Phase Composition of Al-Si Coating from the Initial State to the Hot-Stamped Condition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
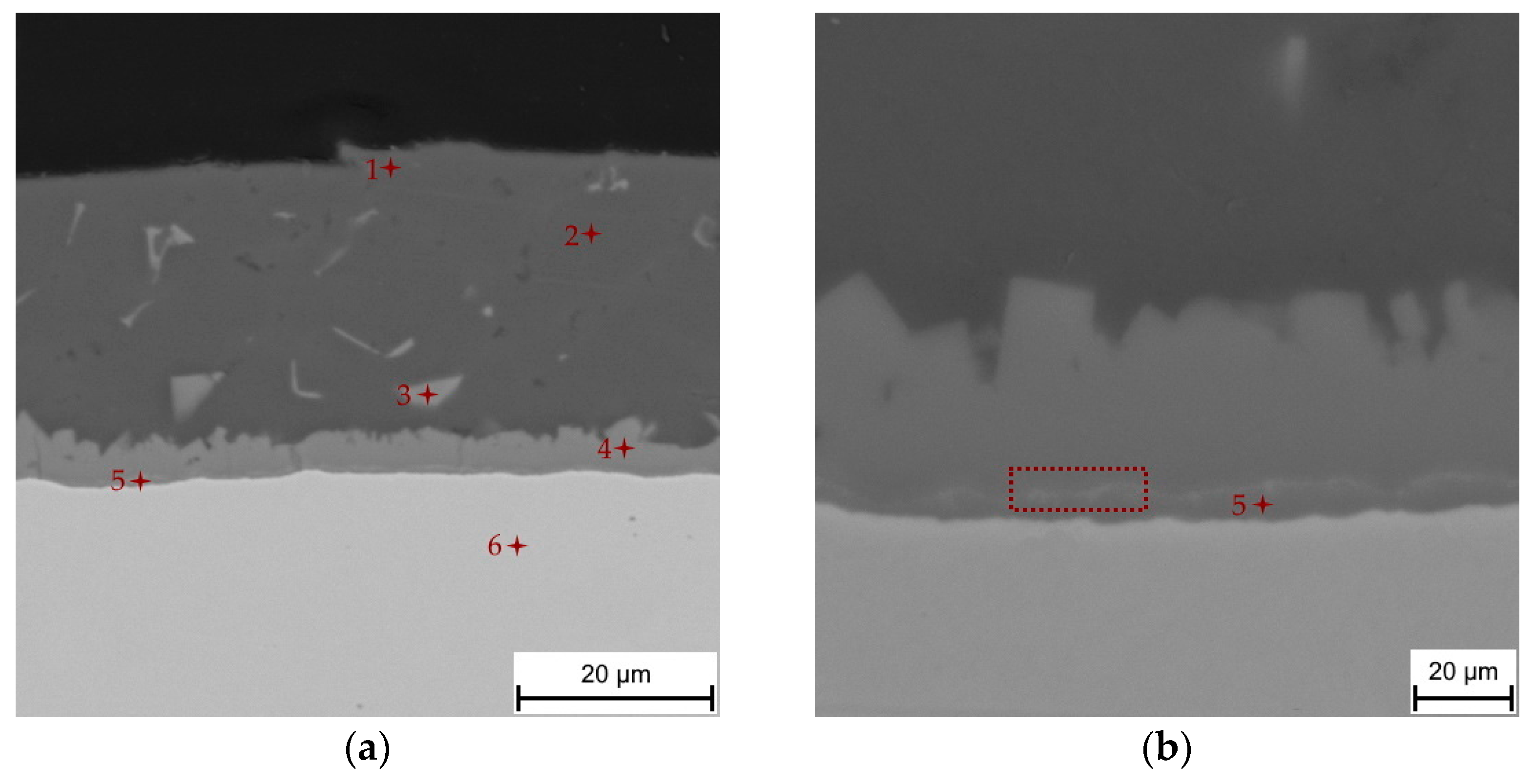
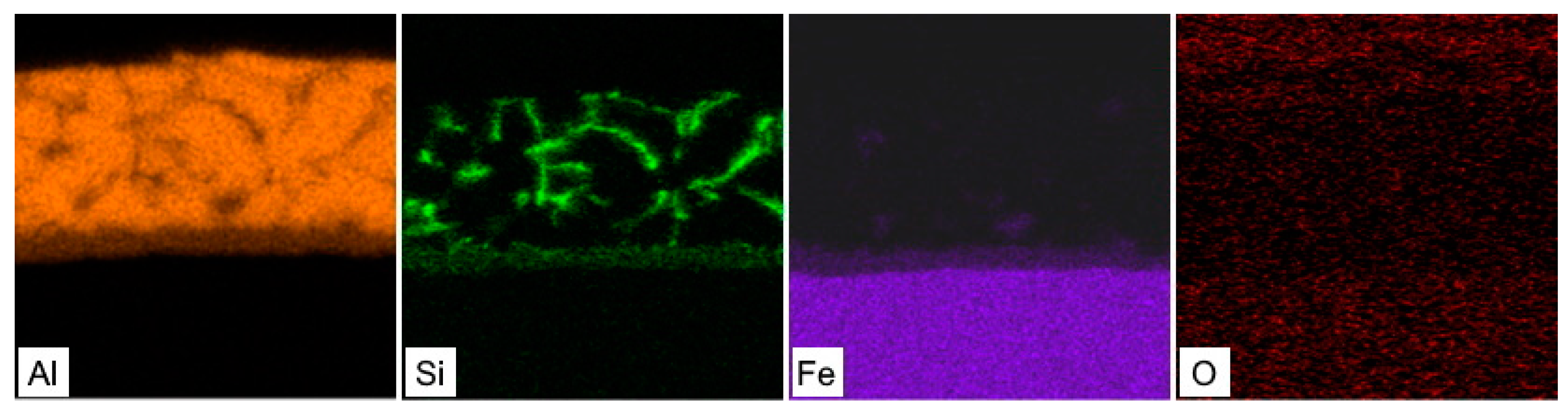
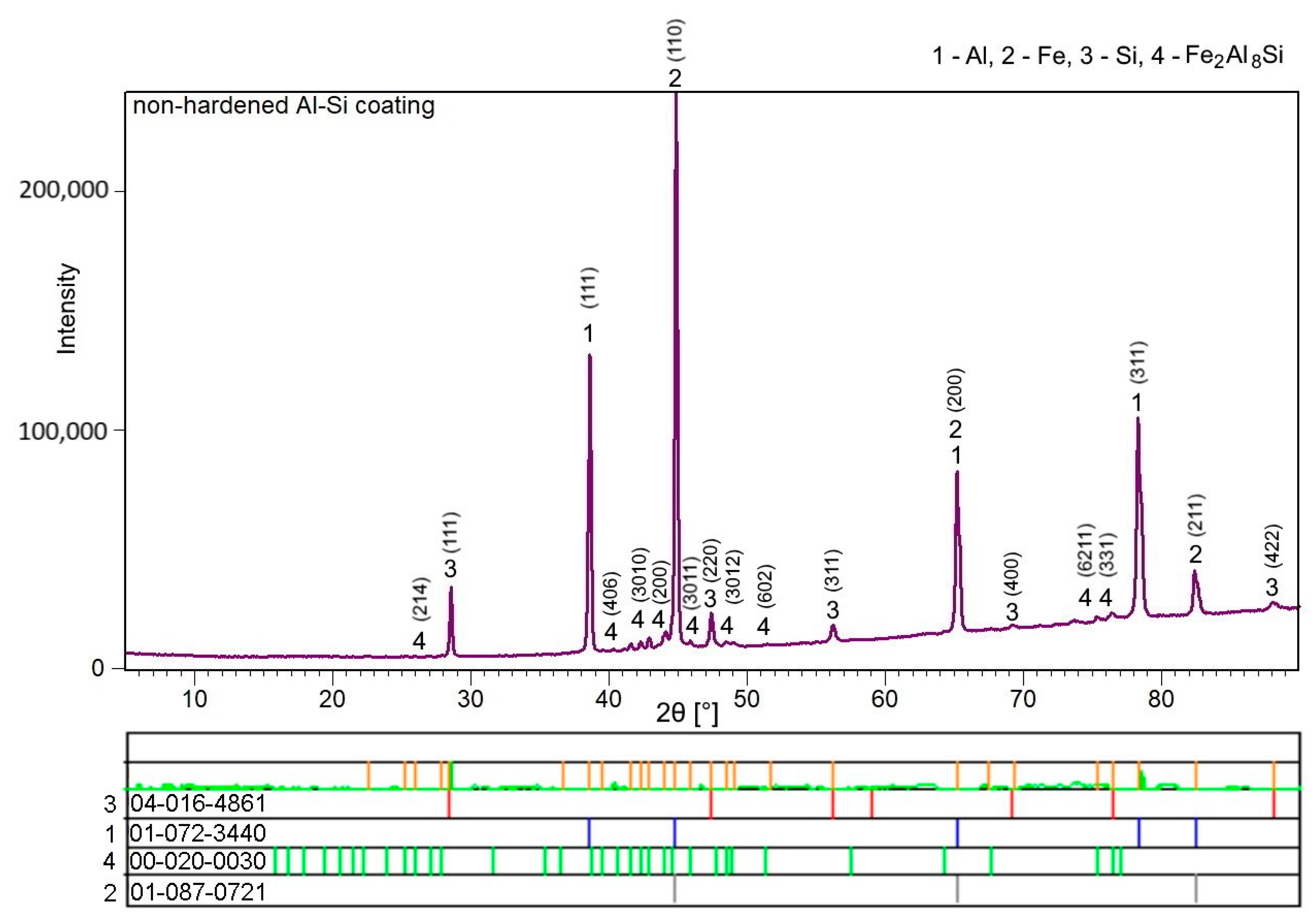
3.1. Chemical and Phase Composition of the As-Received 22MnB5 in the Hot-Dipped Condition
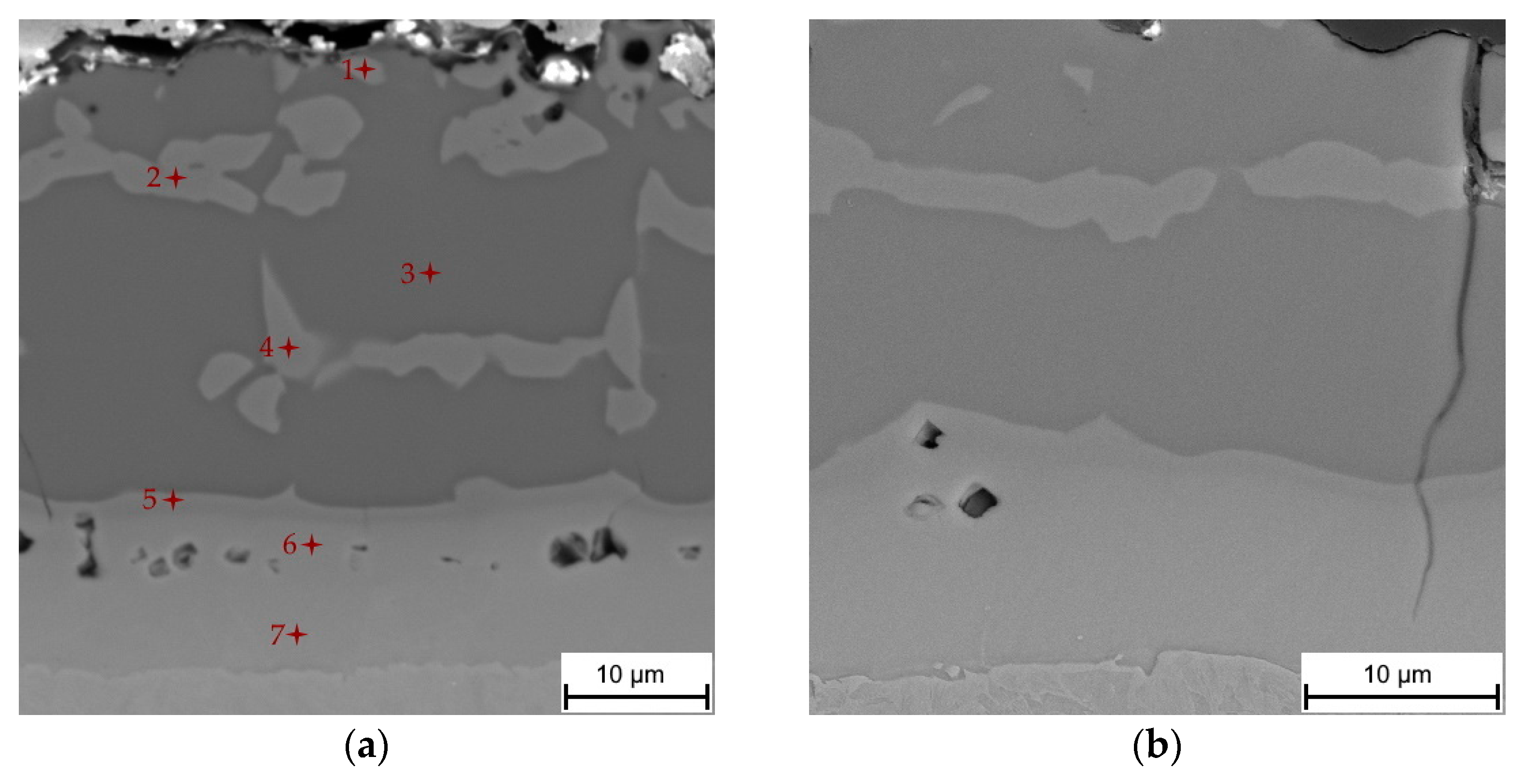
3.2. Chemical and Phase Evolution after Austenitization and Die-Quenching
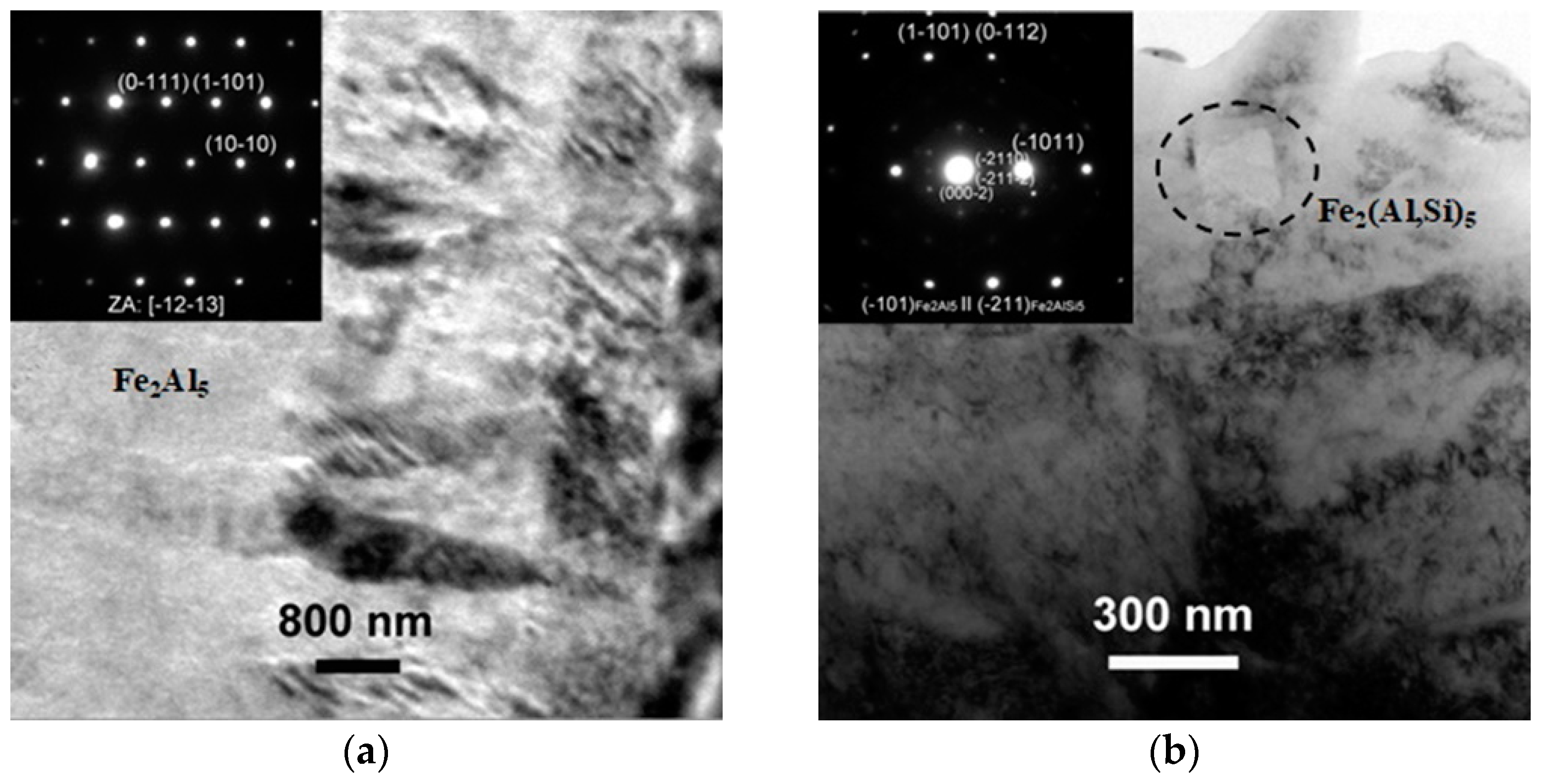
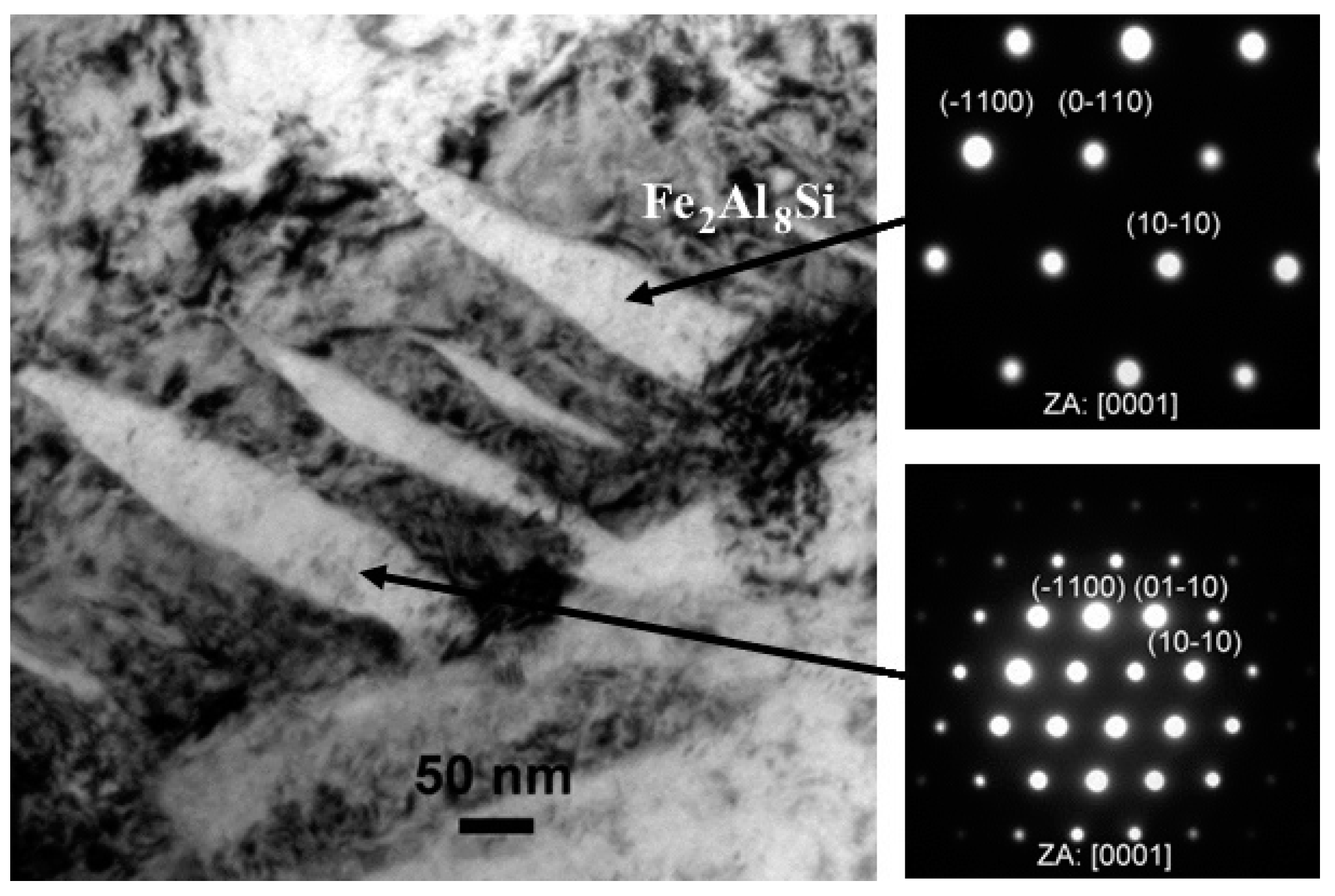
TEM Characterization and Coating Phase Identification
3.3. Changes in the Surface Condition after Die-quenching
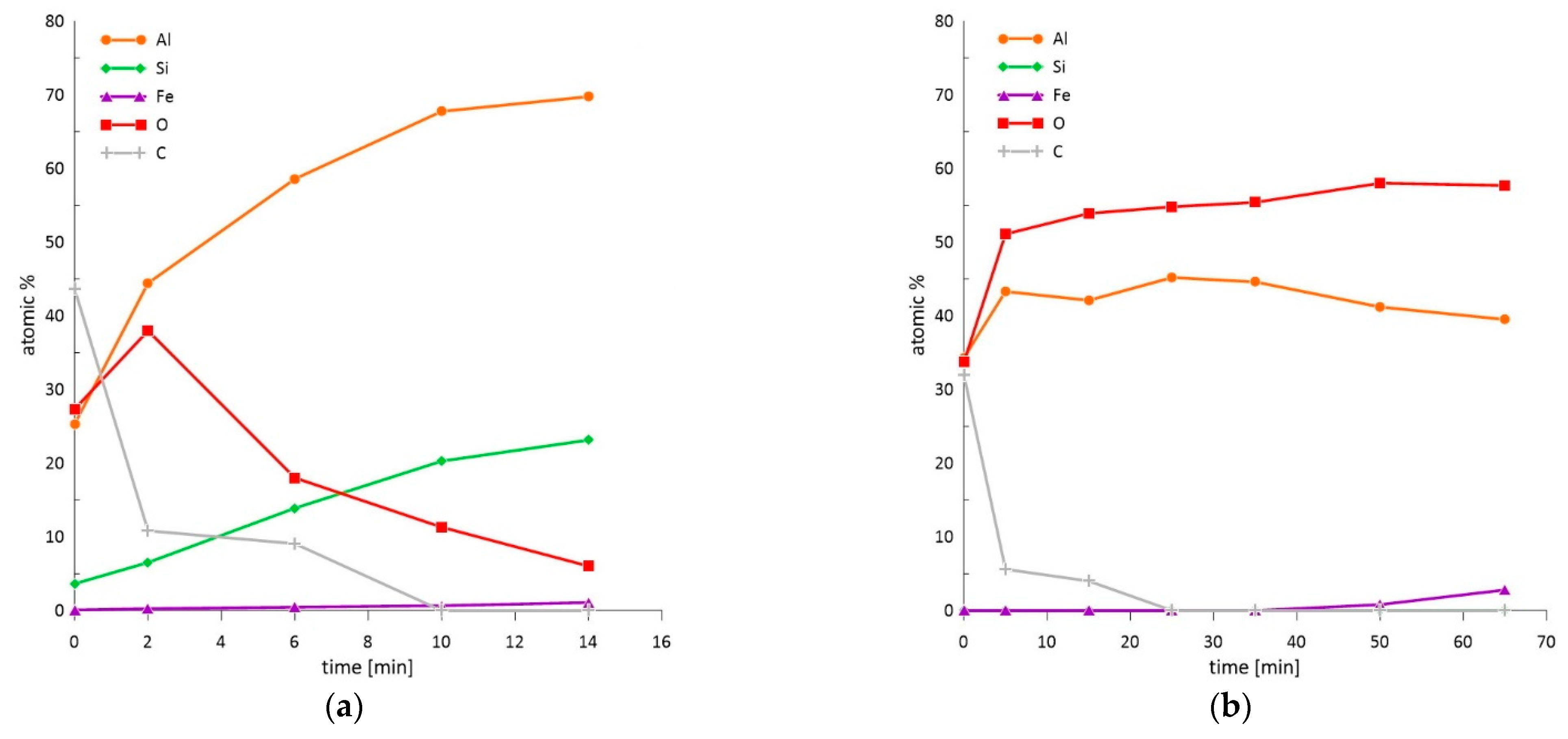
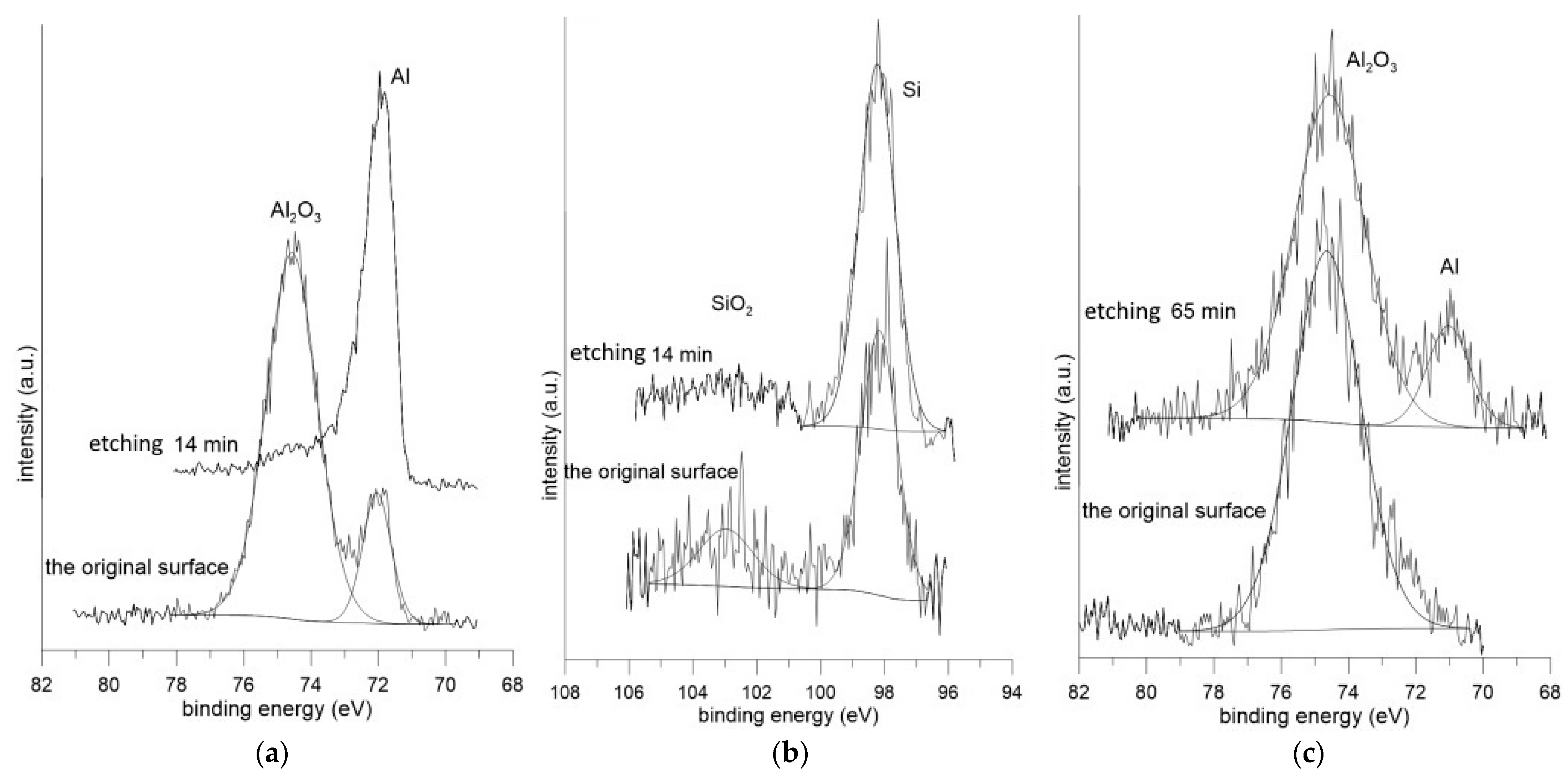
3.3.1. XPS Analysis of the Surface Condition
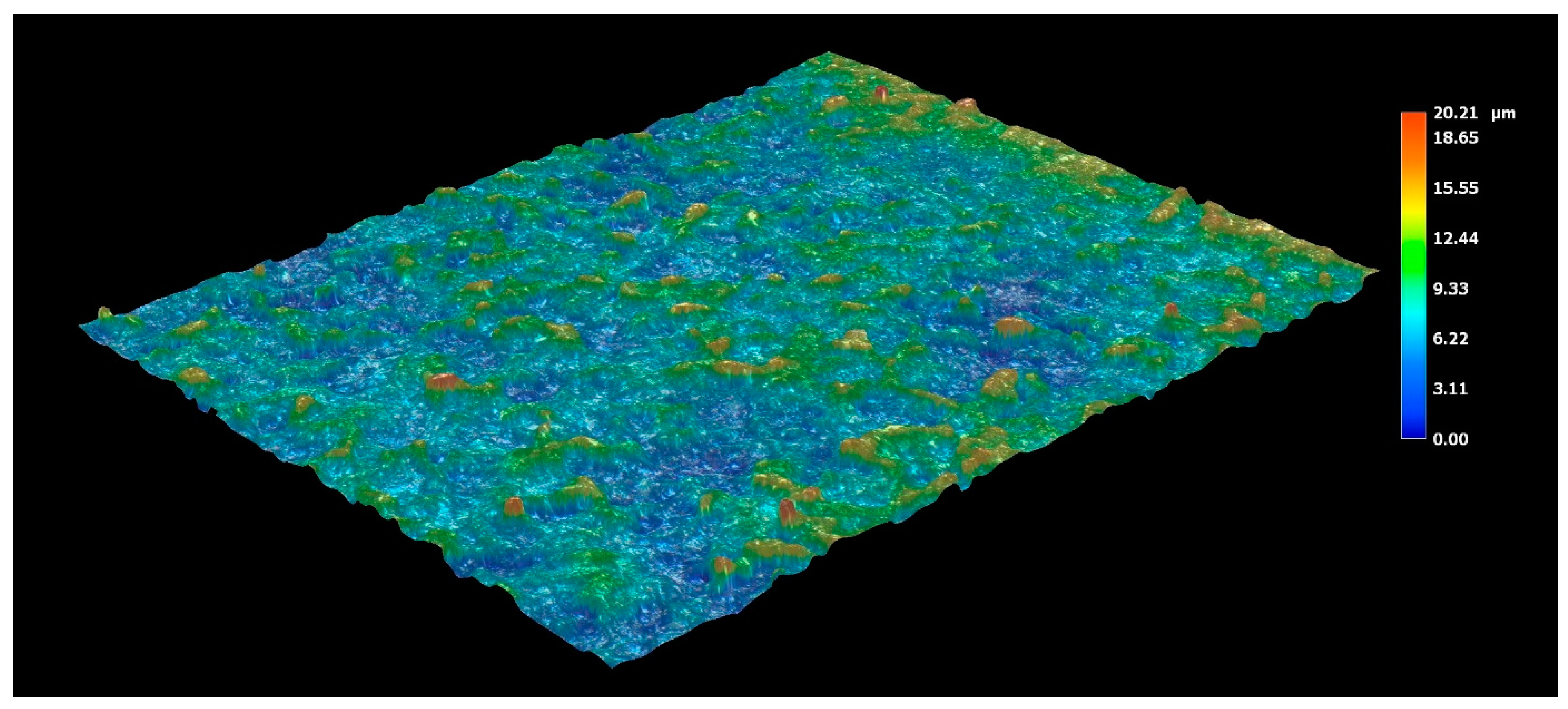
3.3.2. Roughness Measurement of the Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, T.; Fourlaris, G.; Evans, P.; Bright, G. New generation ultrahigh strength boron steel for automotive hot stamping technologies. Mater. Sci. Technol. 2014, 30, 818–826. [Google Scholar] [CrossRef]
