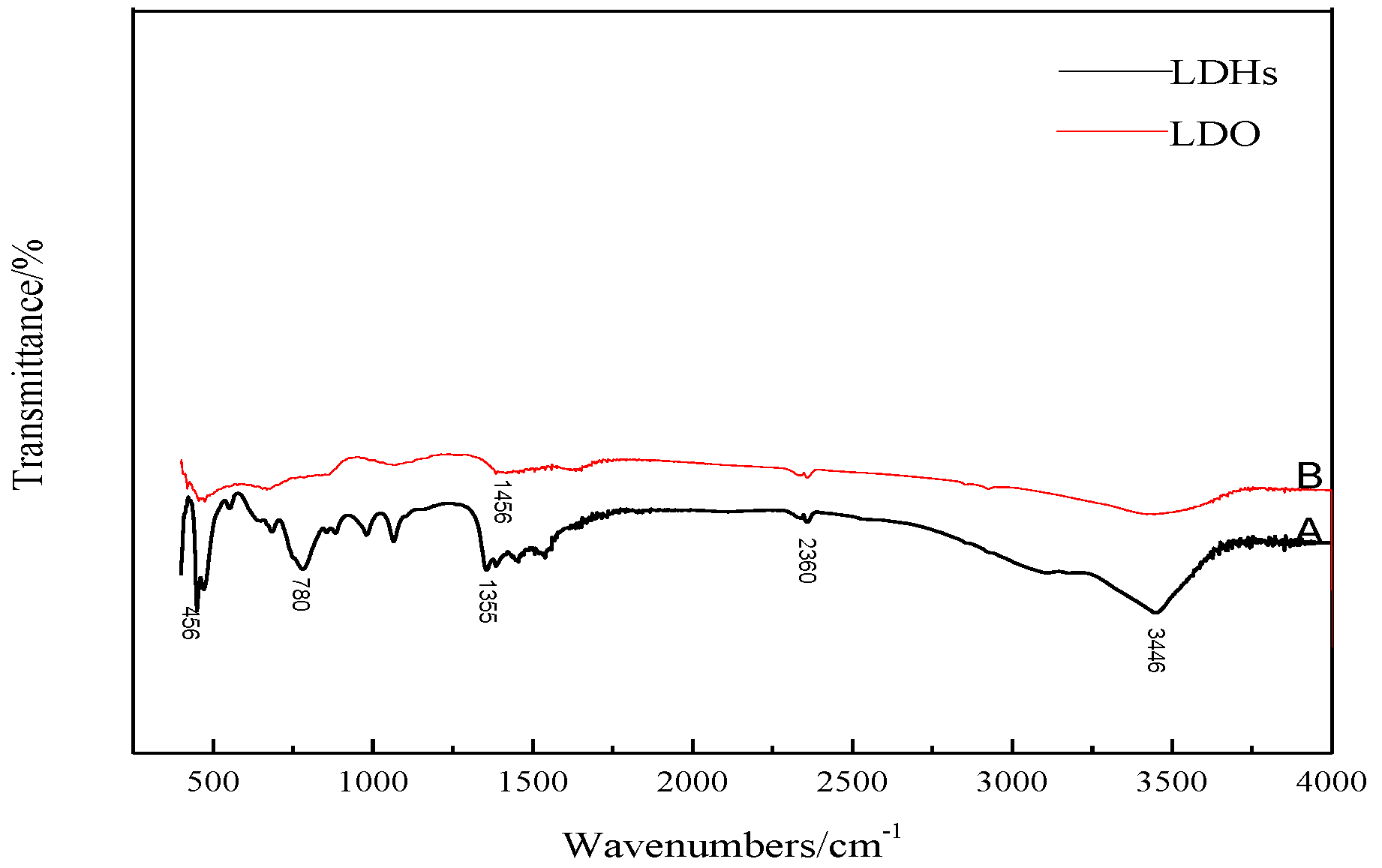
4.1.4. The FTIR 4.1.4 The FT-IR Analysis of LDHs and LDO
4.2. Adsorption Results of LDO in Aqueous Solution
4.2.1. Adsorption Results of LDO in Single Solution
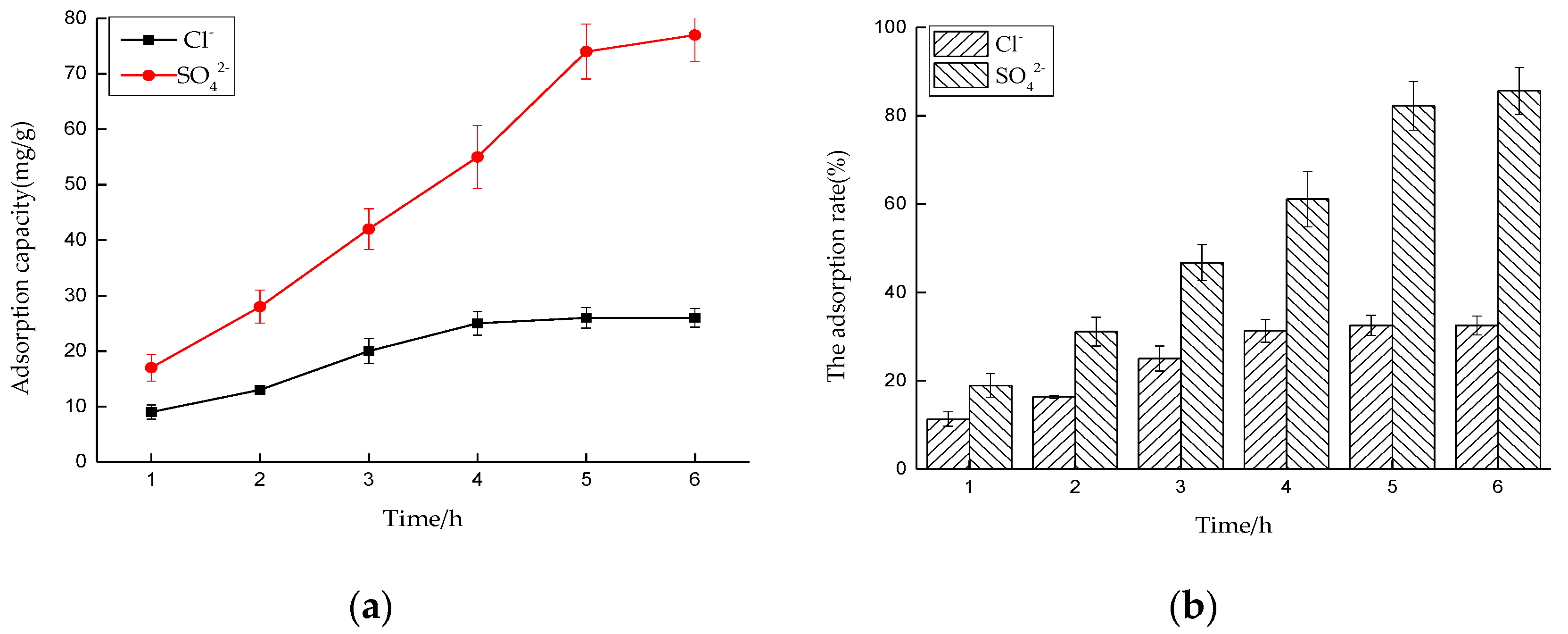
Effect of Time on Adsorption Performance
Effect of Temperature on Adsorption Performance
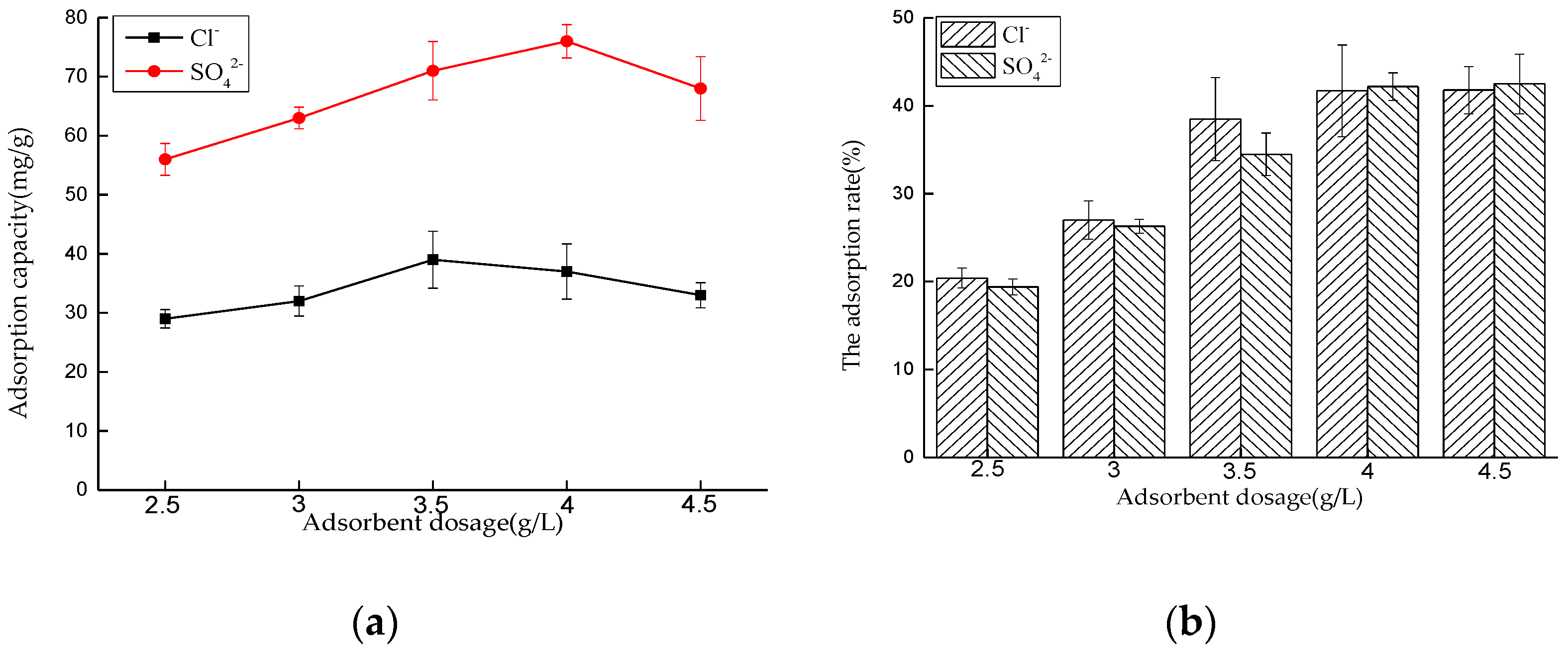
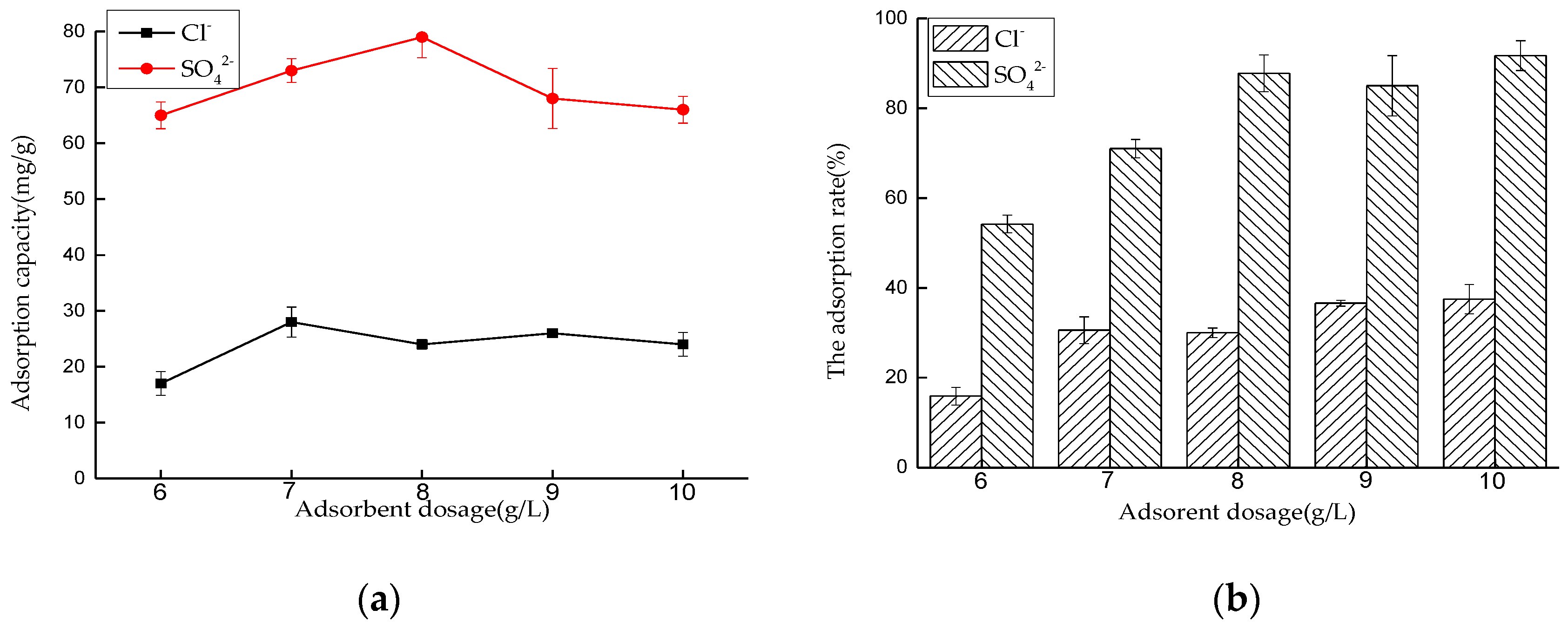
Effect of Dosage on Adsorption Performance
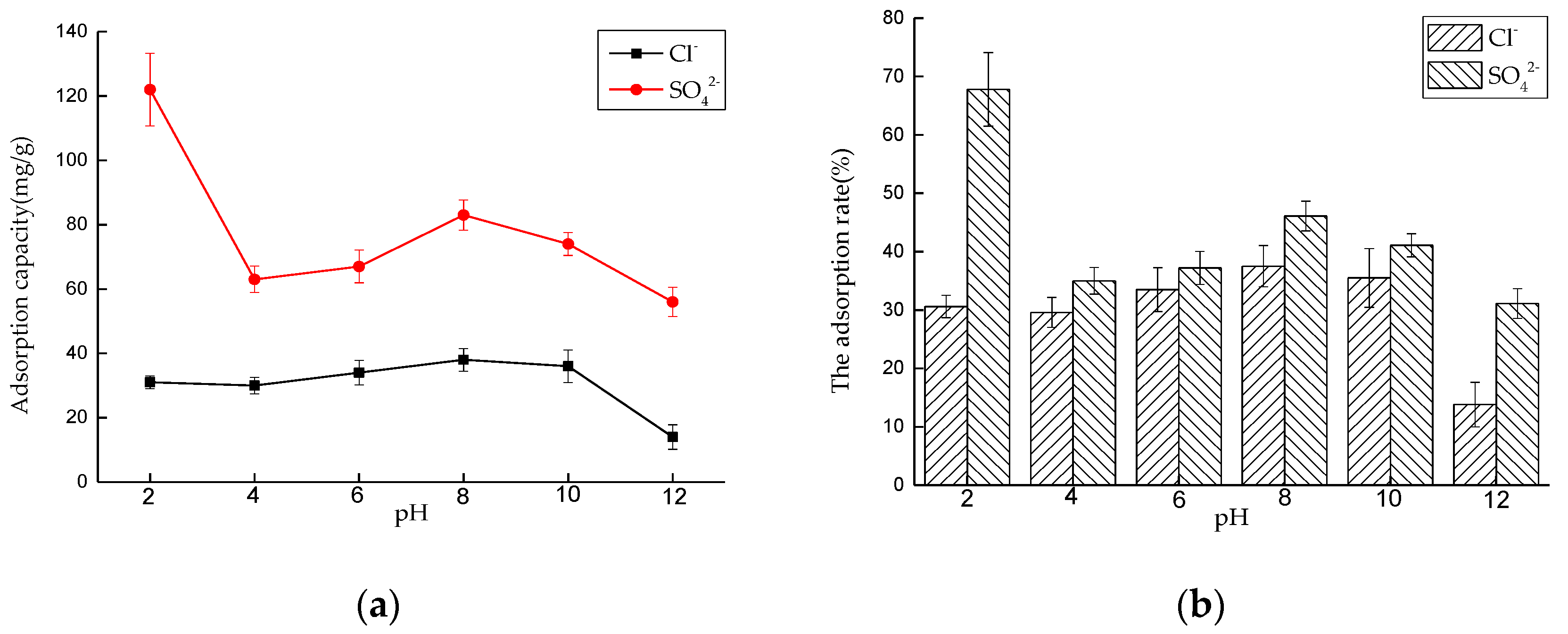
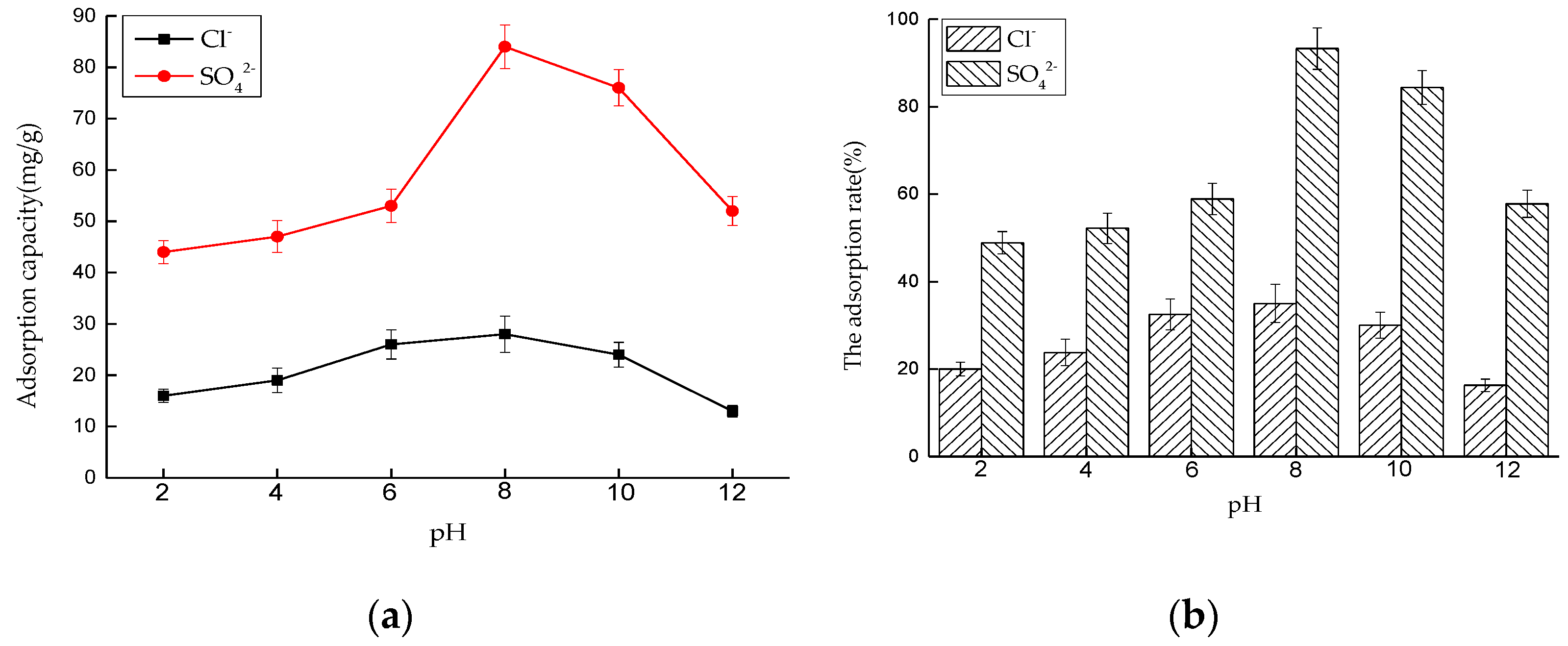
