Antibiofilm and Protein-Repellent Polymethylmethacrylate Denture Base Acrylic Resin for Treatment of Denture Stomatitis
Abstract
1. Introduction
2. Materials and Methods
- (1)
- HIPA resin + 0% MPC (control);
- (2)
- HIPA resin + 1.5% MPC;
- (3)
- HIPA resin + 3% MPC;
- (4)
- HIPA resin + 4.5% MPC.
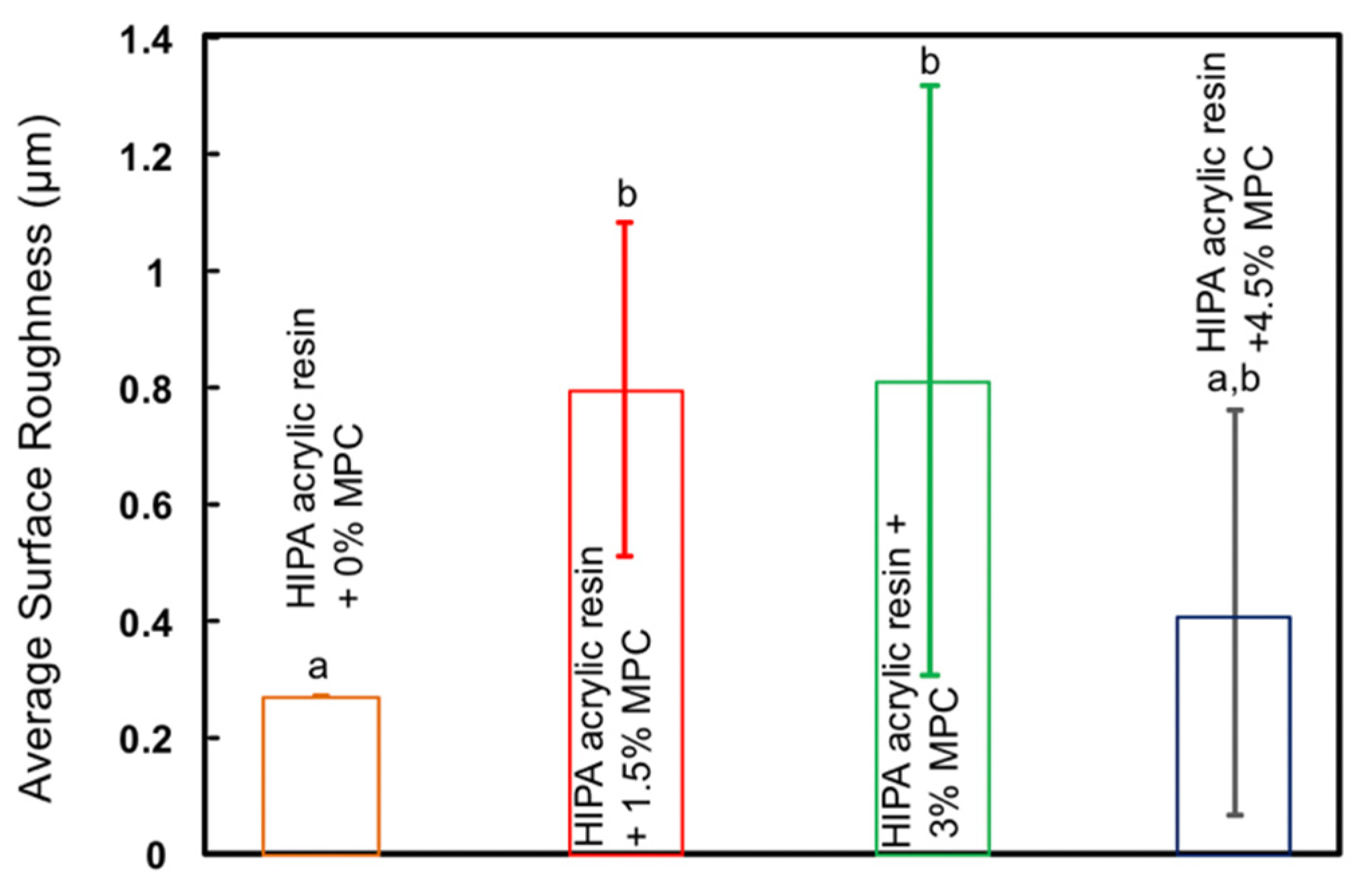
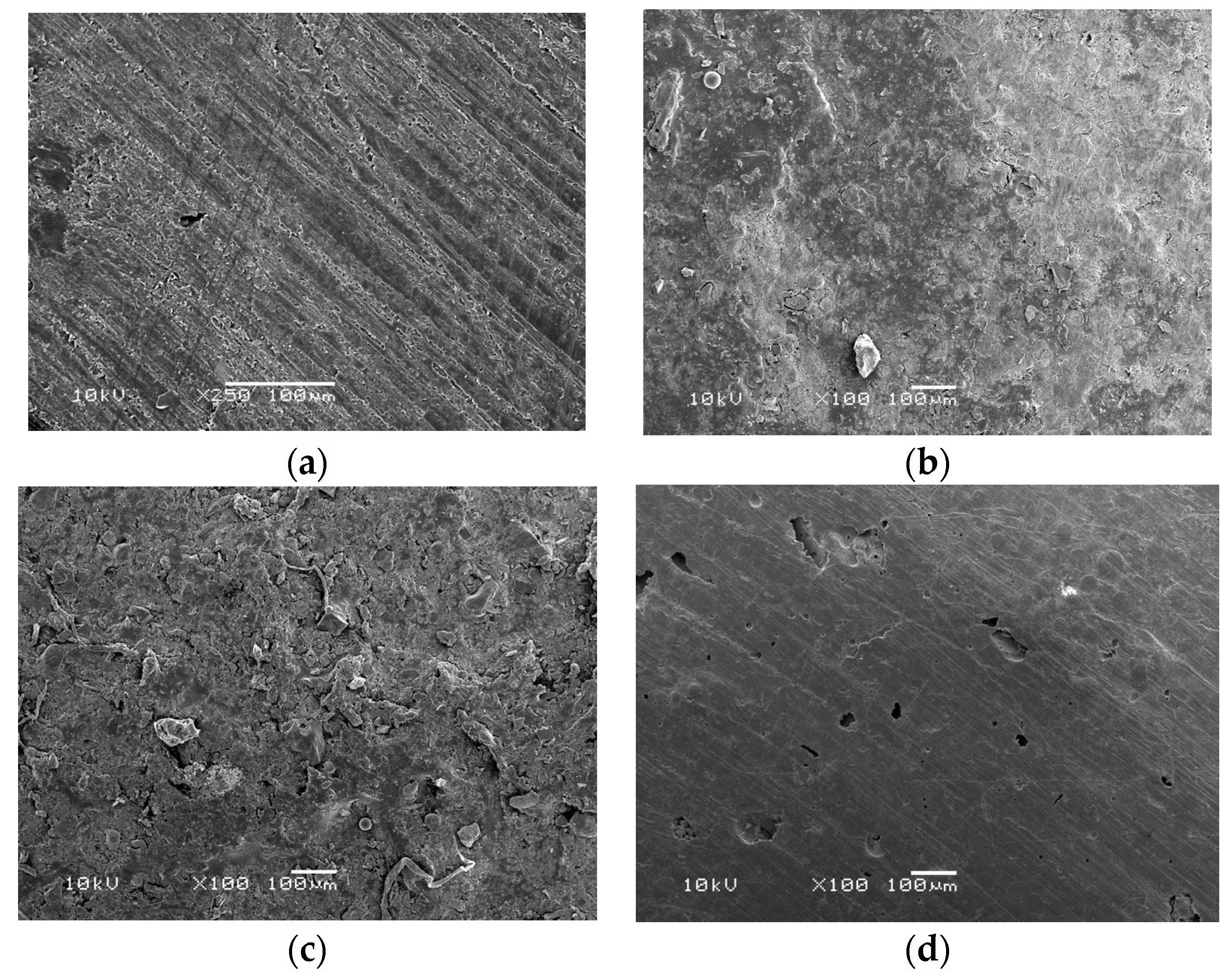
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Pachava, K.R.; Nadendla, L.K.; Alluri, L.S.C.; Tahseen, H.; Sajja, N.P. Invitro antifungal evaluation of denture soft liner incorporated with tea tree oil: A new therapeutic approach towards denture stomatitis. J. Clin. Diagn. Res. 2015, 9, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.M.P.D.C.; Bianchi, H.A.; Tadano, T.; Paula, C.R.D.; Hoffmann-Santos, H.D.; Leite, D.P., Jr.; Hahn, R.C. Factors related to oral candidiasis in elderly users and non-users of removable dental prostheses. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 17. [Google Scholar] [CrossRef]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; López-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for candida biofilms. Oral Surgery. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Zoccolotti, J.D.O.; Tasso, C.O.; Arbeláez, M.I.A.; Malavolta, I.F.; Pereira, E.C.D.S.; Esteves, C.S.G.; Jorge, J.H. Properties of an acrylic resin after immersion in antiseptic soaps: Low-cost, easy-access procedure for the prevention of denture stomatitis. PLoS ONE 2018, 13, e0203187. [Google Scholar]
