Fabrication and Characterization of Viton@FOX-7@Al Spherical Composite with Improved Thermal Decomposition Property and Safety Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results
3.1. XRD Analysis
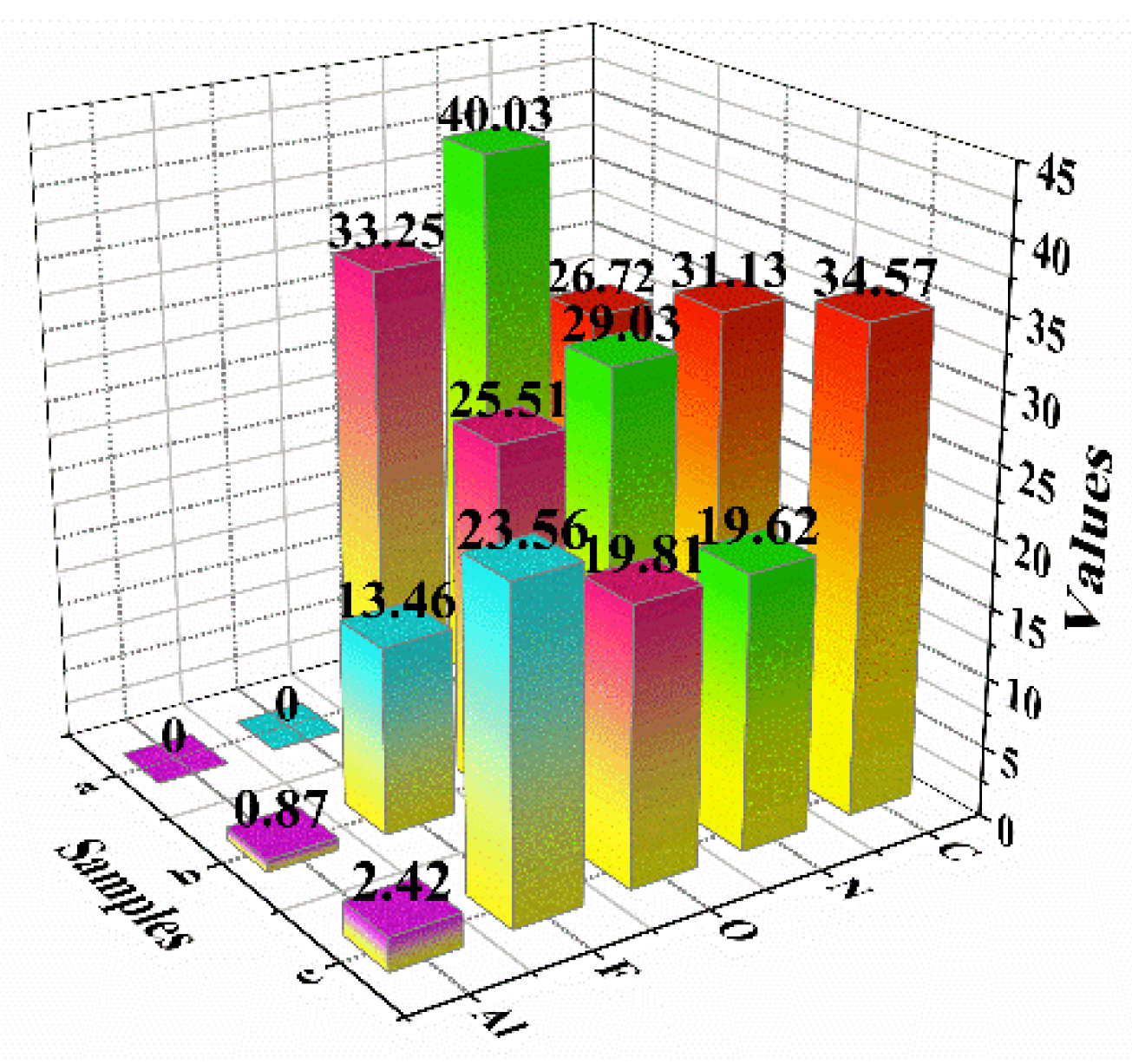
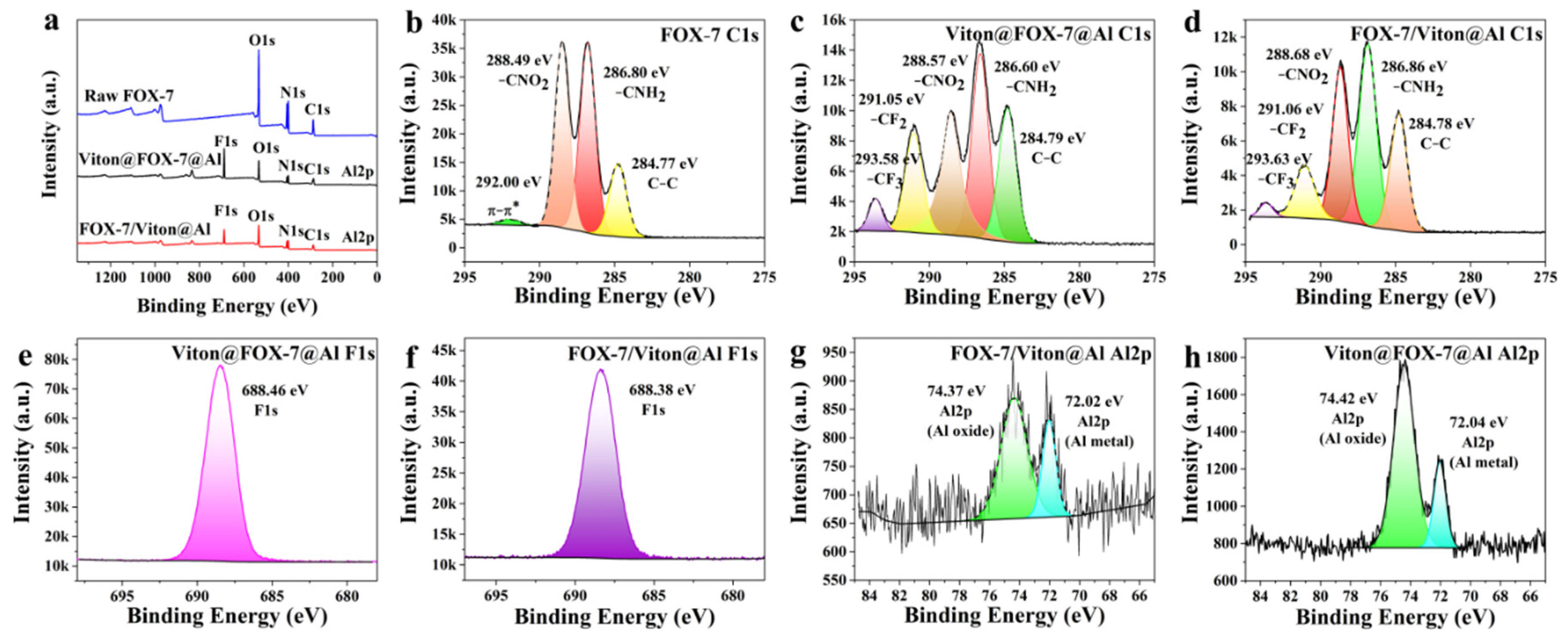
3.2. XPS Analysis
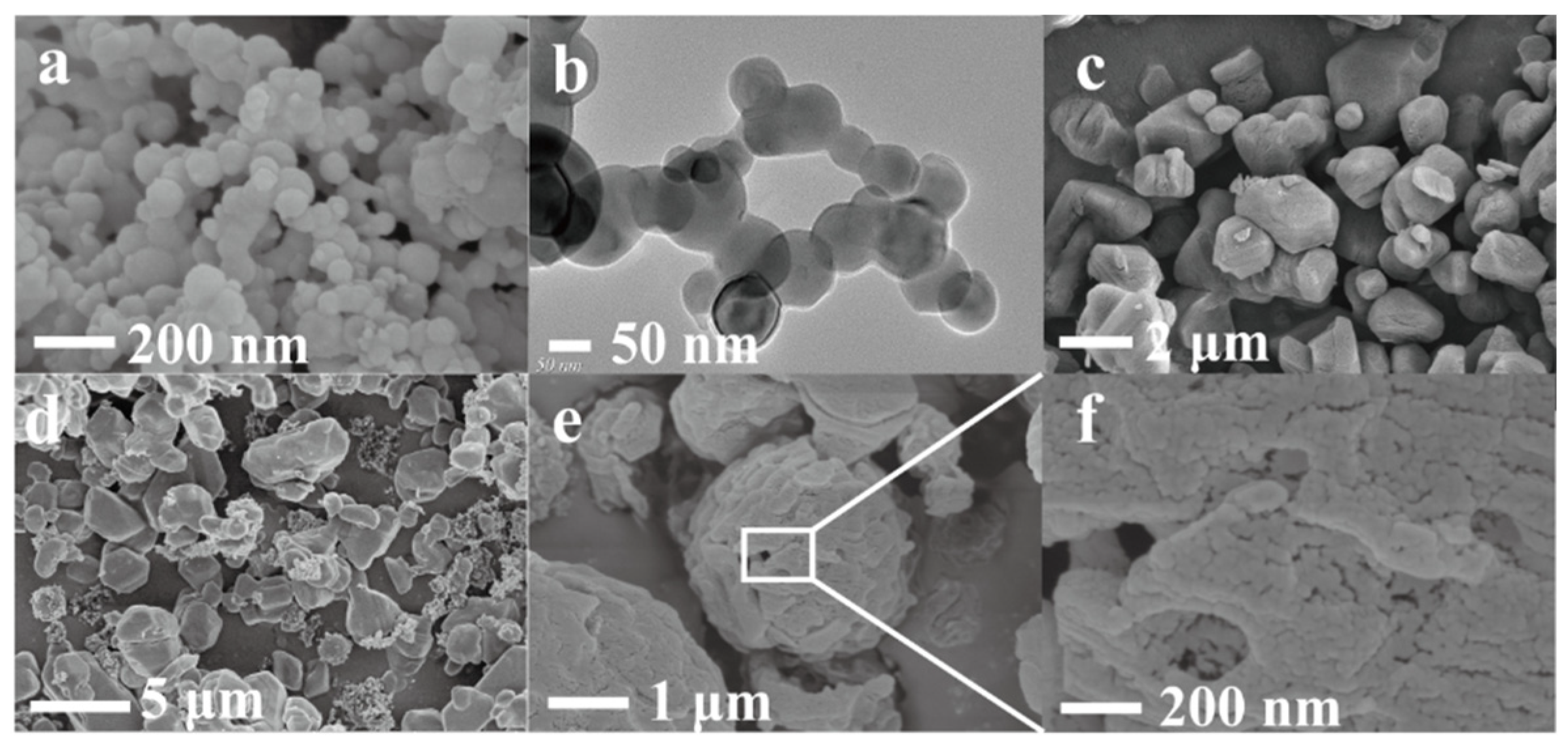
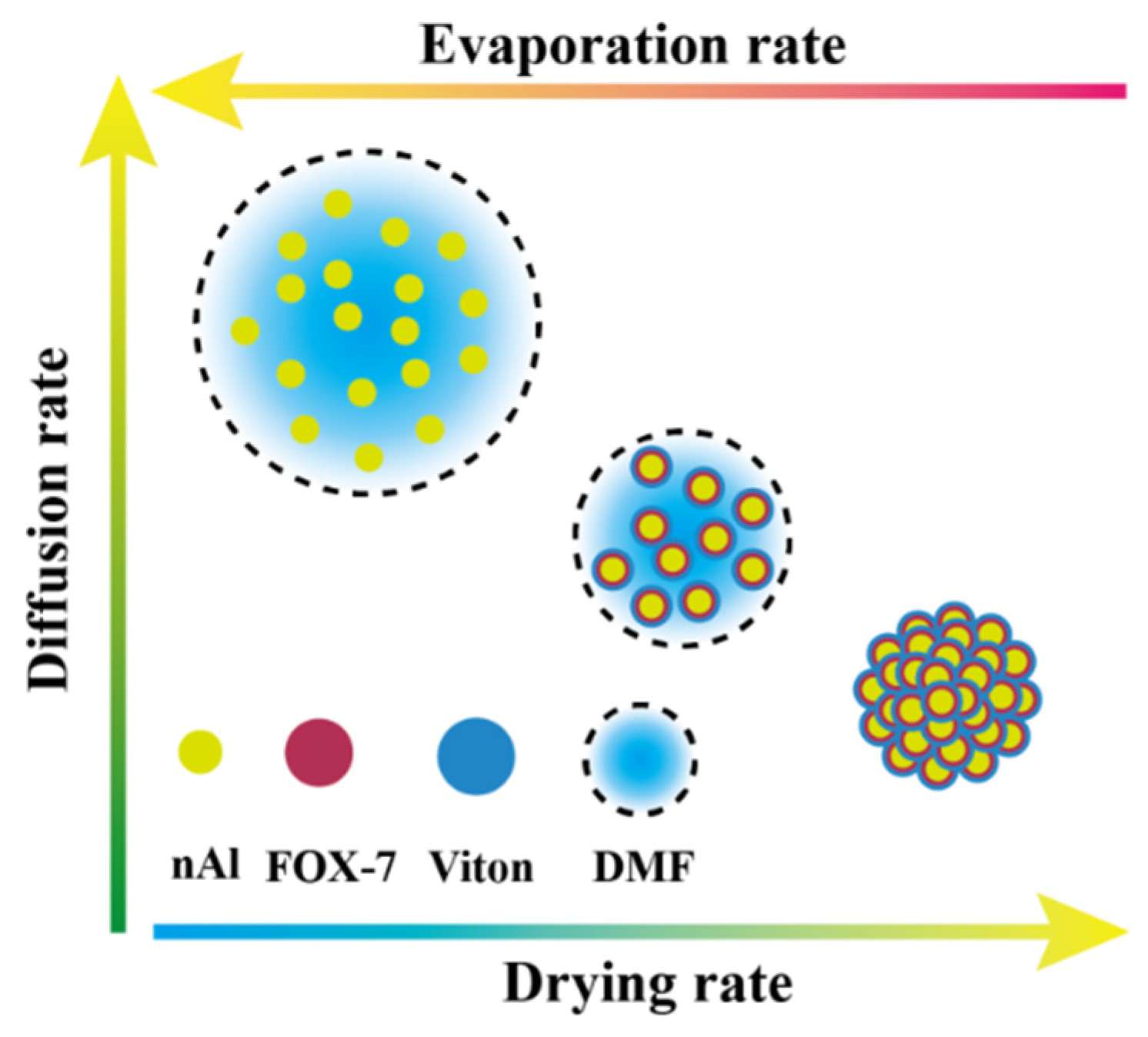
3.3. Morphology Analysis
3.4. DSC Analysis
3.5. Mechanical Sensitivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Soni, P.K.; Sarkar, C.; Mukherjee, N. Thermal reactivity of aluminized polymer-bonded explosives based on non-isothermal thermogravimetry and calorimetry measurements. J. Therm. Anal. Calorim. 2018, 136, 1021–1035. [Google Scholar] [CrossRef]
- Ji, X.; Tang, D.; Li, Y.; Xing, Z.; Wang, Y.; Wang, L.; Gao, Y.; Qin, W. Influence of aluminum nanoparticles and binders on the laser initiation of cyclotrimethylenetrinitramine. Opt. Laser Technol. 2019, 120, 105677. [Google Scholar] [CrossRef]
- Elia, T.; Baudin, G.; Genetier, M.; Lefrançois, A.; Osmont, A.; Catoire, L. Shock to Detonation Transition of Plastic Bonded Aluminized Explosives. Propellants Explos. Pyrotech. 2019, 45, 554–567. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Huang, H.; Ren, H.; Jiao, Q.