In Vitro Evaluation of Desensitizing Agents Containing Bioactive Scaffolds of Nanofibers on Dentin Remineralization
Abstract
:1. Introduction
2. Materials and Methods
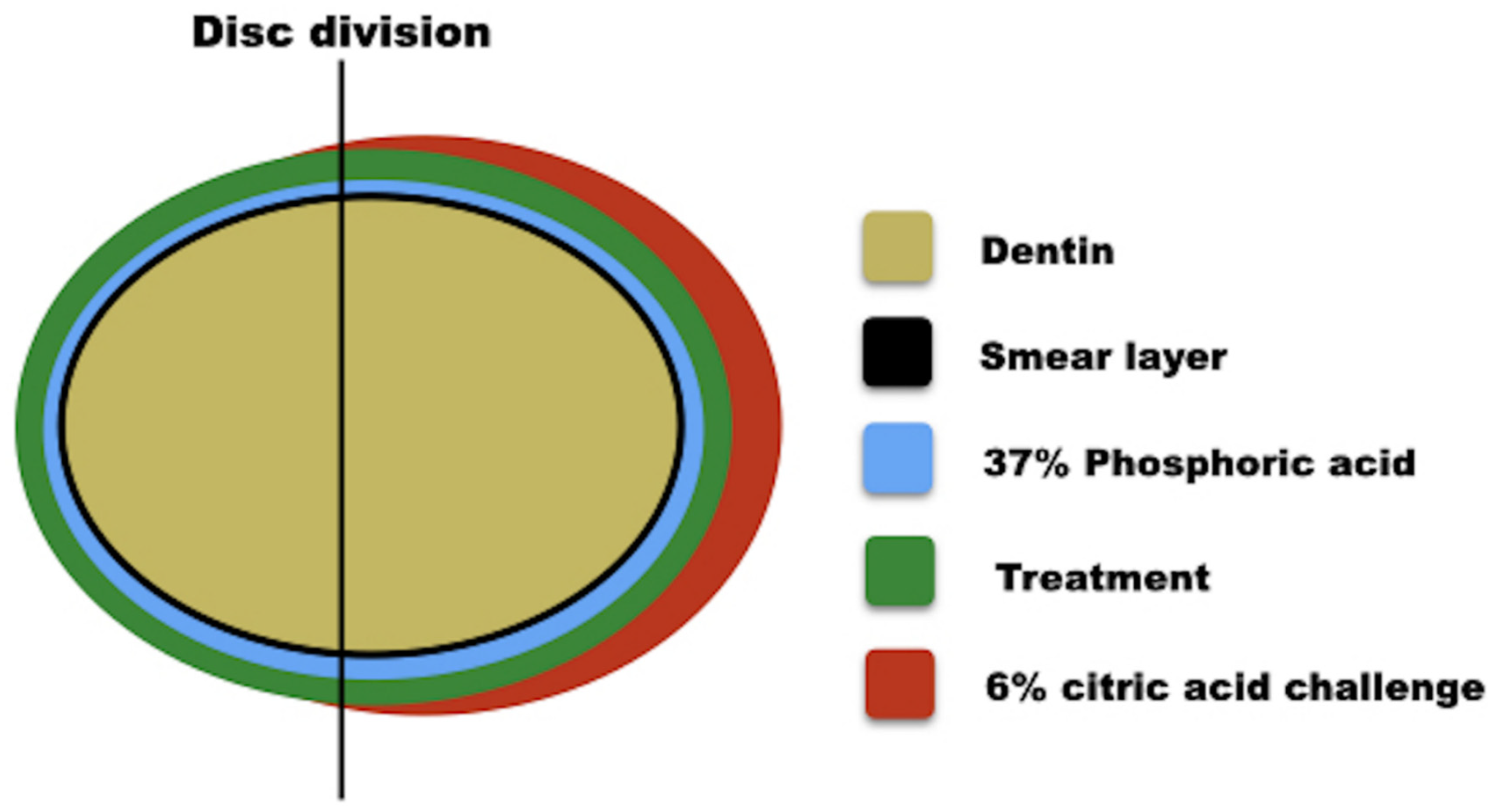
2.1. Experimental Design
2.2. Dentin Sample Preparation
2.3. Nanofibers Fabrication
2.4. Adhesive System and Varnish Samples Preparation
2.5. Experimental Conditions
2.6. Dentin Permeability Analysis
2.7. Scanning Electron Microscopy (SEM) Analysis
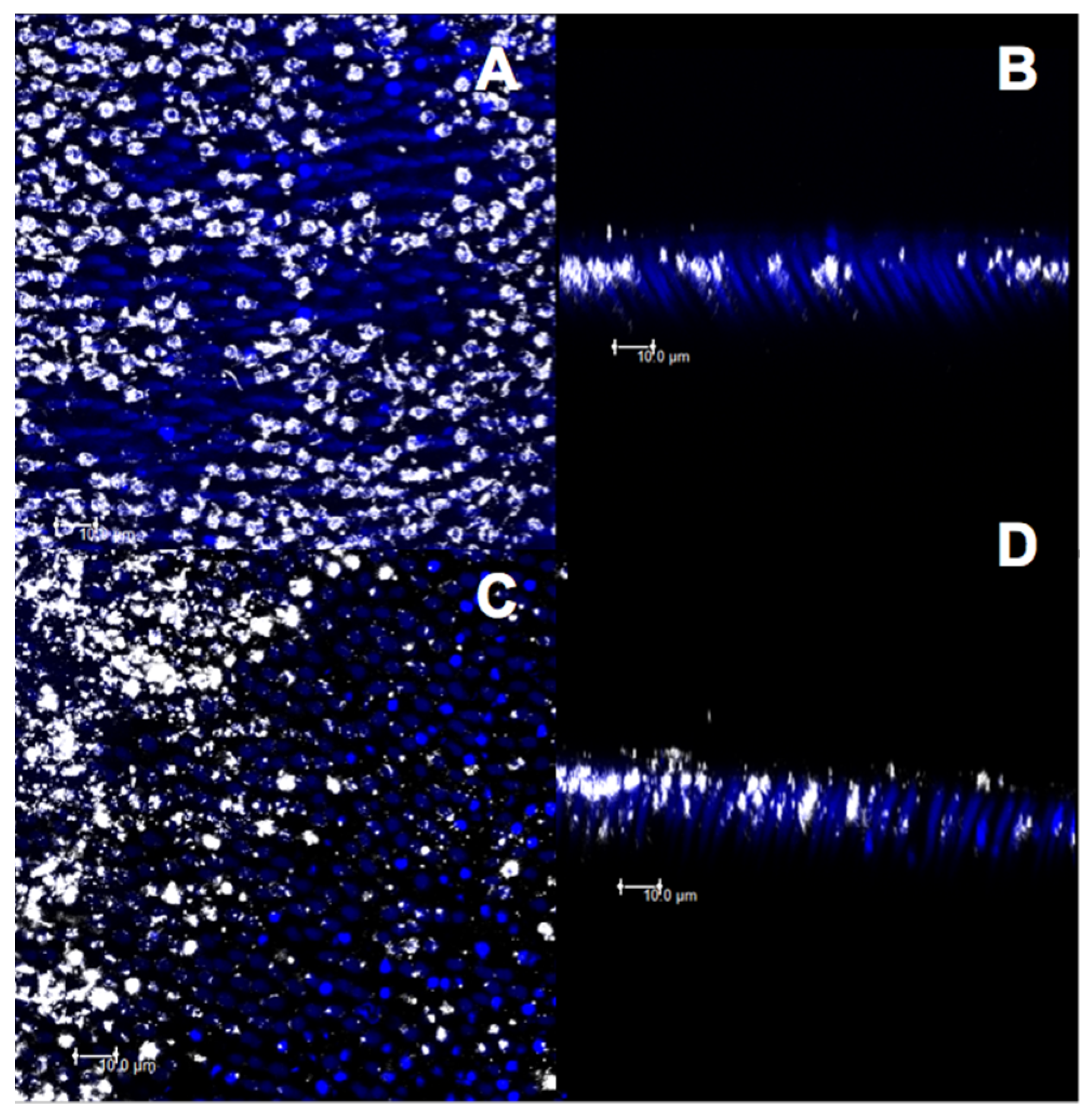
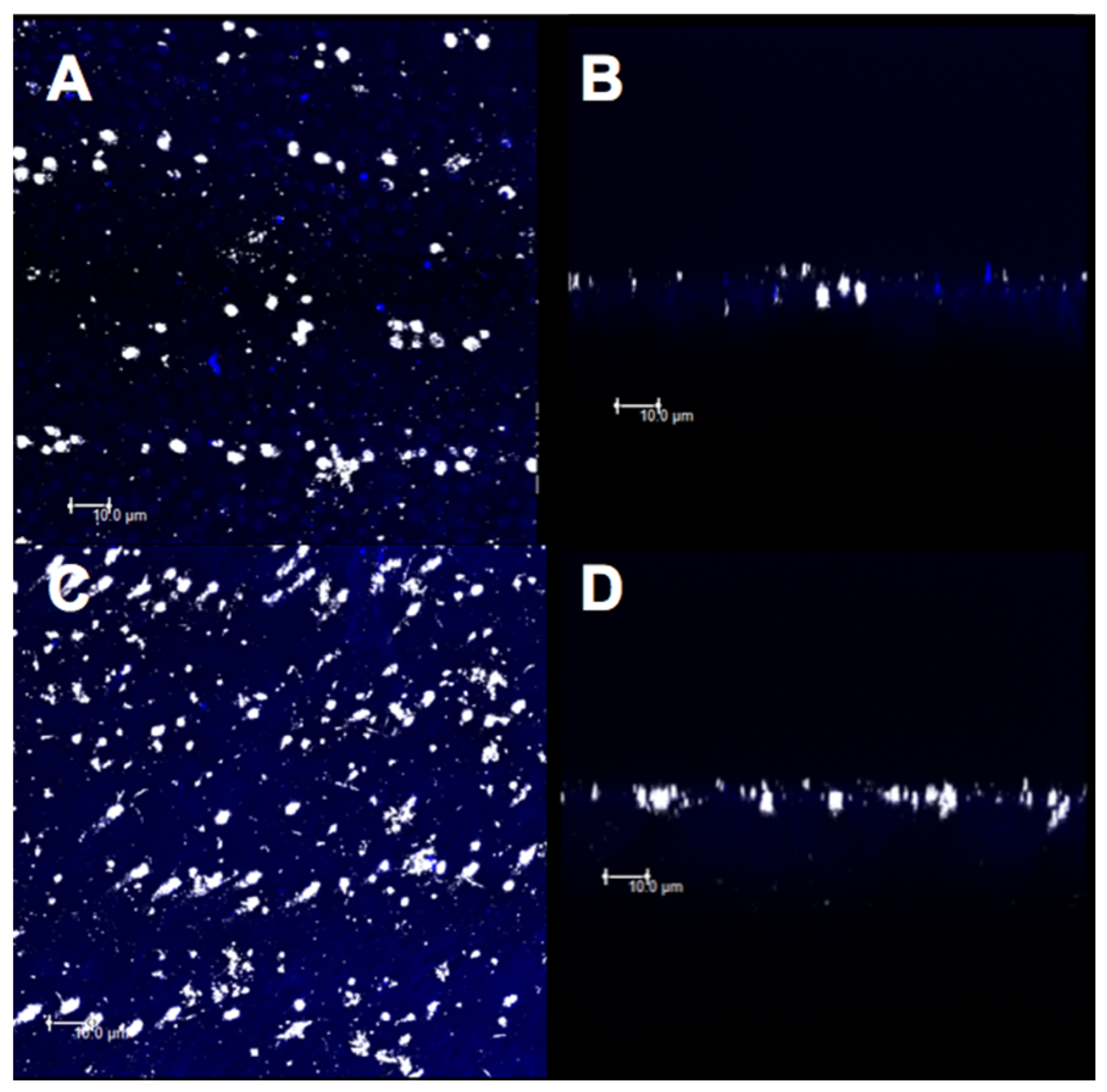
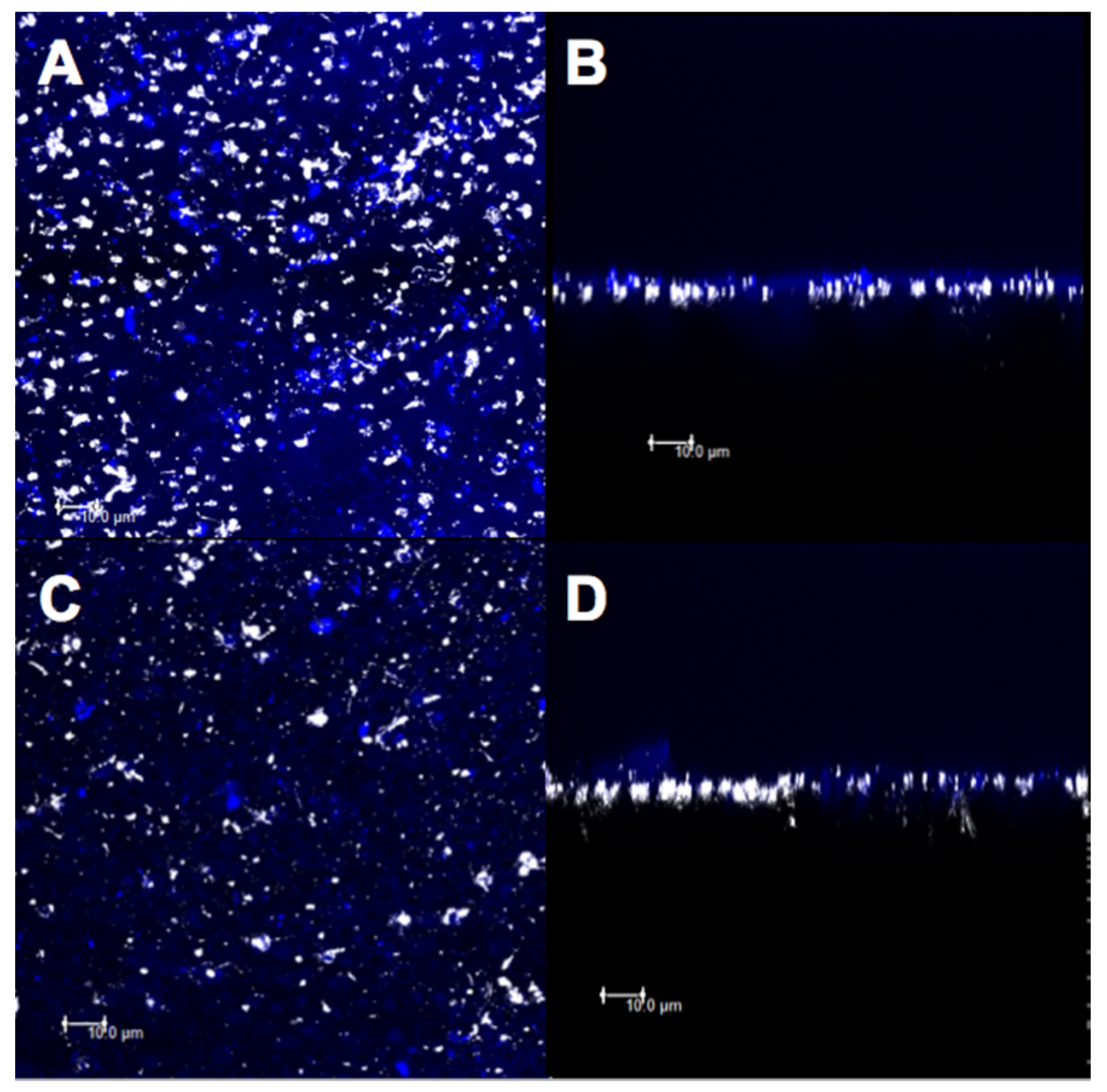
2.8. Confocal Analysis
2.9. Statistical Analysis
3. Results
3.1. Dentine Permeability Measurements
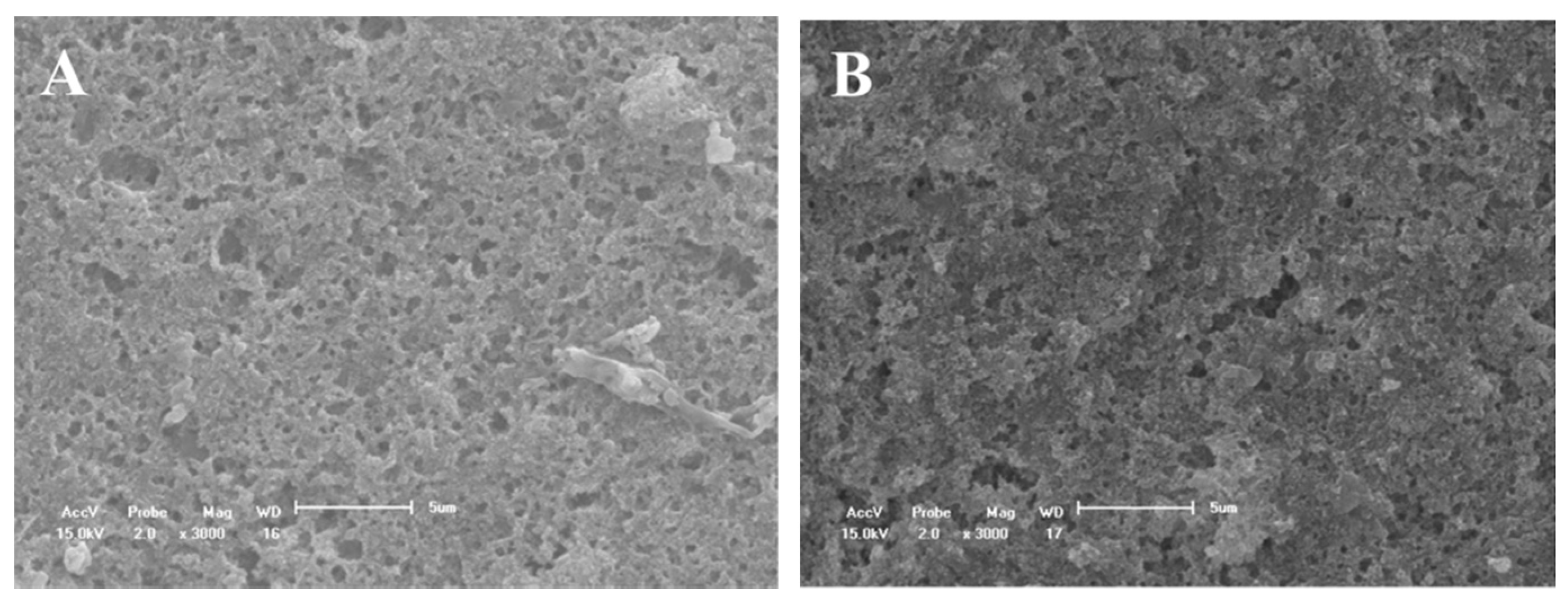
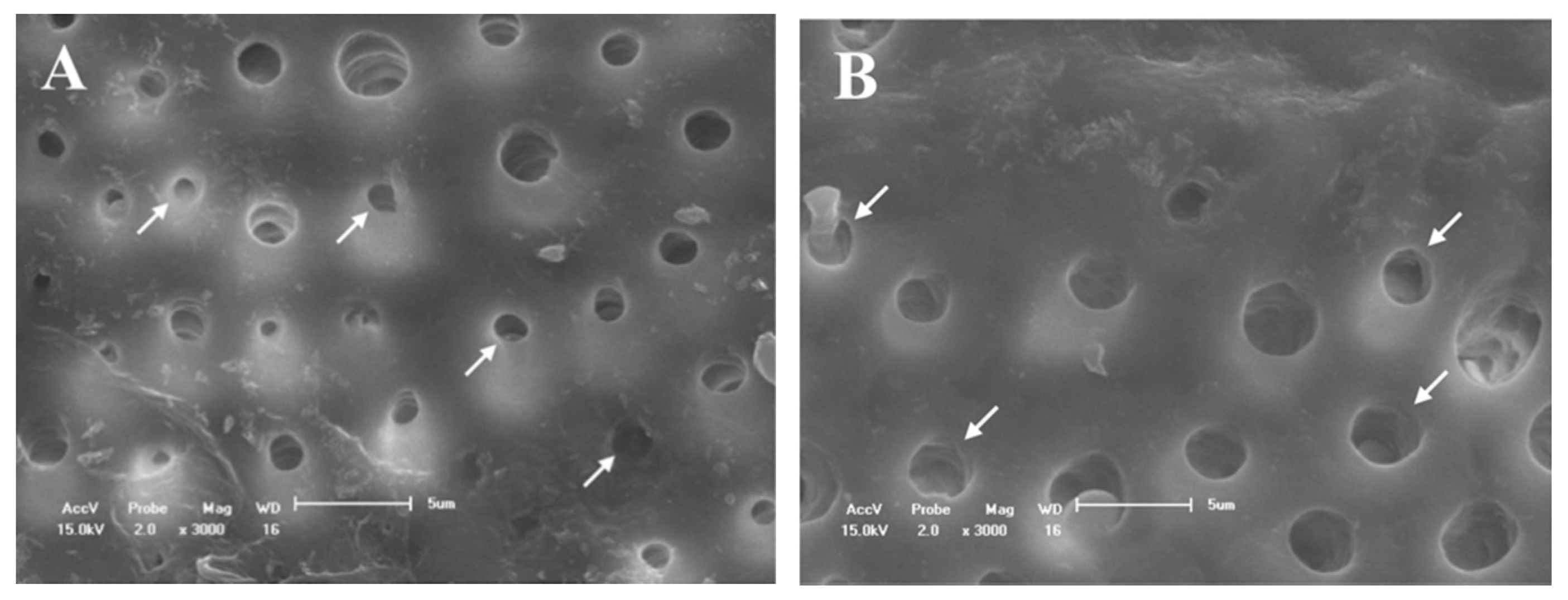
3.2. SEM Evaluation
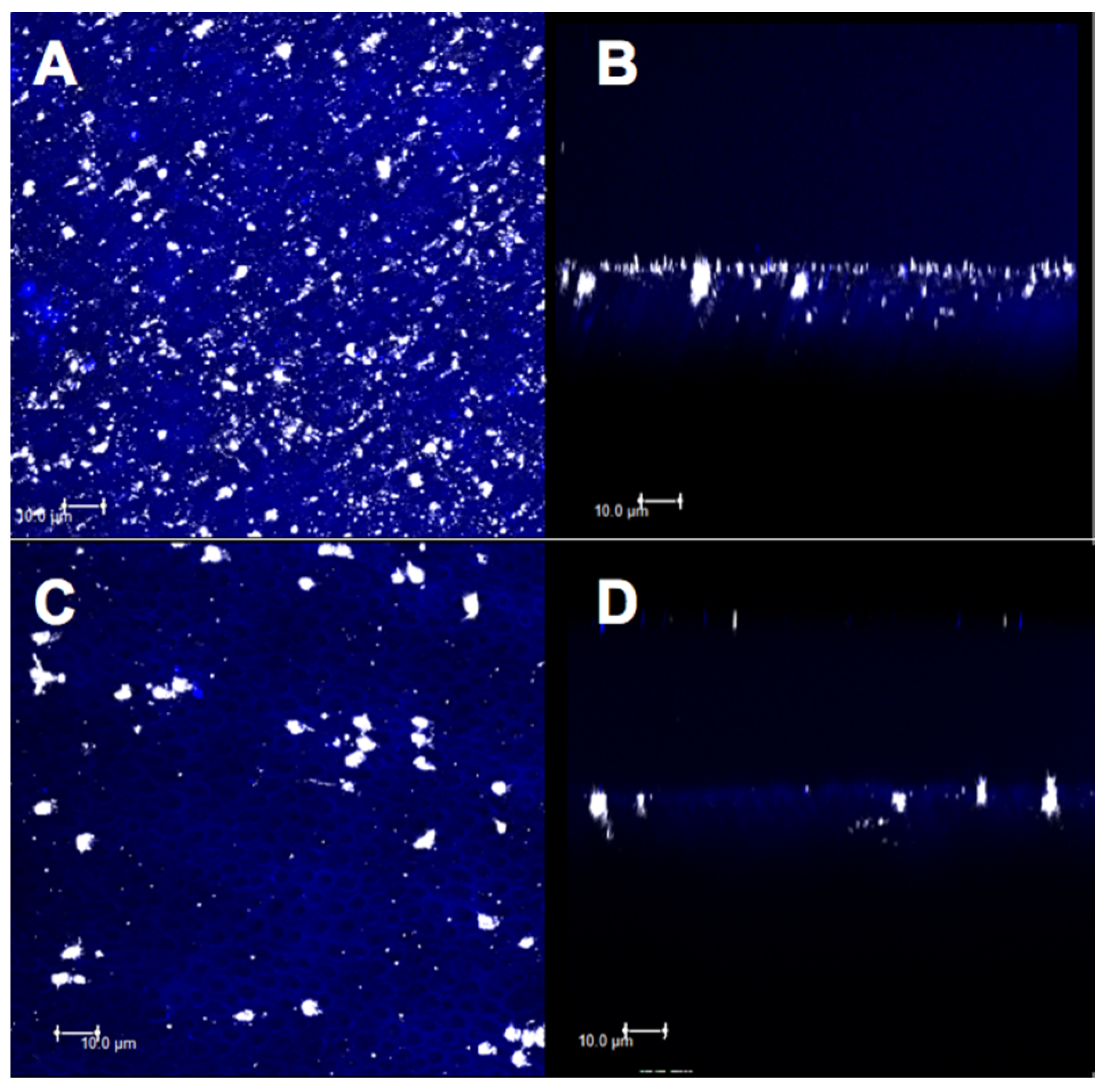
3.3. Confocal Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markowitz, K.; Pashley, D.H. Discovering new treatments for sensitive teeth: The long path from biology to therapy. J. Oral Rehabil. 2008, 35, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Que, K.; Guo, B.; Jia, Z.; Chen, Z.; Yang, J.; Gao, P. A cross-sectional study: Non-carious cervical lesions, cervical dentine hypersensitivity and related risk factors. J. Oral Rehabil. 2012, 40, 24–32. [Google Scholar] [CrossRef]
- West, N.; Seong, J.; Davies, M. Dentine Hypersensitivity. Oral Biofilms 2014, 25, 108–122. [Google Scholar]
- Brannstrom, M.; Linden, L.-A.; Johnson, G. Movement of Dentinal and Pulpal Fluid Caused by Clinical Procedures. J. Dent. Res. 1968, 47, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H. Dentine permeability and its role in the pathobiology of dentine sensitivity. Arch. Oral Biol. 1994, 39, S73–S80. [Google Scholar] [CrossRef]