Effect of pH on Adsorption Performance
4.2.2. Adsorption of LDO in Mixed Solution
Effect of Time on Adsorption Performance in Mixed Solution
Effect of Temperature on Adsorption Performance in Mixed Solution
Effect of Dosage on Adsorption Performance in Mixed Solution
Effect of pH on Adsorption Performance in Mixed Solution
4.3. Experiment of LDO in Cement
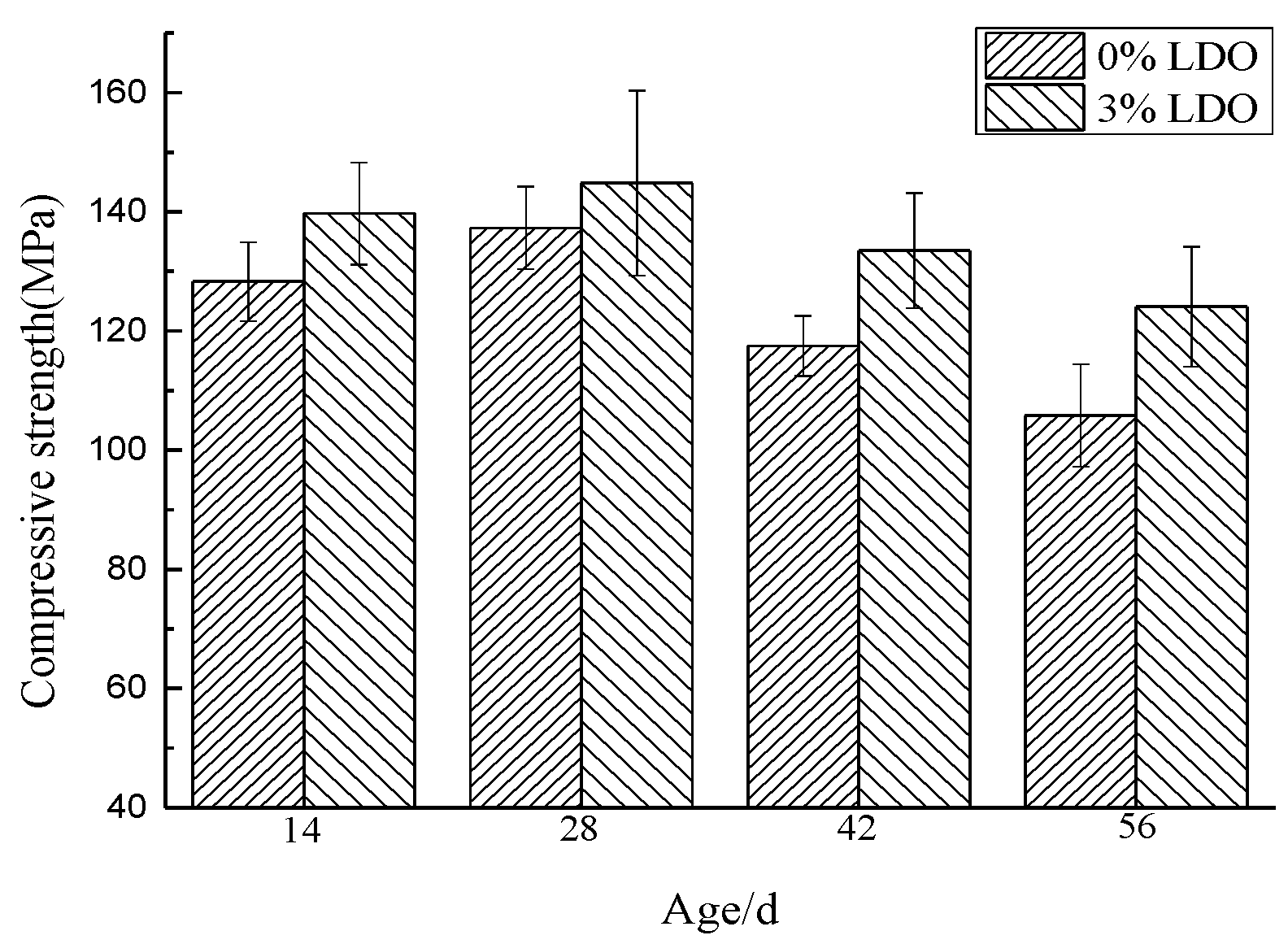
4.3.1. Compressive Strength
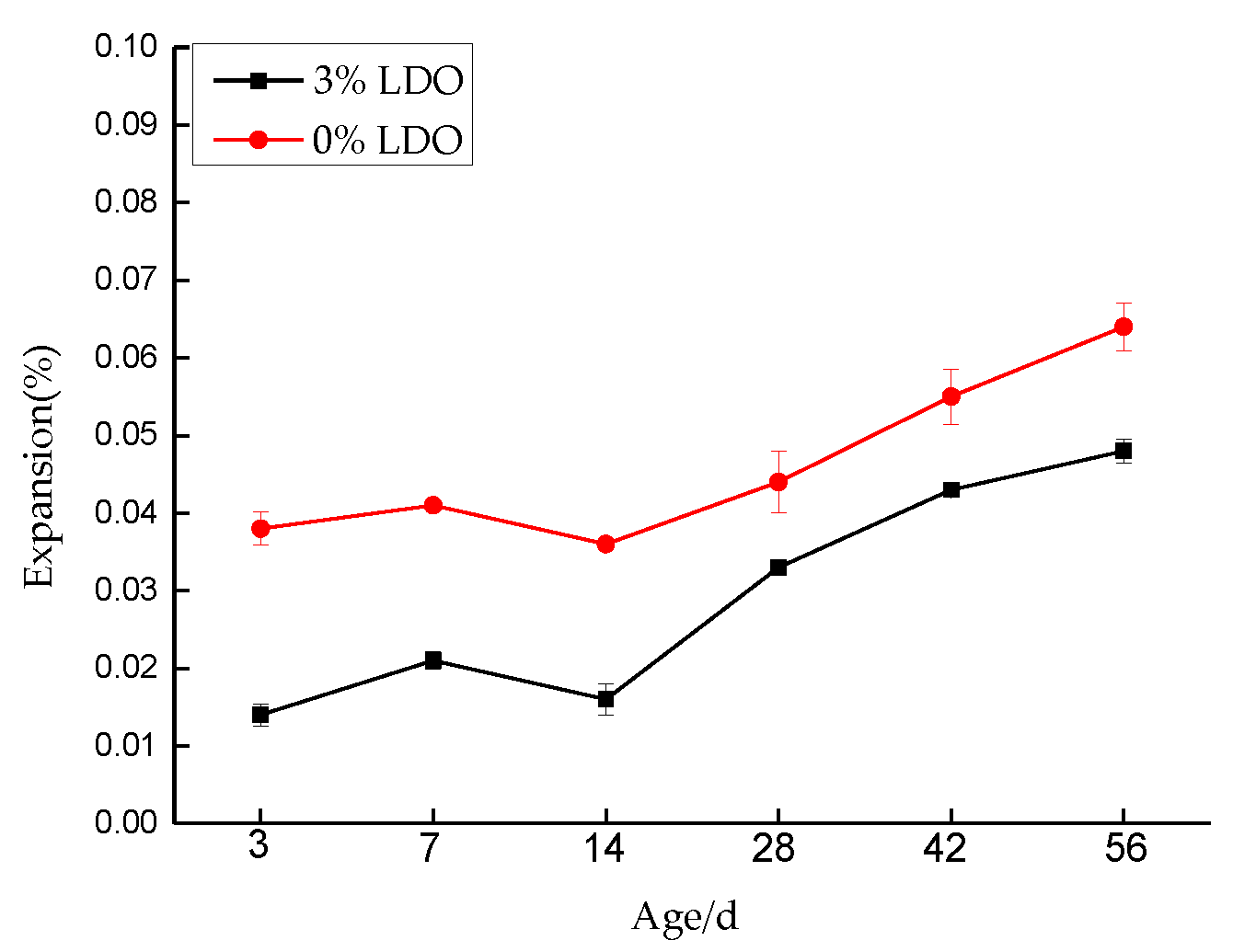
4.3.2. Expansion Measurements
