Foam Glass Lightened Sorel’s Cement Composites Doped with Coal Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Samples
2.2. Conducted Tests and Analyzes
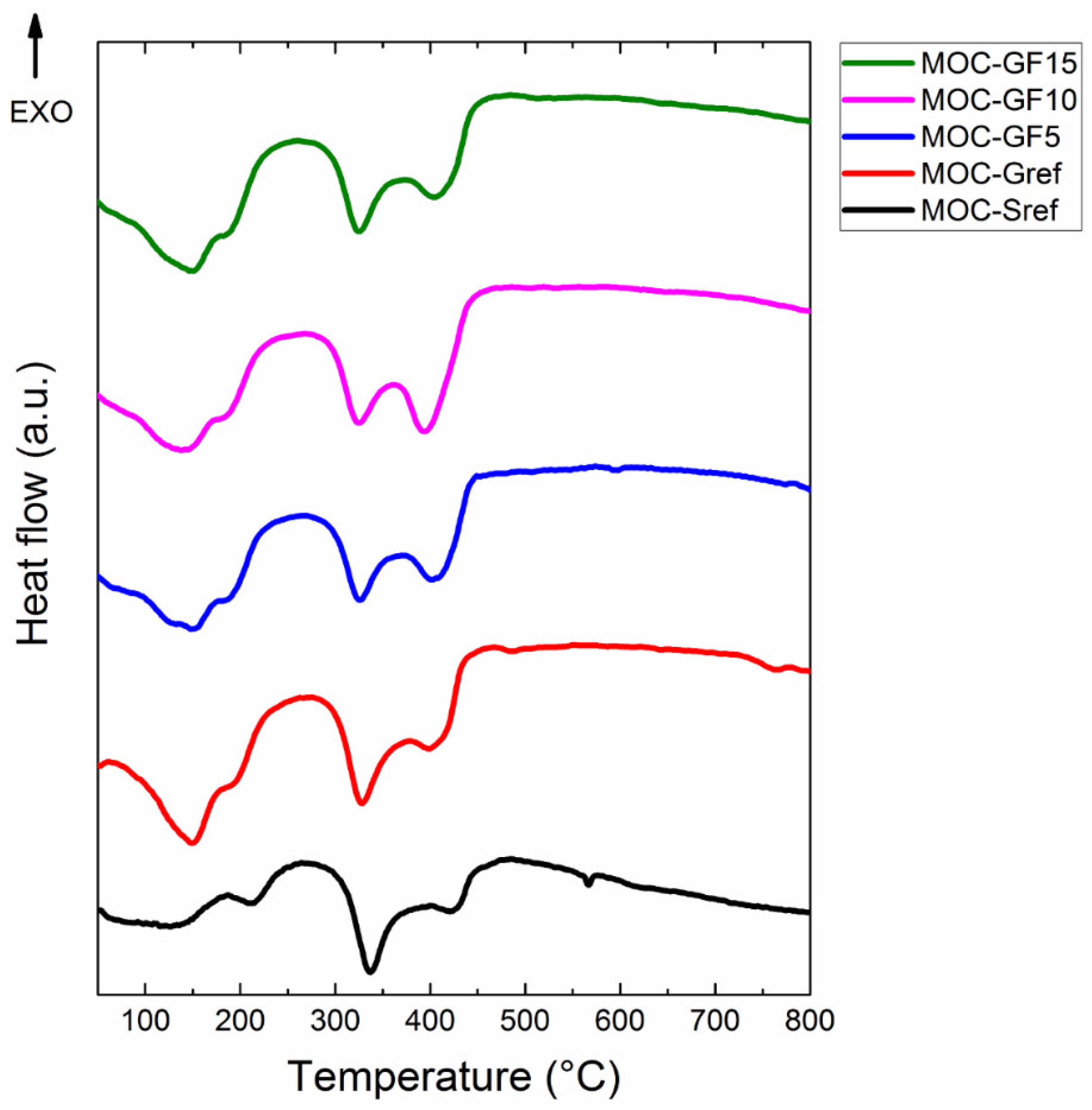
3. Results and Discussion
4. Conclusions
- 1)
- the full replacement of quartz sand by foam glass resulted in a great increase in the total porosity and pore volume, the highest porosity of 29.7% was obtained for composite made of MOC and foam glass;
- 2)
- the admixing of fly ash slightly lowered the total open porosity which decreased with the substitution ratio of glass granulate with fly ash;
- 3)
- the mechanical resistance and stiffness followed the trend of porosity, i.e., with the increase in the porosity the mechanical parameters were significantly reduced;
- 4)
- the use of fly ash in composite mix partially compensated the drop in mechanical parameters caused by the lightening of composites by foam glass;
- 5)
- the use of foam glass greatly reduced the weight of the developed composites and significantly improved their thermal insulation performance, the maximum drop in the thermal conductivity was about 75% compared to the control materials made of MOC and quartz sand;
- 6)
- as the mechanical resistance of composites with foam glass remained still high, the researched materials can be further lightened which will enable to attain materials with highly reduced heat transport ability;
- 7)
- the effect of the fly ash admixing on the hygrothermal function of the developed composites was negligible, therefore it can be simply applied as an alternative, low cost, and eco-efficient filler for MOC-based materials even in the combination with lightweight aggregate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN. World Urbanization Prospects: The 2018 Revision, 1st ed.; UN: New York, NY, USA, 2019; pp. 1–79. [Google Scholar]
- Huang, B.; Gao, X.; Xu, X.; Song, J.; Geng, Y.; Sarkis, J.; Fishman, T.; Kua, H.; Nakatani, J. A Life Cycle Thinking Framework to Mitigate the Environmental Impact of Building Materials. One Earth 2020, 3, 564–573. [Google Scholar] [CrossRef]
- Marinova, S.; Deetman, S.; van der Voet, E.; Daioglou, V. Global construction materials database and stock analysis of residential buildings between 1970. J. Clean. Prod. 2020, 247, 119146. [Google Scholar] [CrossRef]
- IEA. Energy Efficiency. Available online: https://www.iea.org/reports/energy-efficiency-2020 (accessed on 4 January 2021).