- Liang, K.; Xiao, S.; Liu, H.; Shi, W.; Li, J.; Gao, Y.; He, L.; Zhou, X.; Li, J. 8DSS peptide induced effective dentinal tubule occlusion in vitro. Dent. Mater. 2018, 34, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Miglani, S.; Aggarwal, V.; Ahuja, B. Dentin hypersensitivity: Recent trends in management. J. Conserv. Dent. 2010, 13, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Lynch, E.; Brauer, D.S.; Karpukhina, N.; Gillam, D.G.; Hill, R.G. Multi-component bioactive glasses of varying fluoride content for treating dentin hypersensitivity. Dent. Mater. 2012, 28, 168–178. [Google Scholar] [CrossRef]
- Cummins, D. Recent advances in dentin hypersensitivity: Clinically proven treatments for instant and lasting sensitivity relief. Am. J. Dent. 2010, 23. [Google Scholar]
- Ritter, A.V.; De Dias, W.L.; Miguez, P.A.; Caplan, D.J.; Swift, E.J. Treating cervical dentin hypersensitivity with fluoride varnish. J. Am. Dent. Assoc. 2006, 137, 1013–1020. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.L.; Milgrom, P. Fluoride release from varnishes in two in vitro protocols. J. Am. Dent. Assoc. 2004, 135, 1696–1699. [Google Scholar] [CrossRef]
- Yu, X.; Liang, B.; Jin, X.; Fu, B.; Hannig, M. ComparativeIn VivoStudy on the Desensitizing Efficacy of Dentin Desensitizers and One-bottle Self-etching Adhesives. Oper. Dent. 2010, 35, 279–286. [Google Scholar] [CrossRef]
- Orchardson, R.; Gillam, D.G. Managing dentin hypersensitivity. J. Am. Dent. Assoc. 2006, 137, 990–998. [Google Scholar] [CrossRef]
- Du Min, Q.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Burwell, A.K.; Zhong, J.; Tai, B.J. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (novamin) for the treatment of dentin hypersensitivity. Am. J. Dent. 2008, 21, 210–214. [Google Scholar] [PubMed]