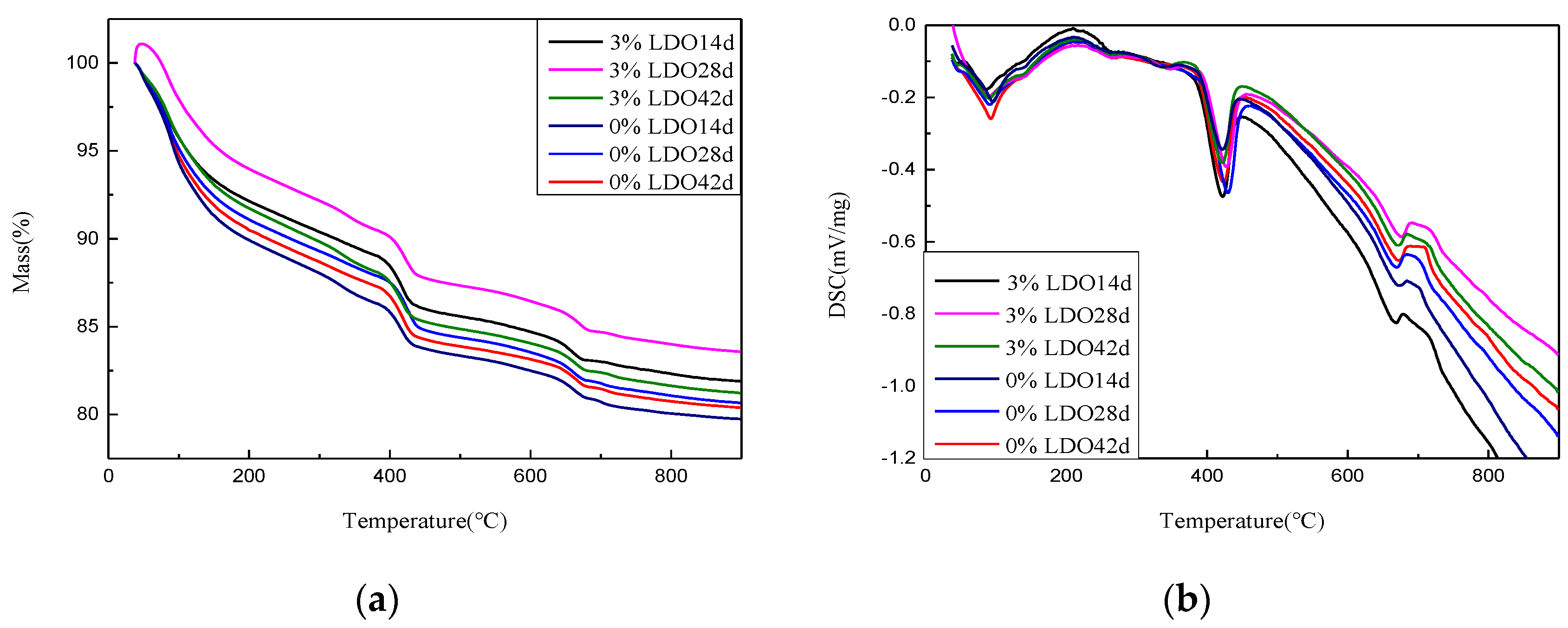
4.3.3. TG–DSC Analysis
5. Conclusions
- LDHs is prepared by a simple hydrothermal synthesis method, and the structure and properties of the materials are analyzed by modern micro-analysis techniques, including phase analysis, morphology observation, and infrared spectroscopy. XRD analysis shows that the peak width of the product is narrow and sharp, there is no obvious impurity peak, and the product purity is high. From the electron microscope, it can be seen that the hexagonal LDHs