- Hossain, M.U.; Ng, S.T. Influence of waste materials on buildings’ life cycle environmental impacts: Adopting resource recovery principle. Resour. Conserv. Recycl. 2019, 142, 10–23. [Google Scholar] [CrossRef]
- Global Alliance for Buildings and Construction (GlobalABC). 2019 Global Status Report for Buildings and Construction: Towards A Zero-Emission, 1st ed.; IEA, United Nations Environment Programme: Paris, France, 2019; pp. 12–16. [Google Scholar]
- Lin, Y.; Du, H. Graphene reinforced cement composites: A review. Constr. Build. Mater. 2020, 265, 120312. [Google Scholar] [CrossRef]
- Awolusi, T.F.; Oke, O.L.; Atoyebi, O.D.; Akinkurolere, O.O.; Sojobi, A.O. Waste tires steel fiber in concrete: A review. Innov. Infrastruct. Solut. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Rao, N.; Suryaprakash, V.; Hemanth, B.; Hemanth Sai Kalyan, B. Investigation on mechanical propeties of concrete by partial replacement of cement with waste glass powder ad fly ash. Int. J. Mech. Prod. Eng. Res. Dev. 2020, 10, 11883–11888. [Google Scholar]
- Fischedick, M.; Roy, J.; Acquaye, A.; Allwood, J.; Ceron, J.-P.; Geng, Y.; Kheshgi, H.; Lanza, A.; Perczyk, D.; Price, L. Industry in Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group iii to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Report; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Qiao, H.; Cheng, Q.; Wang, J.; Shi, Y. The application review of magnesium oxychloride cement. J. Chem. Pharm. Res. 2014, 6, 180–185. [Google Scholar]
- Kusiorowski, R.; Zaremba, T. The use of asbestos wastes as a fillers on sorel cement. Ceram. Silik. 2018, 62, 31–40. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Poon, C.S.; Tsang, D.C. Water resistance of magnesium oxychloride cement wood board with the incorporation of supplementary cementitious materials. Constr. Build. Mater. 2020, 255, 119145. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Influence of supplementary cementitious materials on the performance and environmental impacts of reactive magnesia cement concrete. J. Clean. Prod. 2017, 159, 62–73. [Google Scholar] [CrossRef]
- Gomes, C.M.; Garry, A.-L.; Freitas, E.; Bertoldo, C.; Siqueira, G. Effects of Rice Husk Silica on microstructure and mechanical properties of Magnesium-oxychloride Fiber Cement (MOFC). Constr. Build. Mater. 2020, 241, 118022. [Google Scholar] [CrossRef]
- Aiken, T.A.; Russell, M.; McPolin, D.; Bagnall, L. Magnesium oxychloride boards: Understanding a novel building material. Mater. Struct. 2020, 53, 1–16. [Google Scholar] [CrossRef]
- Ye, Q.; Han, Y.; Zhang, S.; Gao, Q.; Zhang, W.; Chen, H.; Gong, S.; Shi, S.Q.; Xia, C.; Li, J. Bioinspired and biomineralized magnesium oxychloride cement with enhanced compressive strength and water resistance. J. Hazard. Mater. 2020, 383, 121099. [Google Scholar] [CrossRef]
- Jianli, M.; Youcai, Z.; Jinmei, W.; Li, W. Effect of magnesium oxychloride cement on stabilization/solidification of sewage sludge. Constr. Build. Mater. 2010, 24, 79–83. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Using incinerated sewage sludge ash to improve the water resistance of magnesium oxychloride cement (MOC). Constr. Build. Mater. 2017, 147, 519–524. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z. Light-weight wood–magnesium oxychloride cement composite building products made by extrusion. Constr. Build. Mater. 2012, 27, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Xu, B.; Ma, H.; Chen, B.; Li, Z. Micromechanical investigation of magnesium oxychloride cement paste. Constr. Build. Mater. 2016, 105, 496–502. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Yao, N.; Zhang, A. Recent progress of magnesium oxychloride cement: Manufacture, curing, structure and performance. Constr. Build. Mater. 2020, 255, 119381. [Google Scholar] [CrossRef]
- Gomes, C.M.; de Oliveira, A.D.S. Chemical phases and microstructural analysis of pastes based on magnesia cement. Constr. Build. Mater. 2018, 188, 615–620. [Google Scholar] [CrossRef]
- Misra, A.K.; Mathur, R. Magnesium oxychloride cement concrete. Bull. Mater. Sci. 2007, 30, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Di, S.; Gao, X.; Wang, R.; Chen, Z. Strength properties and associated mechanisms of magnesium oxychloride cement-solidified urban river sludge. Constr. Build. Mater. 2020, 250, 118933. [Google Scholar] [CrossRef]
- Beaudoin, J.; Ramachandran, V. Strength development in magnesium oxychloride and other cements. Cem. Concr. Res. 1975, 5, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Jiříčková, A.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Jankovský, O. Synthesis, Structure, and Thermal Stability of Magnesium Oxychloride 5Mg(OH)2∙MgCl2∙8H2O. Appl. Sci. 2020, 10, 1683. [Google Scholar] [CrossRef] [Green Version]
- Lojka, M.; Jankovský, O.; Jiříčková, A.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlík, Z. Thermal stability and kinetics of formation of magnesium oxychloride phase 3Mg(OH)MgCl8H2O. Materials 2020, 13, 767. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.Y.; Wang, F.; Liu, P.; Yu, Q.L.; Brouwers, H.J.H. Super-hydrophobic magnesium oxychloride cement (MOC): From structural control to self-cleaning property evaluation. Mater. Struct. 2020, 53, 1–10. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Yang, S.; Li, Z. Influence of curing regimes on mechanical properties of magnesium oxychloride cement-based composites. Constr. Build. Mater. 2016, 102, 613–619. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, S.; Wang, H.; Chen, R. Effect of 5-phase seed crystal on the mechanical properties and microstructure of magnesium oxychloride cement. Constr. Build. Mater. 2017, 150, 409–417. [Google Scholar] [CrossRef]