- Lopez, T.C.C.; Diniz, I.M.A.; Ferreira, L.S.; Marchi, J.; Borges, R.; De Cara, S.P.H.M.; D’Almeida-Couto, R.; Marques, M.M. Bioactive glass plus laser phototherapy as promise candidates for dentine hypersensitivity treatment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 105, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Jin, G.-Z.; Mahapatra, C.; Patel, K.D.; Chrzanowski, W.; Kim, H.-W. Mesoporous Silica-Layered Biopolymer Hybrid Nanofibrous Scaffold: A Novel Nanobiomatrix Platform for Therapeutics Delivery and Bone Regeneration. Acs Appl. Mater. Interfaces 2015, 7, 8088–8098. [Google Scholar] [CrossRef] [PubMed]
- Bonan, R.F.; Bonan, P.R.; Batista, A.U.; Sampaio, F.C.; Albuquerque, A.J.; Moraes, M.C.; Mattoso, L.H.; Glenn, G.M.; Medeiros, E.S.; Oliveira, J.E. In vitro antimicrobial activity of solution blow spun poly(lactic acid)/polyvinylpyrrolidone nanofibers loaded with Copaiba (Copaifera sp.) oil. Mater. Sci. Eng. C 2015, 48, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Knowles, J.C.; Day, R.M. Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomater. 2008, 4, 264–272. [Google Scholar] [CrossRef]
- De Oliveira, A.A.R.; Gomide, V.S.; Leite, M.D.F.; Mansur, H.S.; Pereira, M.D.M. Effect of polyvinyl alcohol content and after synthesis neutralization on structure, mechanical properties and cytotoxicity of sol-gel derived hybrid foams. Mater. Res. 2009, 12, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Velo, M.M.; Nascimento, T.R.; Scotti, C.K.; Bombonatti, J.F.; Furuse, A.Y.; Silva, V.D.; Simões, T.A.; Medeiros, E.S.; Blaker, J.J.; Silikas, N.; et al. Improved mechanical performance of self-adhesive resin cement filled with hybrid nanofibers-embedded with niobium pentoxide. Dent. Mater. 