prepared with ethanol as raw material have regular and uniform morphology; a cross-supporting structure is formed between the plates, and the dispersion is good. After roasting, the morphology of the samples has little change, and the original structure is still maintained. Infrared analysis shows that the unbaked LDHs material is carbonate hydrotalcite–like material. Compared with LDHs, the stretching vibration of hydroxyl group and the vibration absorption peak of CO32− are weakened, but they still exist, and CO32− is partially converted into CO2 gas to form magnesium–aluminum oxide. These microscopic test results confirm each other, which shows the reliability of the results and lays a foundation for the application of LDO materials in cement concrete.
- The experimental results of LDO in separate solution show that the best conditions for Cl− adsorption are an adsorption time of 4 h, temperature of 35 °C, LDO dosage of 3.5 g/L, and pH of 8. For SO42−, the adsorption time is 6 h, temperature is 35 °C, LDO dosage is 4 g/L, and pH = 8; the adsorption effect is the best.
- The experiment of LDO in mixed solution shows that LDO still has good adsorption effect in the system closer to the actual environment, but the adsorption of two kinds of ions shows some differences. Under the influence of various factors, the adsorption effect of LDO on SO42− is good, but it has a great influence on Cl−. The LDO preferentially adsorbs SO42− when both SO42− and Cl− exist. When the amount of LDO is fixed and the ion concentration in the solution is high, a large amount of SO42− occupies the active sites of hydrotalcite, and the remaining active sites for Cl− adsorption are less, which shows that the adsorption capacity of SO42− is larger than that of Cl−. However, the adsorption capacity of LDO for these two ions in the actual system is less than that in single ion solution. This may be due to the existence of a small amount of other ions in the mixed system, which will compete with SO42− and Cl− for adsorption, thus reducing the adsorption capacity of SO42− and Cl−.