- Karbasian, H.; Tekkaya, A.E. A review on hot stamping. J. Mater. Process. Technol. 2010, 210, 2103–2118. [Google Scholar] [CrossRef]
- Biro, E.; McDermid, J.R.; Vignier, S.; Zhou, Y.N. Decoupling of the softening processes during rapid tempering of a martensitic steel. Mater. Sci. Eng. A 2014, 615, 395–404. [Google Scholar] [CrossRef]
- Fan, D.W.; De Cooman, B.C. State-of-the-Knowledge on Coating Systems for Hot Stamped Parts. Steel Res. Int. 2012, 83, 412–433. [Google Scholar] [CrossRef]
- Guo, M.-X.; Gao, K.-X.; Wang, W.-R.; Wei, X.-C. Microstructural evolution of Al-Si coating and its influence on high temperature tribological behavior of ultra-high strength steel against H13 steel. J. Iron Steel Res. Int. 2017, 24, 1048–1058. [Google Scholar] [CrossRef]
- Windmann, M.; Röttger, A.; Theisen, W. Phase formation at the interface between a boron alloyed steel substrate and an Al-rich coating. Surf. Coat. Technol. 2013, 226, 130–139. [Google Scholar] [CrossRef]
- Suehiro, M.; Kusumi, K.; Maki, J.; Ohgami, M.; Miyakoshi, T. Properties of aluminium coated steels for hot forming. Nippon Steel Tech. Rep. 2003, 88, 16–21. [Google Scholar]