2019, 35, e272–e285. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Müller, T.; Schubert, D.W.; Boccaccini, A.R.; Yao, Q.; Roether, J.A. Electrospun Polyhydroxybutyrate/Poly(ε-caprolactone)/58S Sol–Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. Acs Appl. Mater. Interfaces 2016, 8, 17098–17108. [Google Scholar] [CrossRef]
- Fedorova, N.; Pourdeyhimi, B. High strength nylon micro- and nanofiber based nonwovens via spunbonding. J. Appl. Polym. Sci. 2007, 104, 3434–3442. [Google Scholar] [CrossRef]
- Keul, C.; Liebermann, A.; Roos, M.; Uhrenbacher, J.; Stawarczyk, B. The effect of ceramic primer on shear bond strength of resin composite cement to zirconia. J. Am. Dent. Assoc. 2013, 144, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Hunley, M.T.; Long, T.E. Electrospinning functional nanoscale fibers: A perspective for the future. Polym. Int. 2008, 57, 385–389. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Cury, J.A. Fluoride release from some dental materials in different solutions. Oper. Dent. 1999, 24, 14–19. [Google Scholar]
- Kolbasov, A.; Sinha-Ray, S.; Joijode, A.; Hassan, M.A.; Brown, D.B.; Maze, B.; Pourdeyhimi, B.; Yarin, A.L. Industrial-Scale Solution Blowing of Soy Protein Nanofibers. Ind. Eng. Chem. Res. 2016, 55, 323–333. [Google Scholar] [CrossRef]
- Polat, Y.; Pampal, E.S.; Stojanovska, E.; Simsek, R.; Hassanin, A.H.; Kilic, A.R.; Demir, A.; Yilmaz, S. Solution blowing of thermoplastic polyurethane nanofibers: A facile method to produce flexible porous materials. J. Appl. Polym. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Theoretical and experimental investigation of physical mechanisms responsible for polymer nanofiber formation in solution blowing. Polymer 2015, 56, 452–463. [Google Scholar] [CrossRef]