- Kastiukas, G.; Ruan, S.; Unluer, C.; Liang, S.; Zhou, X. Environmental Assessment of Magnesium Oxychloride Cement Samples: A Case Study in Europe. Sustainable 2019, 11, 6957. [Google Scholar] [CrossRef] [Green Version]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Yang, N.; Scott, A.; Watson, M. Reactive magnesium oxide products: Carbon neutral cement for the future. In Proceedings of the Concrete NZ Conference, Claudelands, Hamilton, OH, USA, 15–17 October 2018. [Google Scholar]
- Power, I.M.; Dipple, G.M.; Francis, P.S. Assessing the carbon sequestration potential of magnesium oxychloride cement building materials. Cem. Concr. Compos. 2017, 78, 97–107. [Google Scholar] [CrossRef]
- Luo, X.; Fan, W.; Li, C.; Wang, Y.; Yang, H.; Liu, X.; Yang, S. Effect of hydroxyacetic acid on the water resistance of magnesium oxychloride cement. Constr. Build. Mater. 2020, 246, 118428. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Comparison of glass powder and pulverized fuel ash for improving the water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2018, 86, 98–109. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Antončík, F.; Pivák, A.; Pavlík, Z. Influence of waste plastic aggregate and water-repellent additive on the properties of lightweight magnesium oxychloride cement composite. Appl. Sci. 2019, 9, 5463. [Google Scholar] [CrossRef] [Green Version]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Bi, W.; Cheeseman, C. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Constr. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- Chau, C.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Pivák, A.; Pavlíková, M.; Záleská, M.; Lojka, M.; Jankovský, O.; Pavlík, Z. Magnesium oxychloride cement composites with silica filler and coal fly ash admixture. Materials 2020, 13, 2537. [Google Scholar] [CrossRef]
- Lauermannová, A.-M.; Antončík, F.; Lojka, M.; Jankovský, O.; Pavlíková, M.; Pivák, A.; Záleská, M.; Pavlík, Z. The impact of graphene and diatomite admixtures on the performance and properties of high-performance magnesium oxychloride cement composites. Materials 2020, 13, 5708. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Cillari, G.; Ricciardi, P.; Miino, M.C.; Torretta, V.; Rada, E.C.; Abbà, A. The Production of Sustainable Concrete with the Use of Alternative Aggregates: A Review. Sustainable 2020, 12, 7903. [Google Scholar] [CrossRef]
- Tosic, N.; Marinkovic, S.; Stojanovic, A.; Nikola, T.; Snežana, M.; Aleksandar, S. Sustainability of the concrete industry: Current trends and future outlook. Tehnika 2017, 72, 38–44. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Non-Critical War Materials Factsheets: Study on the EU’s List of Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Drew, L.J.; Langer, W.H.; Sachs, J.S. Environmentalism and Natural Aggregate Mining. Nat. Resour. Res. 2002, 11, 19–28. [Google Scholar] [CrossRef]
- He, P.; Hossain, U.; Poon, C.S.; Tsang, D.C. Mechanical, durability and environmental aspects of magnesium oxychloride cement boards incorporating waste wood. J. Clean. Prod. 2019, 207, 391–399. [Google Scholar] [CrossRef]
- Biel, T.D.; Lee, H. Magnesium oxychloride cement concrete with recycled tire rubber. Transp. Res. Rec. 1996, 1561, 6–12. [Google Scholar] [CrossRef]
- Pavlíková, M.; Pivák, A.; Záleská, M.; Jankovský, O.; Reiterman, P.; Pavlík, Z. Magnesium oxychloride cement composites lightened with granulated scrap tires and expanded glass. Materials 2020, 13, 4828. [Google Scholar] [CrossRef]
- Sonat, C.; Unluer, C. Development of magnesium-silicate-hydrate (M-S-H) cement with rice husk ash. J. Clean. Prod. 2019, 211, 787–803. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Soe, K.; Hutchison, W.D.; Timmers, H.; Poblete, M.R. Effect of fly ash on mechanical properties of magnesium oxychloride cement under water attack. Struct. Concr. 2020, 21, 1181–1199. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Guan, B.; Xia, Y.; Sheng, Y.; Fang, J. Effect of Fly Ash on Rheological Properties of Magnesium Oxychloride Cement. J. Mater. Civ. Eng. 2019, 31, 04018405. [Google Scholar] [CrossRef]
- AASHTO. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; American Association of State Highway and Transportation Officials: Washington, DC, USA, 2007. [Google Scholar]
- Sanjuán, M.Á.; Suárez-Navarro, J.A.; Argiz, C.; Mora, P. Assessment of natural radioactivity and radiation hazards owing to coal fly ash and natural pozzolan Portland cements. J. Radioanal. Nucl. Chem. 2020, 325, 381–390. [Google Scholar] [CrossRef]
- Font, J.; Casas, M.; Forteza, R.; Cerdà, V.; Garcias, F. Natural radioactive elements and heavy metals in coal, fly ash and bottom ash from a thermal power plant. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1993, 28, 2061–2073. [Google Scholar] [CrossRef]
- Zielinski, R.A.; Budahn, J.R. Radionuclides in fly ash and bottom ash: Improved characterization based on radiography and low energy gamma-ray spectrometry. Fuel 1998, 77, 259–267. [Google Scholar] [CrossRef]
- Erdman, S.; Gapparova, K.; Khudyakova, T.; Tomshina, A. Magnesia Binder Preparation from Local Natural and Technogenic Raw Materials. Procedia Chem. 2014, 10, 310–313. [Google Scholar] [CrossRef] [Green Version]
- EN 933-1: Tests for Geometrical Properties of Aggregates Determination of Particle Size Distribution. Sieving Method; European Committee for Standardization: Brussels, Belgium, 2012. [Google Scholar]
- EN 1015-3: Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table); European Committee for Standardization: Brussels, Belgium, 1999.
- EN 1015-10: Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened 676 Mortar; European Committee for Standardization: Brussels, Belgium, 1999.