- Fan, D.; Kim, H.S.; Birosca, S.; De Cooman, B.C. Critical review of hot stamping technology for automotive steels. In Proceedings of the Materials Science and Technology Conference and Exhibition, MS and T’07—“Exploring Structure, Processing, and Applications Across Multiple Materials Systems”, Detroit, MI, USA, 16–20 September 2007; pp. 98–109. [Google Scholar]
- Dinh, K.-A.; Hong, S.-T.; Luu, T.V.; Kim, M.-J.; Han, H.N. Intermetallic Evolution of Al-Si-Coated Hot Stamping Steel During Modified Electrically Assisted Rapid Heating. Acta Metall. Sin. Engl. Lett. 2018, 31, 1327–1333. [Google Scholar] [CrossRef] [Green Version]
- Cho, L.; Kang, H.; Lee, C.W.; De Cooman, B.C. Microstructure of liquid metal embrittlement cracks on Zn-coated 22MnB5 press-hardened steel. Scr. Mater. 2014, 90–91, 25–28. [Google Scholar] [CrossRef]
- Saha, D.C.; Ji, C.W.; Park, Y.D. Coating behaviour and nugget formation during resistance welding of hot forming steels. Sci. Technol. Weld. Join. 2015, 20, 708–720. [Google Scholar] [CrossRef]
- Panin, S.V.; Vlasov, I.V.; Sergeev, V.P.; Maruschak, P.O.; Sunder, R.; Ovechkin, B.B. Fatigue life improvement of 12Cr1MoV steel by irradiation with Zr + ion beam. Int. J. Fatigue 2015, 76, 3–10. [Google Scholar] [CrossRef]