- Nascimento, T.R.D.L.; Velo, M.M.D.A.C.; Silva, C.F.; Cruz, S.B.S.C.; Gondim, B.L.C.; Mondelli, R.F.L.; Castellano, L.R.C. Current Applications of Biopolymer-based Scaffolds and Nanofibers as Drug Delivery Systems. Curr. Pharm. Des. 2019, 25, 3997–4012. [Google Scholar] [CrossRef] [PubMed]
- Rusin, R.P.; Agee, K.; Suchko, M.; Pashley, D.H. Effect of a new desensitizing material on human dentin permeability. Dent. Mater. 2010, 26, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Watson, T.F.; Thompson, I. Dentine desensitization induced by prophylactic and air-polishing procedures: An in vitro dentine permeability and confocal microscopy study. J. Dent. 2010, 38, 411–422. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Y.; Liu, Y.; Liao, Y.; Xu, R.; Hedin, N.E.; Fong, H. Bis-GMA/TEGDMA dental composites reinforced with electrospun nylon 6 nanocomposite nanofibers containing highly aligned fibrillar silicate single crystals. Polymer 2007, 48, 2720–2728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Magalhães, A.C.; Francisconi-Dos-Rios, L.F.; Calabria, M.P.; Araújo, D.; Buzalaf, M.; Lauris, J.; Pereira, J.C. Treatment of Dentin Hypersensitivity Using Nano-Hydroxyapatite Pastes: A Randomized Three-Month Clinical Trial. Oper. Dent. 2016, 41, E93–E101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabria, M.; Porfirio, R.; Fernandes, S.; Wang, L.; Buzalaf, M.; Pereira, J.; Magalhães, A. Comparative In Vitro Effect of TiF4 to NaF and Potassium Oxalate on Reduction of Dentin Hydraulic Conductance. Oper. Dent. 2014, 39, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiller, K.