- Pavlik, Z.; Keppert, M.; Pavlíková, M.; Žumár, J.; Fořt, J.; Černý, R. Mechanical, hygric, and durability properties of cement mortar with mswi bottom ash as partial silica sand replacement. Cem. Wapno Beton 2014, 2014, 67–80. [Google Scholar]
- Pavlíková, M.; Zemanová, L.; Pokorný, J.; Záleská, M.; Jankovský, O.; Lojka, M.; Sedmidubský, D.; Pavlík, Z. Valorization of wood chips ash as an eco-friendly mineral admixture in mortar mix design. Waste Manag. 2018, 80, 89–100. [Google Scholar] [CrossRef]
- EN 1015-11, Methods of Test for Mortar for Masonry-Part 10: Determination of Flexural and Compressive Strength 678 of Hardened Mortar; European Committee for Standardization: Brussels, Belgium, 1999.
- EN 1015-18: Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar; European Committee for Standardization: Brussels, Belgium, 2002.
- Feng, C.; Guimarães, A.S.; Ramos, N.; Sun, L.; Gawin, D.; Konca, P.; Hall, C.; Zhao, J.; Hirsch, H.; Grunewald, J.; et al. Hygric properties of porous building materials (VI): A round robin campaign. Build. Environ. 2020, 185, 107242. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zheng, L.; Wen, J.; Wu, C.; Tan, Y. Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr. Build. Mater. 2013, 38, 1–7. [Google Scholar] [CrossRef]
- Gong, W.; Wang, N.; Zhang, N. Effect of fly ash and metakaolin on the macroscopic and microscopic characterizations of magnesium oxychloride cement. Constr. Build. Mater. 2021, 267, 120957. [Google Scholar] [CrossRef]
- Ma, Z.; Tang, Q.; Wu, H.; Xu, J.; Liang, C. Mechanical properties and water absorption of cement composites with various fineness and contents of waste brick powder from C&D waste. Cem. Concr. Compos. 2020, 114, 103758. [Google Scholar] [CrossRef]
- Yue, D.-T.; Tan, Z.-C.; Di, Y.-Y.; Lv, X.-R.; Sun, L.-X. Specific Heat Capacity and Thermal Conductivity of Foam Glass (Type 150P) at Temperatures from 80 to 400 K. Int. J. Thermophys. 2006, 27, 270–281. [Google Scholar] [CrossRef]
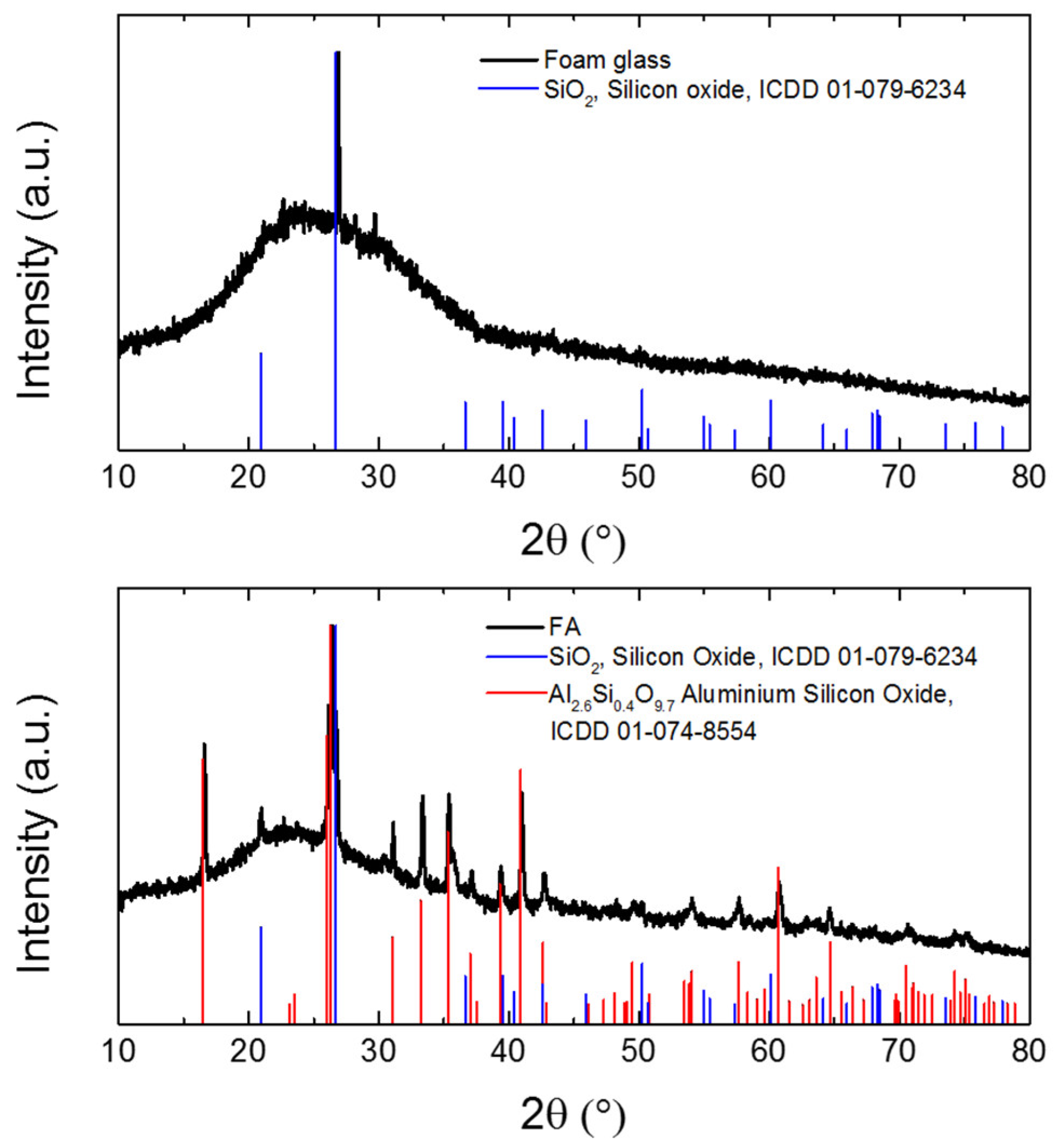
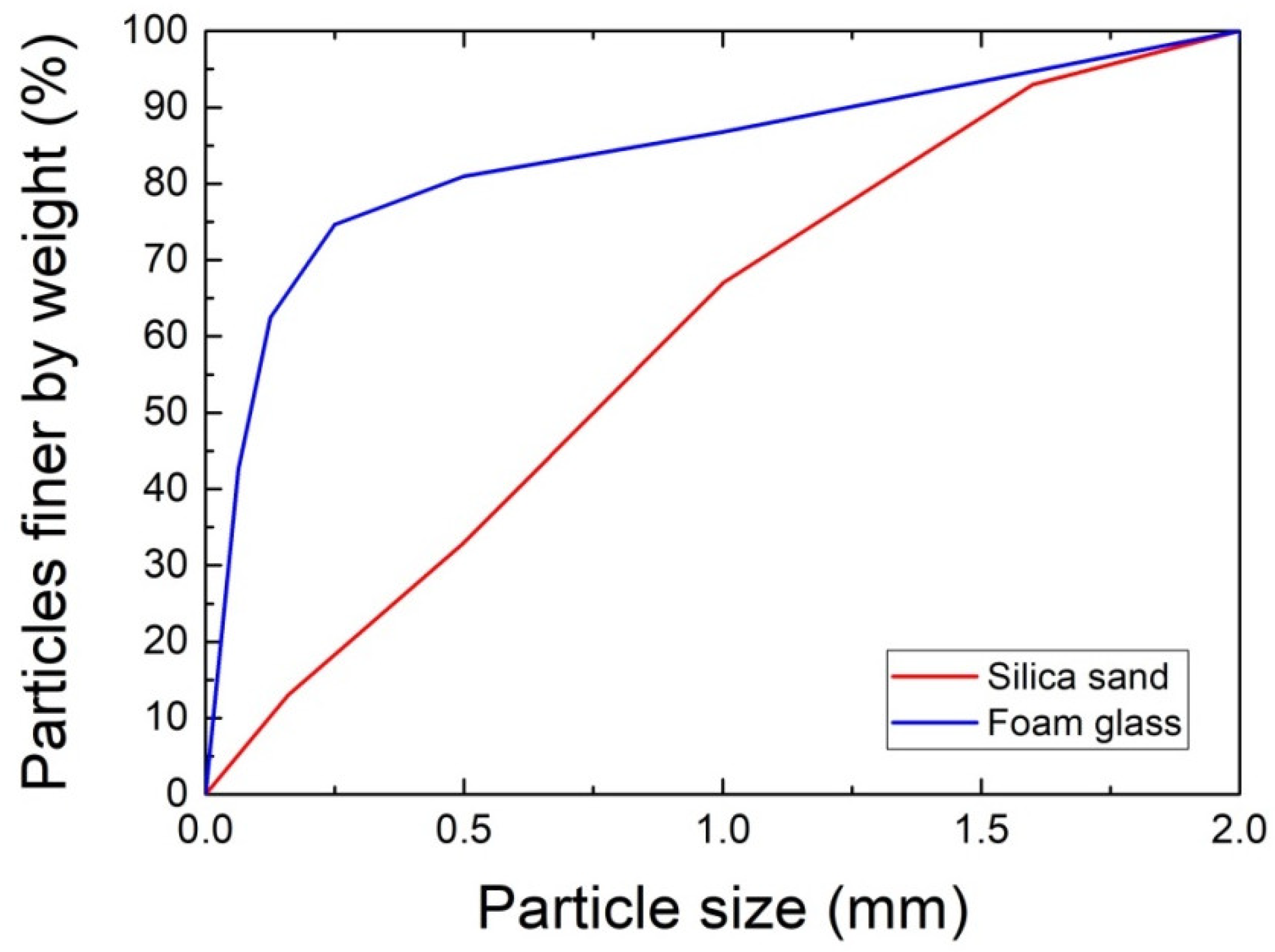
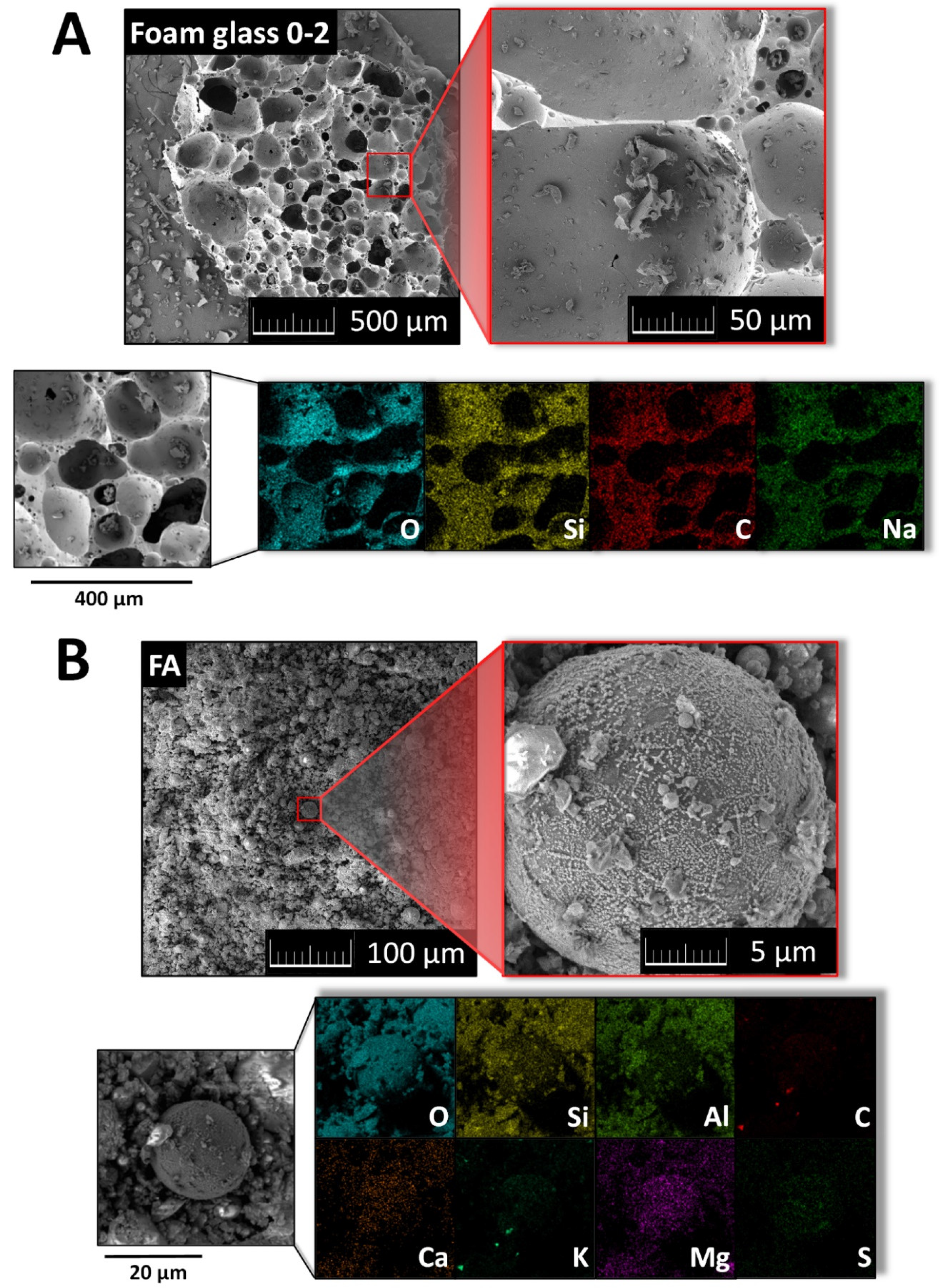