- Peng, N.; Cai, P.; Mortimer, M.; Wu, Y.; Gao, C.; Huang, Q. The exopolysaccharide-eDNA interaction modulates 3D architecture of Bacillus subtilis biofilm. BMC Microbiol. 2020, 20, 115. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical appearance of oral candida infection and therapeutic strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef]
- Garcia, I.M.; Rodrigues, S.B.; Gama, M.E.R.; Leitune, V.C.B.; Melo, M.A.; Collares, F.M. Guanidine derivative inhibits C. albicans biofilm growth on denture liner without promote loss of materials’ resistance. Bioact. Mater. 2020, 5, 228–232. [Google Scholar] [CrossRef]
- Bakhshi, M.; Taheri, J.B.; Basir Shabestari, S.; Tanik, A.; Pahlevan, R. Comparison of therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatitis. Gerodontology 2012, 29, e680–e684. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Fouda, S.M. Current perspectives and the future of Candida albicans-associated denture stomatitis treatment. Dent. Med. Probl. 2020, 57, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.; Machado, A.L.; Vergani, C.E.; Giampaolo, E.T.; Pavarina, A.C.; Magnani, R. Effect of disinfectants on the hardness and roughness of reline acrylic resins. J. Prosthodont. 2006, 15, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.F.; Mendes, E.M.; Arthur, R.A.; de Negrini, T.C.; Lamers, M.L.; Mengatto, C.M. Antimicrobial effect and cytotoxic activity of vinegar-hydrogen peroxide mixture: A possible alternative for denture disinfection. J. Prosthet. Dent. 2019, 121, 966. [Google Scholar] [CrossRef]