-J. Influence of Aluminum Particle Size on Thermal Decomposition of RDX. J. Energetic Mater. 2013, 31, 178–191. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Y.; Wang, X.; Hao, G.; Liu, J.; Ke, X.; Chen, T.; Jiang, W. Preparation of a superfine RDX/Al composite as an energetic material by mechanical ball-milling method and the study of its thermal properties. RSC Adv. 2018, 8, 38047–38055. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Wu, P.; Song, C.; Yang, Y. Preparation and Properties of RDX/Aluminum Composites by Spray-Drying Method. J. Nanomater. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Hao, W.; Li, G.; Niu, L.; Gou, R.-J.; Zhang, C. Molecular Dynamics Insight into the Evolution of Al Nanoparticles in the Thermal Decomposition of Energetic Materials. J. Phys. Chem. C 2020, 124, 10783–10792. [Google Scholar] [CrossRef]
- Yan, T.; Ren, H.; Liu, J.; Jiao, Q. Facile preparation and synergetic energy releasing of nano-Al@RDX@Viton hollow microspheres. Chem. Eng. J. 2020, 379, 122333. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Zainuddin, M.I.; Tanaka, S.; Furushima, R.; Uematsu, K. Correlation between slurry properties and structures and properties of granules. J. Eur. Ceram. Soc. 2010, 30, 3291–3296. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, B.; Wu, P.; Shi, J.; Qiao, Z.; Yang, Z.; Yang, G.; Huang, B.; Nie, F. Facile, continuous and large-scale production of core–shell HMX@TATB composites with superior mechanical properties by a spray-drying process. RSC Adv. 2015, 5, 21042–21049. [Google Scholar] [CrossRef]
- Ji, W.; Li, X.; Wang, J.; Ye, B.; Wang, C. Preparation and Characterization of the Solid Spherical HMX/F2602by the Suspension Spray-Drying Method. J. Energetic Mater. 2016, 34, 357–367. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Liu, H. Recrystallize FOX-7 by Spray Recrystallization Method and Its Performance Characterization. J. Propellants, Explos. 2020, 43, 662–668. (In Chinese) [Google Scholar]
- Technology and Industry for National Defense of China. Experimental Methods of Sensitivity and Safety; GJB/772A-97; National Military Standard of China. Costind Army Standard Publishing House: Beijing, China, 1997. (In Chinese) [Google Scholar]
- Arora, N.; Jagirdar, B.R. Monodispersity and stability: Case of ultrafine aluminium nanoparticles (<5 nm) synthesized by the solvated metal atom dispersion approach. J. Mater. Chem. 2012, 22, 9058–9063. [Google Scholar] [CrossRef]
- Bellamy, A.J. FOX-7 (1,1-Diamino-2,2-dinitroethene). Family Med. 2007, 125, 1–33. [Google Scholar] [CrossRef]