- Gromov, V.E.; Gorbunov, S.V.; Ivanov, Y.F.; Vorobiev, S.V.; Konovalov, S.V. Formation of surface gradient structural-phase states under electron-beam treatment of stainless steel. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2011, 5, 974. [Google Scholar] [CrossRef]
- Wang, H.; Song, G.; Tang, G. Evolution of surface mechanical properties and microstructure of Ti6Al4V alloy induced by electropulsing-assisted ultrasonic surface rolling process. J. Alloys Compd. 2016, 681, 146–156. [Google Scholar] [CrossRef]
- Marker, M.C.J.; Skolyszewska-Kühberger, B.; Effenberger, H.S.; Schmetterer, C.; Richter, K.W. Phase equilibria and structural investigations in the system Al-Fe-Si. Intermetallics 2011, 19, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Zhe, M.; Dezellus, O.; Gardiola, B.; Braccini, M.; Viala, J.C. Chemical Changes at the Interface Between Low Carbon Steel and an Al-Si Alloy During Solution Heat Treatment. J. Phase Equilibria Diffus. 2011, 32, 486–497. [Google Scholar] [CrossRef] [Green Version]
- Windmann, M.; Röttger, A.; Theisen, W. Formation of intermetallic phases in Al-coated hot-stamped 22MnB5 sheets in terms of coating thickness and Si content. Surf. Coat. Technol. 2014, 246, 17–25. [Google Scholar] [CrossRef]
- Shin, D.; Lee, J.-Y.; Heo, H.; Kang, C.Y. TEM Microstructural Evolution and Formation Mechanism of Reaction Layer for 22MnB5 Steel Hot-Dipped in Al–10% Si. Coatings 2018, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.W.; Kim, H.S.; De Cooman, B.C. A Review of the Physical Metallurgy related to the Hot Press Forming of Advanced High Strength Steel. Steel Res. Int. 2009, 80, 241–248. [Google Scholar]
- Gui, Z.; Liang, W.; Zhang, Y. Enhancing ductility of the Al-Si coating on hot stamping steel by controlling the Fe-Al phase transformation during austenitization. Sci. China Technol. Sci. 2014, 57, 1785–1793. [Google Scholar] [CrossRef]
- Liang, W.K.; Tao, W.J.; Zhu, B.; Zhang, Y.S. Influence of heating parameters on properties of the Al-Si coating applied to hot stamping. Sci. China Technol. Sci. 2017, 60, 1088–1102. [Google Scholar] [CrossRef]