- Ali, A.A.; Alharbi, F.A.; Suresh, C.S. Effectiveness of coating acrylic resin dentures on preventing candida adhesion. J. Prosthodont. 2013, 22, 445–450. [Google Scholar] [CrossRef]
- Yodmongkol, S.; Chantarachindawong, R.; Thaweboon, S.; Thaweboon, B.; Thaweboon, B.; Amornsakchai, T.; Srikhirin, T. The effects of silane-SiO2 nanocomposite films on Candida albicans adhesion and the surface and physical properties of acrylic resin denture base material. J. Prosth. Dent. 2014, 112, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Lazarin, A.A.; Zamperini, C.A.; Vergani, C.E.; Wady, A.F.; Giampaolo, E.T.; Machado, A.L. Candida albicans adherence to an acrylic resin modified by experimental photopolymerised coatings: An in vitro study. Gerodontology 2014, 31, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Ishihara, K.; Nomura, H.; Mihara, T.; Kurita, K.; Iwasaki, Y.; Nakabayashi, N. Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 1998, 39, 323–330. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, C.; Melo, M.A.S.; Bai, Y.X.; Cheng, L.; Xu, H.H.K. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int. J. Oral Sci. 2015, 7, 103–109. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Chen, C.; Liu, J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent. Mater. 2015, 31, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Al-Rafee, M. The epidemiology of edentulism and the associated factors: A literature Review. J. Fam. Med. Prim. Care 2020, 9, 1841. [Google Scholar] [CrossRef] [PubMed]
- SPSS Statistics, version 20.0; IBM: Armonk, NY, USA, 2018.