-A.; Buchalla, W.; Grillmeier, I.; Neubauer, C.; Schmalz, G. In vitro effects of hydroxyapatite containing toothpastes on dentin permeability after multiple applications and ageing. Sci. Rep. 2018, 8, 4888. [Google Scholar] [CrossRef]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin Hypersensitivity: Etiology, Diagnosis and Treatment; A Literature Review. J. Dent. 2013, 14, 136–145. [Google Scholar]
- Cate, J.M.T. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur. J. Oral Sci. 1997, 105, 461–465. [Google Scholar] [CrossRef]
- Gaffar, A. Treating hypersensitivity with fluoride varnishes. Compend. Contin. Educ. Dent. (Jamesburg, N.J. 1995) 1998, 19, 1088–1090. [Google Scholar]
- Yilmaz, H.G.; Kurtulmus-Yilmaz, S.; Cengiz, E. Long-Term Effect of Diode Laser Irradiation Compared to Sodium Fluoride Varnish in the Treatment of Dentine Hypersensitivity in Periodontal Maintenance Patients: A Randomized Controlled Clinical Study. Photomed. Laser Surg. 2011, 29, 721–725. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, J.; Li, X.; Yin, W.; He, T.; Hu, D.; Liao, Y.; Yao, X.; Wang, Y. Effect of a novel bioactive glass-ceramic on dentinal tubule occlusion: Anin vitrostudy. Aust. Dent. J. 2014, 60, 96–103. [Google Scholar] [CrossRef]
- Canali, G.D.; Ignacio, S.A.; Rached, R.N.; Souza, E.M. Clinical efficacy of resin-based materials for dentin hypersensitivity treatment. Am. J. Dent 2017, 30, 201–204. [Google Scholar] [PubMed]
- Osmari, D.; Fraga, S.; de Ferreira, A.C.O.; de Eduardo, C.P.; Marquezan, M.; Silveira, B.L. da In-office Treatments for Dentin Hypersensitivity: A Randomized Split-mouth Clinical Trial. Oral Health Prev. Dent. 2018, 16, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Nikaido, T.; Sonoda, H.; Foxton, R.M.; Tagami, J. Ultrastructure of the dentin-adhesive interface after acid-base challenge. J. Adhes Dent. 2004, 6, 183–190. [Google Scholar] [PubMed]