- The experiment of LDO in cement shows that, compared with ordinary cement paste specimens, the specimens doped with LDO have less of an ettringite formation, a stronger ability to cure SO42− and Cl−, and the LDO enhances the compressive strength and stability of the specimens.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L. Synthesis of Mg–Al Layered Double Hydroxides and Their Adsorption Capability of Chloride Ion in Cement Paste. Ph.D. Thesis, University of South China, Hengyang, China, 2018. (In Chinese). [Google Scholar]
- Gong, X. The Experimental Research of Sulfate Attack Resistance of Concrete. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2008. (In Chinese). [Google Scholar]
- Duan, P. Research on Modification Mechanism and the Application of Layered Double Hydroxides for Durability of Concrete. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2014. (In Chinese). [Google Scholar]
- Mo, D. Preferential Intercalation in Layered Double Hydroxides and Mechanism Study. Ph.D. Thesis, Beijing University of Chemical Technology, Beijing, China, 2004. (In Chinese). [Google Scholar]
- Evans, D.G.; Slade, R. Structural Aspects of layered double hydoxides. In Structure and Bonding; Duan, X., Evans, D.G., Eds.; Springer: Heidelberg, Germany, 2006; Volume 119, pp. 1–87. [Google Scholar]
- Li, F.; Duan, X. (Eds.) Applications of layered double hydroxides. In Structure and Bonding; Springer: Heidelberg, Germany, 2006; Volume 119, pp. 193–223. [Google Scholar]
- Jone, L. Process for Removing Heavy Metal Ions from Solutions Using Adsorbents Containing Activated Hydrotalcite. U.S. Patent 4752397, 30 June 1988. [Google Scholar]
- Orthman, J.; Zhu, H.; Lu, G. Use of anion clay hydrotalcite to remove coloured organics from aqueous solutions. Sep. Purif. Technol. 2003, 31, 53–59. [Google Scholar] [CrossRef]
- Ulibarri, M.; Pavlovic, I.; Hermosín, M.; Cornejo, J. Hydrotalcite-like compounds as potential sorbents of phenols from water. Appl. Clay Sci. 1995, 10, 131–145. [Google Scholar] [CrossRef]
- Tatematsu, H.; Akamura, T.; Koshimuzu, H.; Morishita, T.; Kotaki, H. Cement additive for inhibiting concrete deteriora-tion. Ziolites 1996, 17, 404. [Google Scholar]
- Shui, Z.; Ma, J.; Chen, W.; Chen, X. Chloride Binding Capacity of Cement Paste Containing Layered Double Hydroxide (LDH). J. Test. Eval. 2012, 40, 796–800. [Google Scholar]
- Shui, Z.; Ma, J. The effect of layered double hydroxides on the concrete resistance of chloride-ion penetration. Key Eng. Mater. 2012, 509, 99–105. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Uptake of chloride and carbonate by Mg-Al and Ca-Al layered double hydroxides in simulated pore solutions of alkali-activated slag cement. Cem. Concr. Res. 2017, 100, 1–13. [Google Scholar] [CrossRef]
- Yang, L.; Chen, M.; Lu, Z.; Huang, Y.; Wang, J.; Lu, L.; Cheng, X. Synthesis of CaFeAl layered double hydroxides 2D nanosheets and the adsorption behaviour of chloride in simulated marine concrete. Cem. Concr. Compos. 2020, 114, 103817. [Google Scholar] [CrossRef]
- Yoon, S.; Moon, J.; Bae, S.; Duan, X.; Giannelis, E.P.; Monteiro, P.M. Chloride adsorption by calcined layered double hydroxides in hardened Portland cement paste. Mater. Chem. Phys. 2014, 145, 376–386. [Google Scholar] [CrossRef]
- Chen, Y.; Shui, Z.; Chen, W.; Chen, G. Chloride binding of synthetic Ca–Al–NO3 LDHs in hardened cement paste. Constr. Build. Mater. 2015, 93, 1051–1058. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Polder, R. Laboratory investigation of the influence of two types of modified hydrotalcites on chloride ingress into cement mortar. Cem. Concr. Compos. 2015, 58, 105–113. [Google Scholar] [CrossRef]
- Tong, M. Preparation and performance study on Mg–Al-hydrotalcite. Inorg. Chem. Ind. 2014, 46, 22–25. [Google Scholar]





| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | Na2O | SO3 |
|---|---|---|---|---|---|---|
| 19.58 | 63.85 | 4.63 | 2.91 | 2.45 | 0.14 | 3.27 |
| Samples | Element | Weight/% | Atom/% |
|---|---|---|---|
| LDHs | C | 16.92 | 24.20 |
| O | 49.40 | 53.05 | |
| Mg | 18.68 | 13.20 | |
| Al | 15.00 | 9.55 | |
| LDO | C | 4.04 | 6.75 |
| O | 37.39 | 47.52 | |
| Mg | 31.89 | 25.90 | |
| Al | 26.68 | 19.83 |
| Sample | 0%LDO 14 d | 0%LDO 28 d | 0%LDO 42 d | 3%LDO 14 d | 3%LDO 28 d | 3%LDO 42 d |
|---|---|---|---|---|---|---|
| Ettringite (%) | 11.14 | 12.53 | 14.24 | 3.57 | 8.51 | 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, B.; Mao, Z.; Wang, Y. Study on Adsorption Properties of Calcined Mg–Al Hydrotalcite for Sulfate Ion and Chloride Ion in Cement Paste. Materials 2021, 14, 994. https://doi.org/10.3390/ma14040994
Wang J, Huang B, Mao Z, Wang Y. Study on Adsorption Properties of Calcined Mg–Al Hydrotalcite for Sulfate Ion and Chloride Ion in Cement Paste. Materials. 2021; 14(4):994. https://doi.org/10.3390/ma14040994
Chicago/Turabian StyleWang, Jun, Bei Huang, Zhongyang Mao, and Yu Wang. 2021. "Study on Adsorption Properties of Calcined Mg–Al Hydrotalcite for Sulfate Ion and Chloride Ion in Cement Paste" Materials 14, no. 4: 994. https://doi.org/10.3390/ma14040994