- Slade, G.D.; Akinkugbe, A.A.; Sanders, A.E. Projections of U.S. Edentulism prevalence following 5 decades of decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Davenport, J.C. The oral distribution of Candida in denture stomatitis. Brit. Dent. J. 1970, 129, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Millsap, K.W.; Bos, R.; Mei, H.C.V.D.; Busscher, H.J. Adhesion and surface-aggregation of Candida albicans from saliva on acrylic surfaces with adhering bacteria as studied in a parallel plate flow chamber. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 1999, 75, 351–359. [Google Scholar]
- Izumida, F.E.; Moffa, E.B.; Vergani, C.E.; Machado, A.L.; Jorge, J.H.; Giampaolo, E.T. In vitro evaluation of adherence of Candida albicans, Candida glabrata, and Streptococcus mutans to an acrylic resin modified by experimental coatings. Biofouling 2014, 30, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Sibarani, J.; Takai, M.; Ishihara, K. Surface modification on microfluidic devices with 2-methacryloyloxyethyl phosphorylcholine polymers for reducing unfavorable protein adsorption. Colloids Surf. B Biointerfaces 2007, 54, 88–93. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, J.; Melo, M.A.S.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234. [Google Scholar] [CrossRef]
- Xie, X.; Wang, L.; Xing, D.; Zhang, K.; Weir, M.D.; Liu, H.; Bai, Y.; Xu, H.H. Novel dental adhesive with triple benefits of calcium phosphate recharge, protein-repellent and antibacterial functions. Dent. Mater. 2017, 33, 553–563. [Google Scholar] [CrossRef]
- Mitwalli, H.; Balhaddad, A.A.; AlSahafi, R.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel CaF2 nanocomposites with antibacterial function and fluoride and calcium ion release to inhibit oral biofilm and protect teeth. J. Funct. Biomater. 2020, 11, 56. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Imazato, S.; Weir, M.D.; Reynolds, M.A.; Xu, H.H. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater. Sci. Eng. C 2016, 67, 702–710. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Melo, M.A.S.; Chen, C.; Fouad, A.F.; Bai, Y.; Xu, H.H.K. Novel protein-repellent and biofilm-repellent orthodontic cement containing 2-methacryloyloxyethyl phosphorylcholine. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Melo, M.A.S.; Bai, Y.; Xu, H.H.K. Novel protein-repellent dental adhesive containing 2-methacryloyloxyethyl phosphorylcholine. J. Dent. 2014, 42, 1284–1291. [Google Scholar] [CrossRef]
- Emami, E.; Kabawat, M.; Rompre, P.H.; Feine, J.S. Linking evidence to treatment for denture stomatitis: A meta-analysis of randomized controlled trials. J. Dent. 2014, 42, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.E.; Uy, C.E.; Ramani, R.S.; Waddell, J.N. Evaluation of surface roughness, hardness and elastic modulus of nanoparticle containing light-polymerized denture glaze materials. J. Mech. Behav. Biomed. Mater. 2020, 103, 103601. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Kirk, M.F. pH as a primary control in environmental microbiology: 1. Thermodynamic perspective. Front. Environ. Sci. 2018, 6, 21. [Google Scholar] [CrossRef]
- Goettsche, Z.S.; Ettinger, R.L.; Wefel, J.S.; Hogan, M.M.; Harless, J.D.; Qian, F. In vitro assessment of 3 dentifrices containing fluoride in preventing demineralization of overdenture abutments and root surfaces. J. Prosthet. Dent. 2014, 112, 1257–1264. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajunaid, S.O.; Baras, B.H.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K. Antibiofilm and Protein-Repellent Polymethylmethacrylate Denture Base Acrylic Resin for Treatment of Denture Stomatitis. Materials 2021, 14, 1067. https://doi.org/10.3390/ma14051067
Bajunaid SO, Baras BH, Balhaddad AA, Weir MD, Xu HHK. Antibiofilm and Protein-Repellent Polymethylmethacrylate Denture Base Acrylic Resin for Treatment of Denture Stomatitis. Materials. 2021; 14(5):1067. https://doi.org/10.3390/ma14051067
Chicago/Turabian StyleBajunaid, Salwa O., Bashayer H. Baras, Abdulrahman A. Balhaddad, Michael D. Weir, and Hockin H. K. Xu. 2021. "Antibiofilm and Protein-Repellent Polymethylmethacrylate Denture Base Acrylic Resin for Treatment of Denture Stomatitis" Materials 14, no. 5: 1067. https://doi.org/10.3390/ma14051067
APA StyleBajunaid, S. O., Baras, B. H., Balhaddad, A. A., Weir, M. D., & Xu, H. H. K. (2021). Antibiofilm and Protein-Repellent Polymethylmethacrylate Denture Base Acrylic Resin for Treatment of Denture Stomatitis. Materials, 14(5), 1067. https://doi.org/10.3390/ma14051067