- McMahon, B.W.; Perez, J.P.L.; Yu, J.; Boatz, J.A.; Anderson, S.L. Synthesis of Nanoparticles from Malleable and Ductile Metals Using Powder-Free, Reactant-Assisted Mechanical Attrition. ACS Appl. Mater. Interfaces 2014, 6, 19579–19591. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, W.D.; Liu, W.; Selomulya, C.; Chen, X.D.; Zhao, D. A General “Surface-Locking” Approach toward Fast Assembly and Processing of Large-Sized, Ordered, Mesoporous Carbon Microspheres. Angew. Chem.-Int. Ed. 2013, 52, 13764–13768. [Google Scholar] [CrossRef]
- Liu, D.; Xue, N.; Wei, L.; Zhang, Y.; Qin, Z.; Li, X.; Binks, B.P.; Yang, H. Surfactant Assembly within Pickering Emulsion Droplets for Fabrication of Interior-Structured Mesoporous Carbon Microspheres. Angew. Chem.-Int. Ed. 2018, 57, 10899–10904. [Google Scholar] [CrossRef]
- Carné-Sánchez, A.; Imaz, I.; Cano-Sarabia, M.; Maspoch, D. A spray-drying strategy for synthesis of nanoscale metal–organic frameworks and their assembly into hollow superstructures. Nat. Chem. 2013, 5, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, C.; Jia, X.; Wang, J.; Tan, Y. Fabrication of Nanoparticle-Stacked 1,1-Diamino-2,2-Dinitroethylene (FOX-7) Microspheres with Increased Thermal Stability. J. Nanomater. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Chatragadda, K.; Vargeese, A.A. A Kinetics Investigation on the Nitro-Nitrite Rearrangement Mediated Thermal Decomposition of High Temperature Monoclinic Phase of 1,1-Diamino-2,2-Dinitroethylene (γ-Fox-7). J. Chem. Sci. 2017, 129, 281–288. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Yang, W.; Song, C.; Sun, H.; Wang, J. Design and Characterization of a Cook-Off Resistant High-Energy Booster Explosive Based on CL-20/FOX-7. Propellants, Explos. Pyrotech. 2019, 44, 550–556. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Starink, M. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Barrie, P.J. The mathematical origins of the kinetic compensation effect: 1. The effect of random experimental errors. Phys. Chem. Chem. Phys. 2012, 14, 318–326. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Thermal degradation characteristics, kinetics, thermodynamic, and reaction mechanism analysis of pistachio shell pyrolysis for its bioenergy potential. Biomass Convers. Biorefinery 2020, 1–15. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.-D.; Sun, Y.-T.; Tian, J.-A.; Liu, H.-M.; Wu, B.-D.; Wang, J.-Y. Preparation and characterization of HMX/EVA/hBNNSs micro-composites with improved thermal stability and reduced sensitivity. Def. Technol. 2020. [Google Scholar] [CrossRef]