- Hoppe, A.; Mouriño, V.; Boccaccini, A.R. Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci. 2012, 1, 254–256. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, T.; Wang, X.P.; Fang, Q.F. Electrospun submicron bioactive glass fibers for bone tissue scaffold. J. Mater. Sci. Mater. Med. 2009, 20, 793–798. [Google Scholar] [CrossRef]
- Shiau, H.J. Dentin Hypersensitivity. J. Evid. Based Dent. Pract. 2012, 12, 220–228. [Google Scholar] [CrossRef]
- Marto, C.M.; Paula, A.B.; Nunes, T.; Pimenta, M.; Abrantes, A.M.; Pires, A.S.; Laranjo, M.; Coelho, A.; Donato, H.; Botelho, M.F.; et al. Evaluation of the efficacy of dentin hypersensitivity treatments-A systematic review and follow-up analysis. J. Oral Rehabil. 2019, 46, 952–990. [Google Scholar] [CrossRef]




| Material | Manufacturer | Composition | Procedure |
|---|---|---|---|
| Clearfil SE Bond | Kuraray, Sakazu, Kurashiki, Okayama, Japan | Primer: Water, MDP, HEMA, hydrophilic dimethacrylates, camphorquinone. Bond: MDP, Bis-GMA, HEMA, camphorquinone hydrophobic dimethacrylate, N/N-diethanol p-toluidine bond, colloidal silica. | Apply primer (20 s), and gently air dry Apply adhesive, gently air dry, light cure (10 s) |
| Duraphat | Colgate®, Sao Bernardo do Campo, SP, Brazil | 1 mL of this suspension contains 50 mg sodium fluoride, equivalent to 22.6 mg fluoride, in an alcoholic solution of natural resins. | Passively apply for 4 min |
| Material | Minimum (With Smear Layer) | Maximum (After Acid Etching) | Treatment | Final (After Erosive Challenge) |
|---|---|---|---|---|
| Clearfil SE Bond C | 55.86 (37.28) Aa | 100 Ab | 89.33 (40.56) Aab | 87.72 (42.80) Aab |