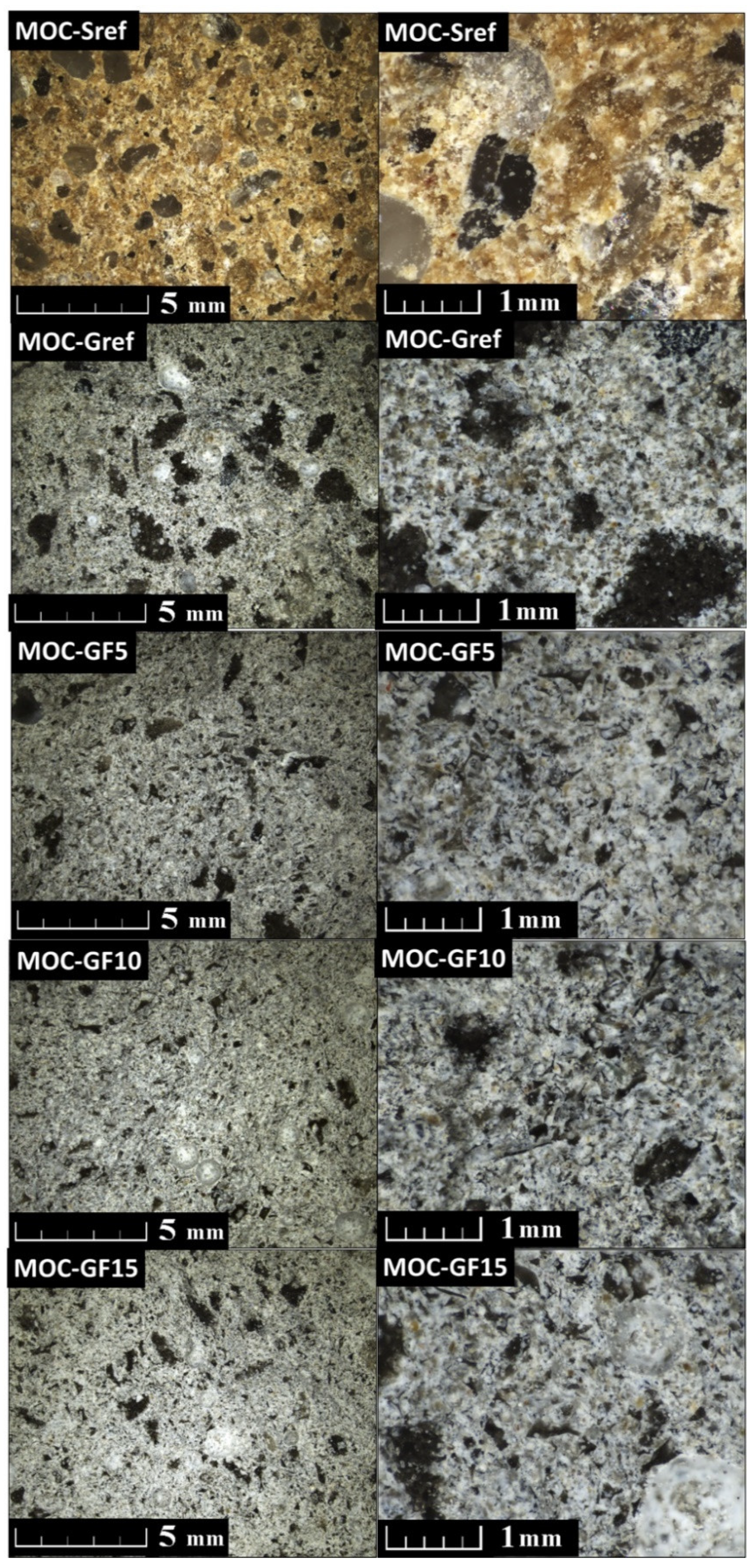

| Composite | MgO | MgCl2∙6H2O | Water | Sand | Foam Glass | Fly Ash |
|---|---|---|---|---|---|---|
| MOC-Sref | 1541.25 | 682.85 | 567.25 | 3 × 1125 | - | - |
| MOC-Gref | 1541.25 | 682.85 | 850.88 | - | 1564.65 | - |
| MOC-GF5 | 1541.25 | 682.85 | 850.88 | - | 1486.42 | 95.54 |
| MOC-GF10 | 1541.25 | 682.85 | 850.88 | - | 1408.18 | 191.07 |
| MOC-GF15 | 1541.25 | 682.85 | 850.88 | - | 1329.95 | 286.61 |
| Material | d10 (μm) | d50 (μm) | d90 (μm) |
|---|---|---|---|
| MgO | 1.09 | 7.36 | 23.04 |
| FA | 23.05 | 42.61 | 67.1 |
| Material | Dry Specific Density (kg∙m−3) | Dry Loose Bulk Density (kg∙m−3) | Blaine Fineness (m2∙kg−1) |
|---|---|---|---|
| MgO | 3329 | 839 | 702 |
| FA | 2057 | 791 | 403 |
| Material | Spread Diameter (mm) | ρb (kg∙m−3) | ρs (kg∙m−3) | Ψ (%) | Spread Diameter (mm) |
|---|---|---|---|---|---|
| MOC-Sref | 160/165 ± 5 | 2127 ± 30 | 2430 ± 29 | 12.5 ± 0.3 | 160 × 165 |
| MOC-Gref | 150/150 ± 5 | 1431 ± 20 | 2035 ± 24 | 29.7 ± 0.6 | 150 × 150 |