- Field, J.E. Hot spot ignition mechanisms for explosives. Accounts Chem. Res. 1992, 25, 489–496. [Google Scholar] [CrossRef]
- He, G.; Yang, Z.; Zhou, X.; Zhang, J.; Pan, L.; Liu, S. Polymer bonded explosives (PBXs) with reduced thermal stress and sensitivity by thermal conductivity enhancement with graphene nanoplatelets. Compos. Sci. Technol. 2016, 131, 22–31. [Google Scholar] [CrossRef]
- Wang, J.; Ye, B.; An, C.; Wu, B.; Li, H.; Wei, Y. Preparation and Properties of Surface-Coated HMX with Viton and Graphene Oxide. J. Energetic Mater. 2016, 34, 235–245. [Google Scholar] [CrossRef]


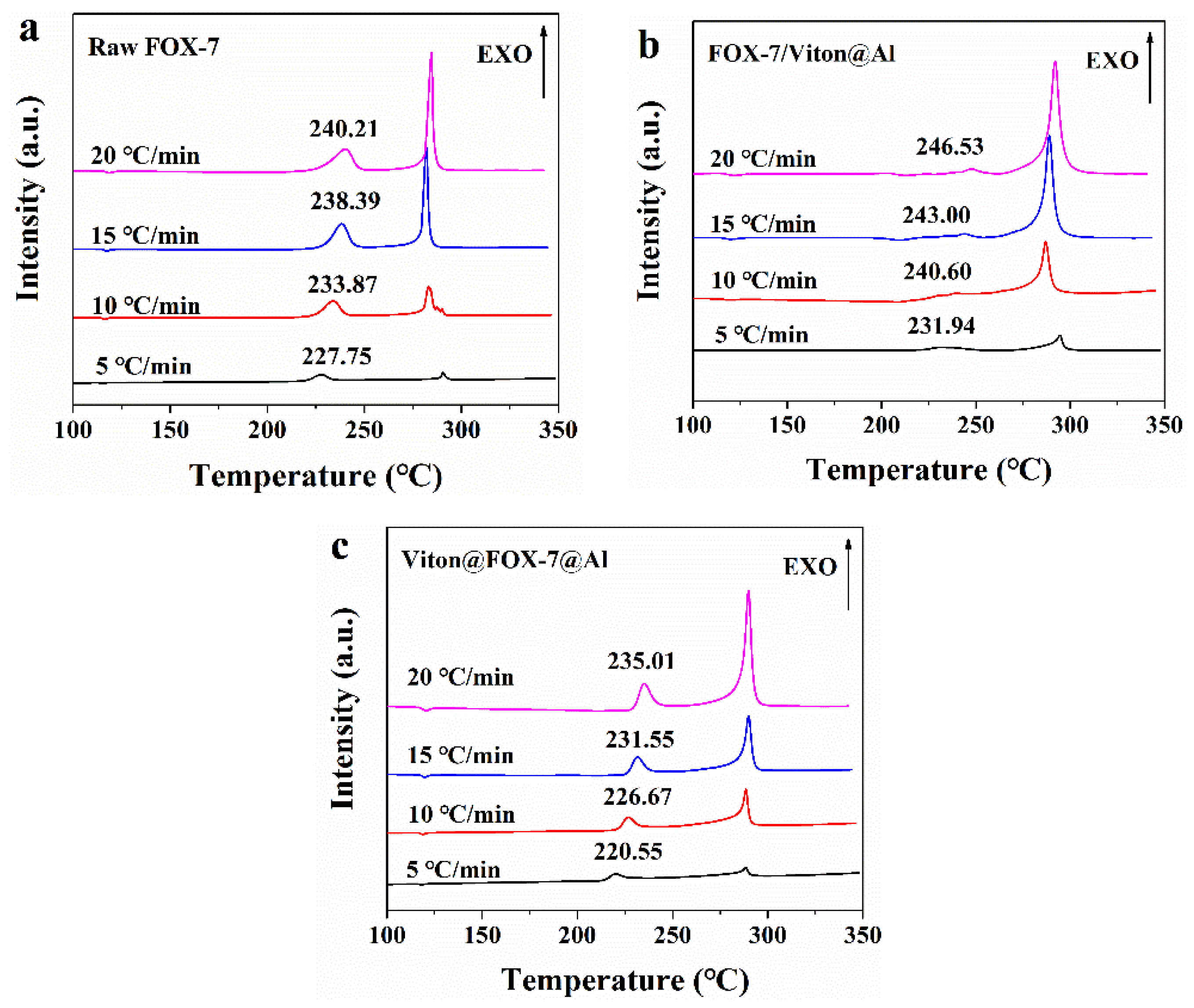
| Samples | Ea (kJ·mol−1) | Averaged Ea (kJ·mol−1) | ln A (s−1) | r2 | ||
|---|---|---|---|---|---|---|
| Kissinger | Starink | Ozawa | ||||
| Recrystallized FOX-7 | 294.56 | 303.13 | 295.42 | 297.70 | 68.87 | 0.9678 |
| FOX-7/Viton@Al | 200.40 | 208.91 | 201.25 | 203.52 | 46.95 | 0.9980 |
| Viton@FOX-7@Al | 190.75 | 199.08 | 191.59 | 193.81 | 45.75 | 0.9914 |
| Samples | T0 (°C) | Tb (°C) | ΔS≠/(J·mol−1·K−1) | ΔH≠/(kJ·mol−1) | ΔG≠/(kJ·mol−1) |
|---|---|---|---|---|---|
| Recrystallized FOX-7 | 223.55 | 230.64 | 315.25 | 290.43 | 133.84 |
| FOX-7/Viton@Al | 216.63 | 224.55 | 133.03 | 196.38 | 132.16 |
| Viton@FOX-7@Al | 213.37 | 223.97 | 123.11 | 186.71 | 126.81 |
| Samples | Impact Sensitivity H50 (cm) | Friction Sensitivity (%) |