| Clearfil SE Bond + nanofiber CN | 47.57 (41.23) Aa | 100 Ab | 103.35 (28.19) Ab | 106.41 (43.13) Ab |
| Duraphat varnish D | 79.19 (46.60) Aa | 100 Aa | 106.07 (47.92) Aa | 120.61 (65.89) Aa |
| Duraphat varnish + nanofiberDN | 59.79 (27.52) Aa | 100 Ab | 116.06 (23.87) Ab | 130.86 (32.06) Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos-Bitencourt, N.; Velo, M.; Nascimento, T.; Scotti, C.; da Fonseca, M.G.; Goulart, L.; Castellano, L.; Ishikiriama, S.; Bombonatti, J.; Sauro, S. In Vitro Evaluation of Desensitizing Agents Containing Bioactive Scaffolds of Nanofibers on Dentin Remineralization. Materials 2021, 14, 1056. https://doi.org/10.3390/ma14051056
Bastos-Bitencourt N, Velo M, Nascimento T, Scotti C, da Fonseca MG, Goulart L, Castellano L, Ishikiriama S, Bombonatti J, Sauro S. In Vitro Evaluation of Desensitizing Agents Containing Bioactive Scaffolds of Nanofibers on Dentin Remineralization. Materials. 2021; 14(5):1056. https://doi.org/10.3390/ma14051056
Chicago/Turabian StyleBastos-Bitencourt, Natália, Marilia Velo, Tatiana Nascimento, Cassiana Scotti, Maria Gardennia da Fonseca, Luiz Goulart, Lucio Castellano, Sergio Ishikiriama, Juliana Bombonatti, and Salvatore Sauro. 2021. "In Vitro Evaluation of Desensitizing Agents Containing Bioactive Scaffolds of Nanofibers on Dentin Remineralization" Materials 14, no. 5: 1056. https://doi.org/10.3390/ma14051056
APA StyleBastos-Bitencourt, N., Velo, M., Nascimento, T., Scotti, C., da Fonseca, M. G., Goulart, L., Castellano, L., Ishikiriama, S., Bombonatti, J., & Sauro, S. (2021). In Vitro Evaluation of Desensitizing Agents Containing Bioactive Scaffolds of Nanofibers on Dentin Remineralization. Materials, 14(5), 1056. https://doi.org/10.3390/ma14051056