| MOC-GF5 | 155/150 ± 5 | 1453 ± 30 | 2041 ± 25 | 28.8 ± 0.6 | 155 × 150 |
| MOC-GF10 | 155/160 ± 5 | 1467 ± 21 | 2069 ± 25 | 28.1 ± 0.6 | 155 × 160 |
| MOC-GF15 | 160/165 ± 5 | 1495 ± 21 | 2056 ± 25 | 27.3 ± 0.5 | 160 × 165 |
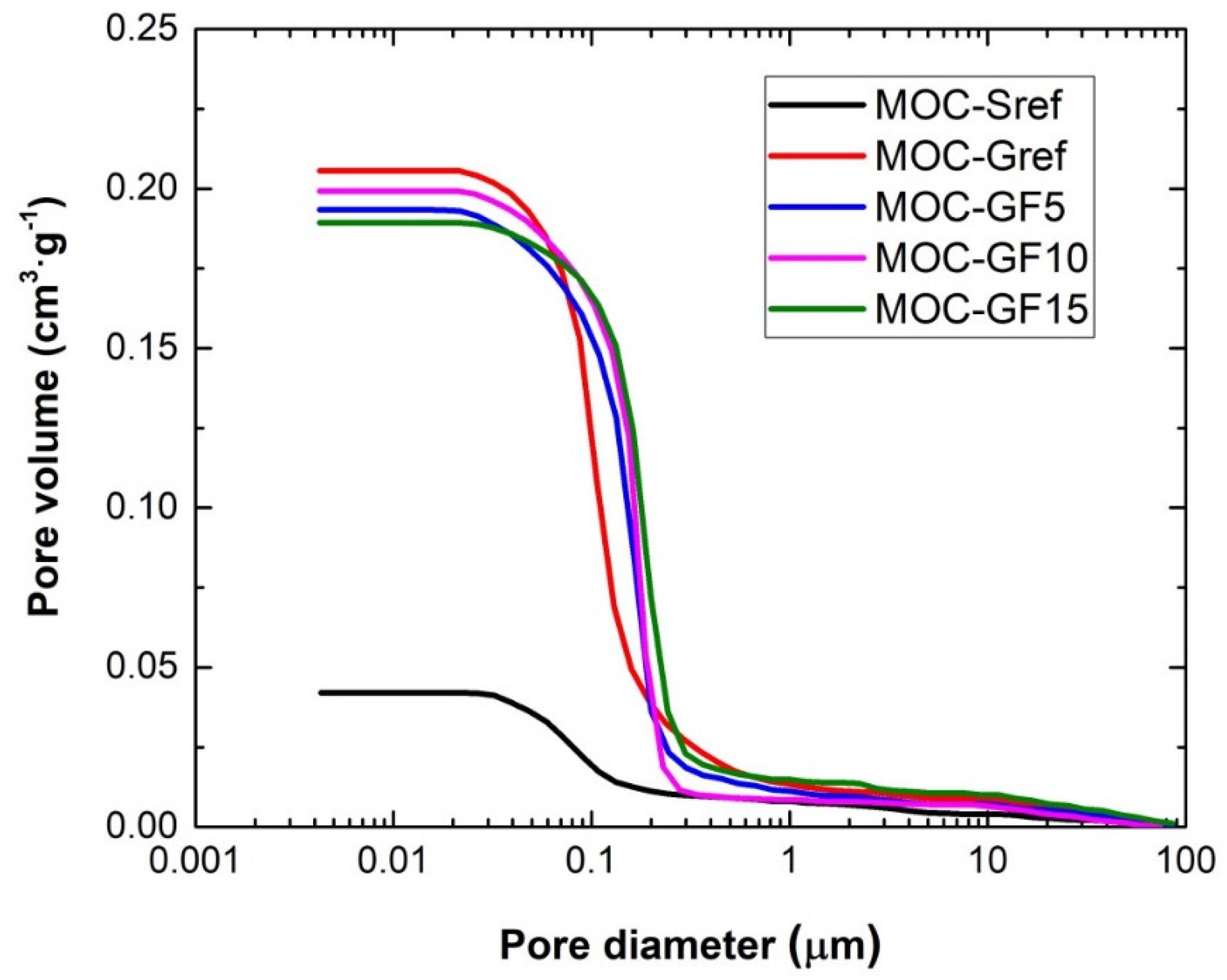
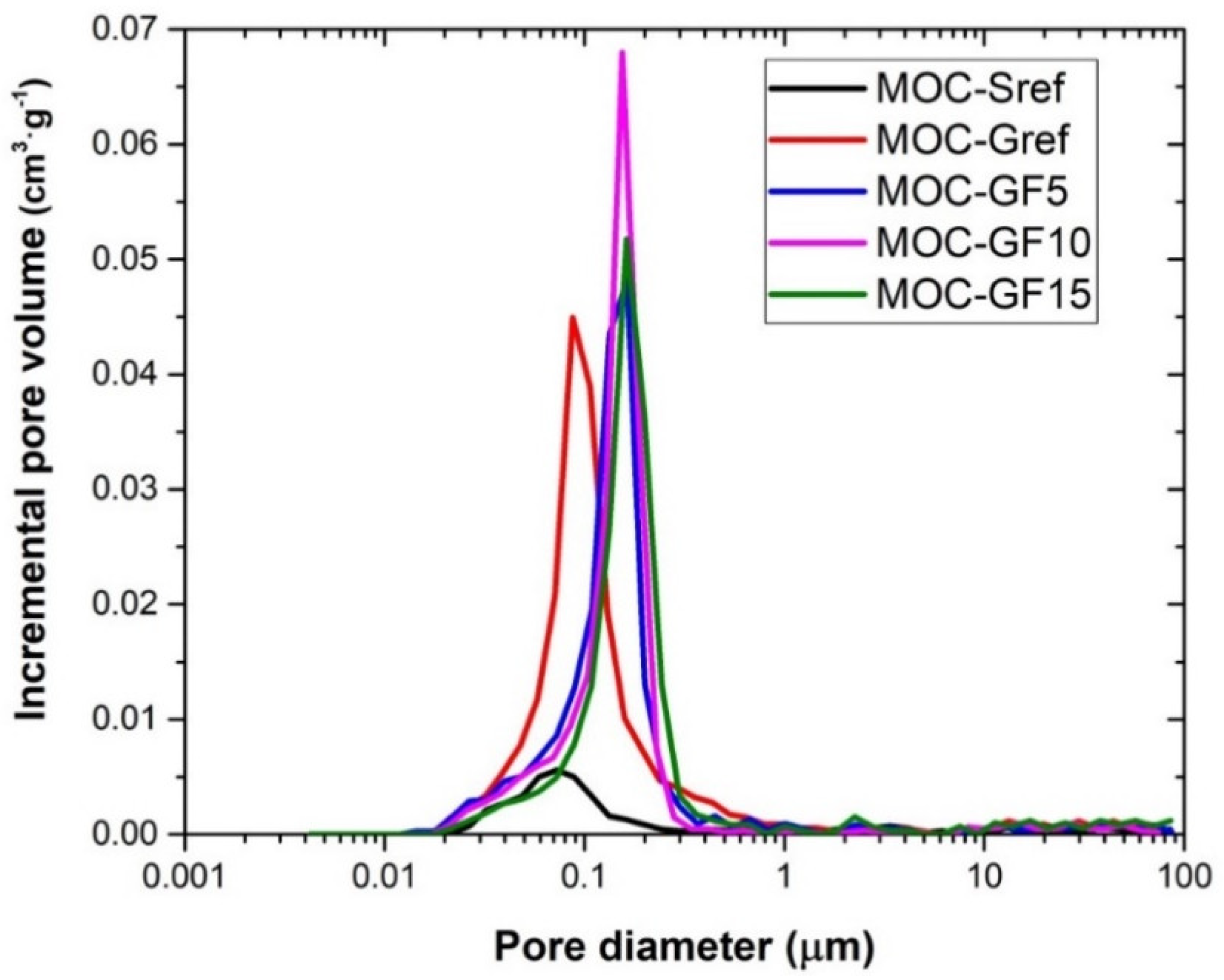
| Material | Total Pore Volume (cm3∙g−1) | Total Hg Porosity (%) | Average Pore Diameter (μm) |
|---|---|---|---|
| MOC-Sref | 0.0627 | 11.8 | 0.072 |
| MOC-Gref | 0.2057 | 29.6 | 0.087 |
| MOC-GF5 | 0.1935 | 28.9 | 0.163 |
| MOC-GF10 | 0.1993 | 28.4 | 0.162 |
| MOC-GF15 | 0.1894 | 26.7 | 0.154 |
| Material | ff (MPa) | fc (MPa) | Ed (GPa) | Aw (kg∙m−2∙s−1/2) |
|---|---|---|---|---|
| MOC-Sref | 23.8 ± 0.3 | 69.9 ± 1.0 | 31.2 ± 0.7 | 0.017 ± 4 × 10−5 |
| MOC-Gref | 9.3 ± 0.1 | 43.7 ± 0.6 | 10.7 ± 0.2 | 0.057 ± 1× 10−4 |
| MOC-GF5 | 9.4 ± 0.1 | 43.9 ± 0.6 | 10.9 ± 0.2 | 0.054 ± 1× 10−4 |
| MOC-GF10 | 9.5 ± 0.1 | 44.2 ± 0.6 | 11.2 ± 0.2 | 0.056 ± 1× 10−4 |
| MOC-GF15 | 9.8 ± 0.1 | 45.7 ± 0.6 | 11.3 ± 0.2 | 0.059 ± 1× 10−4 |
| Material | λ (W∙m−1∙K−1) | a × 10−6 (m2∙s−1) | cv × 106 (J∙m−3∙K−1) |