- Grigorieva, R.; Drillet, P.; Mataigne, J.M.; Redjaïmia, A. Phase Transformations in the Al-Si Coating during the Austenitization Step. Solid State Phenom. 2011, 172–174, 784–790. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, S.-M.; Zhang, Q.-F.; Liu, C.-S. Effect of Temperature on Microstructure and Formability of Al-10 mass% Si Coatings. J. Iron Steel Res. Int. 2016, 23, 270–275. [Google Scholar] [CrossRef]
- Cho, L.; Golem, L.; Seo, E.J.; Bhattacharya, D.; Speer, J.G.; Findley, K.O. Microstructural characteristics and mechanical properties of the Al-Si coating on press hardened 22MnB5 steel. J. Alloys Compd. 2020, 846, 156349. [Google Scholar] [CrossRef]
- Jordan, C.E.; Goggins, K.M.; Benscoter, A.O.; Marder, A.R. Metallographic preparation technique for hot-dip galvanized and galvannealed coatings on steel. Mater. Charact. 1993, 31, 107–114. [Google Scholar] [CrossRef]
- Richards, R.W.; Jones, R.D.; Clements, P.D.; Clarke, H. Metallurgy of continuous hot dip aluminizing. Int. Mater. Rev. 1994, 39, 191–212. [Google Scholar] [CrossRef]
- Pelcastre, L.; Hardell, J.; Rolland, A.; Prakash, B. Influence of microstructural evolution of Al-Si coated UHSS on its tribological behaviour against tool steel at elevated temperatures. J. Mater. Process. Technol. 