|---|---|---|
| Recrystallized FOX-7 | 128.3 | 19 |
| FOX-7/Viton@Al | 135.4 | 12 |
| Viton@FOX-7@Al | >160 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, Y.; Song, C.; Sun, Y.; Han, Y.; Zhao, Y.; Wang, J. Fabrication and Characterization of Viton@FOX-7@Al Spherical Composite with Improved Thermal Decomposition Property and Safety Performance. Materials 2021, 14, 1093. https://doi.org/10.3390/ma14051093
Li X, Yang Y, Song C, Sun Y, Han Y, Zhao Y, Wang J. Fabrication and Characterization of Viton@FOX-7@Al Spherical Composite with Improved Thermal Decomposition Property and Safety Performance. Materials. 2021; 14(5):1093. https://doi.org/10.3390/ma14051093
Chicago/Turabian StyleLi, Xiaodong, Yue Yang, Changgui Song, Yantao Sun, Yuanqi Han, Yue Zhao, and Jingyu Wang. 2021. "Fabrication and Characterization of Viton@FOX-7@Al Spherical Composite with Improved Thermal Decomposition Property and Safety Performance" Materials 14, no. 5: 1093. https://doi.org/10.3390/ma14051093
APA StyleLi, X., Yang, Y., Song, C., Sun, Y., Han, Y., Zhao, Y., & Wang, J. (2021). Fabrication and Characterization of Viton@FOX-7@Al Spherical Composite with Improved Thermal Decomposition Property and Safety Performance. Materials, 14(5), 1093. https://doi.org/10.3390/ma14051093