|---|---|---|---|
| MOC-Sref | 3.17 | 2.07 | 1.55 |
| MOC-Gref | 0.97 | 1.74 | 0.56 |
| MOC-GF5 | 0.80 | 1.75 | 0.45 |
| MOC-GF10 | 0.81 | 1.74 | 0.46 |
| MOC-GF15 | 0.79 | 1.79 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivák, A.; Pavlíková, M.; Záleská, M.; Lojka, M.; Lauermannová, A.-M.; Faltysová, I.; Jankovský, O.; Pavlík, Z. Foam Glass Lightened Sorel’s Cement Composites Doped with Coal Fly Ash. Materials 2021, 14, 1103. https://doi.org/10.3390/ma14051103
Pivák A, Pavlíková M, Záleská M, Lojka M, Lauermannová A-M, Faltysová I, Jankovský O, Pavlík Z. Foam Glass Lightened Sorel’s Cement Composites Doped with Coal Fly Ash. Materials. 2021; 14(5):1103. https://doi.org/10.3390/ma14051103
Chicago/Turabian StylePivák, Adam, Milena Pavlíková, Martina Záleská, Michal Lojka, Anna-Marie Lauermannová, Ivana Faltysová, Ondřej Jankovský, and Zbyšek Pavlík. 2021. "Foam Glass Lightened Sorel’s Cement Composites Doped with Coal Fly Ash" Materials 14, no. 5: 1103. https://doi.org/10.3390/ma14051103