2016, 228, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Sundman, B.; Ohnuma, I.; Dupin, N.; Kattner, U.R.; Fries, S.G. An assessment of the entire Al-Fe system including D03 ordering. Acta Mater. 2009, 57, 2896–2908. [Google Scholar] [CrossRef]
- Gui, Z.-X.; Wang, K.; Zhang, Y.-S.; Zhu, B. Cracking and interfacial debonding of the Al-Si coating in hot stamping of pre-coated boron steel. Appl. Surf. Sci. 2014, 316, 595–603. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Microstructural evolution of intermetallic layer in hot-dipped aluminide mild steel with silicon addition. Surf. Coat. Technol. 2011, 205, 4726–4731. [Google Scholar] [CrossRef]
- Fan, D.; De Cooman, B. Formation of an Aluminide Coating on Hot Stamped Steel. ISIJ Int. 2010, 50, 1713–1718. [Google Scholar] [CrossRef] [Green Version]
- Matysik, P.; Jóźwiak, S.; Czujko, T. Characterization of Low-Symmetry Structures from Phase Equilibrium of Fe-Al System—Microstructures and Mechanical Properties. Materials 2015, 8, 914–931. [Google Scholar] [CrossRef]
- Grauer, S.J.; Caron, E.J.F.R.; Chester, N.L.; Wells, M.A.; Daun, K.J. Investigation of melting in the Al-Si coating of a boron steel sheet by differential scanning calorimetry. J. Mater. Process. Technol. 2015, 216, 89–94. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Effect of silicon on the formation of intermetallic phases in aluminide coating on mild steel. Intermetallics 2011, 19, 1455–1460. [Google Scholar] [CrossRef]
- Mehrer, H.; Luckabauer, M.; Sprengel, W. Self- and Solute Diffusion, Interdiffusion and Thermal Vacancies in the System Iron-Aluminium. Defect Diffus. Forum 2013, 333, 1–25. [Google Scholar] [CrossRef]
- Taylor, T.; Clough, A. Critical review of automotive hot-stamped sheet steel from an industrial perspective. Mater. Sci. Technol. 2018, 34, 809–861. [Google Scholar] [CrossRef]
- Ji, C.-W.; Lee, H.J.; Kim, Y.D.; Jo, I.G.; Choi, I.D.; Park, Y.D. Effects of surface coating on weld growth of resistance spot-welded hot-stamped boron steels. J. Mater. Process. Technol. 2014, 28, 4761–4769. [Google Scholar] [CrossRef]
- Ruan, Y.; Yan, N.; Zhu, H.Z.; Zhou, K.; Wei, B. Thermal performance determination of binary Fe-Al alloys at elevated temperatures. J. Alloys Compd. 2017, 701, 676–681. [Google Scholar] [CrossRef]
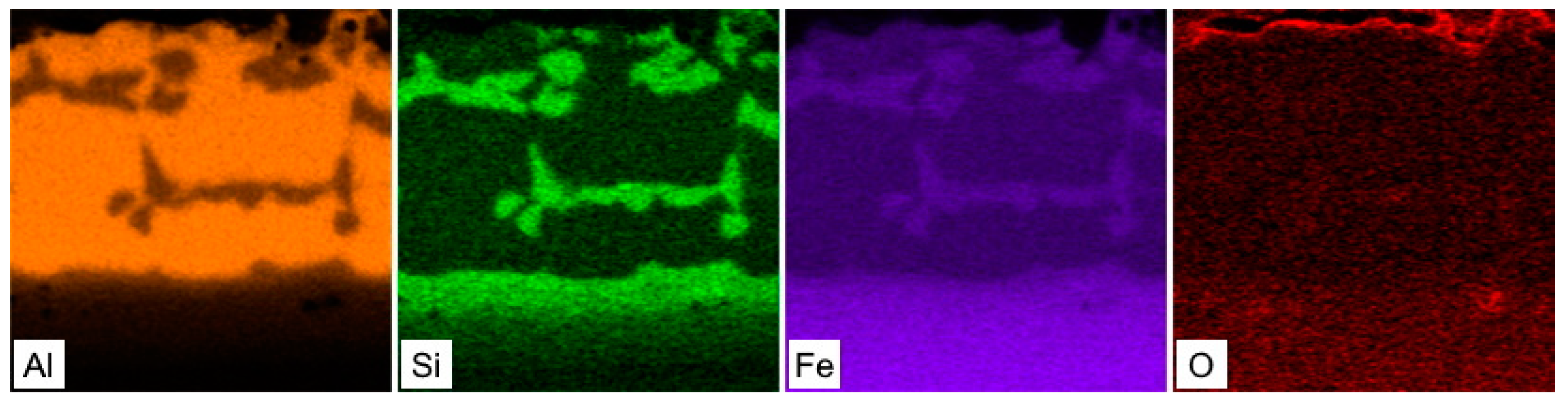
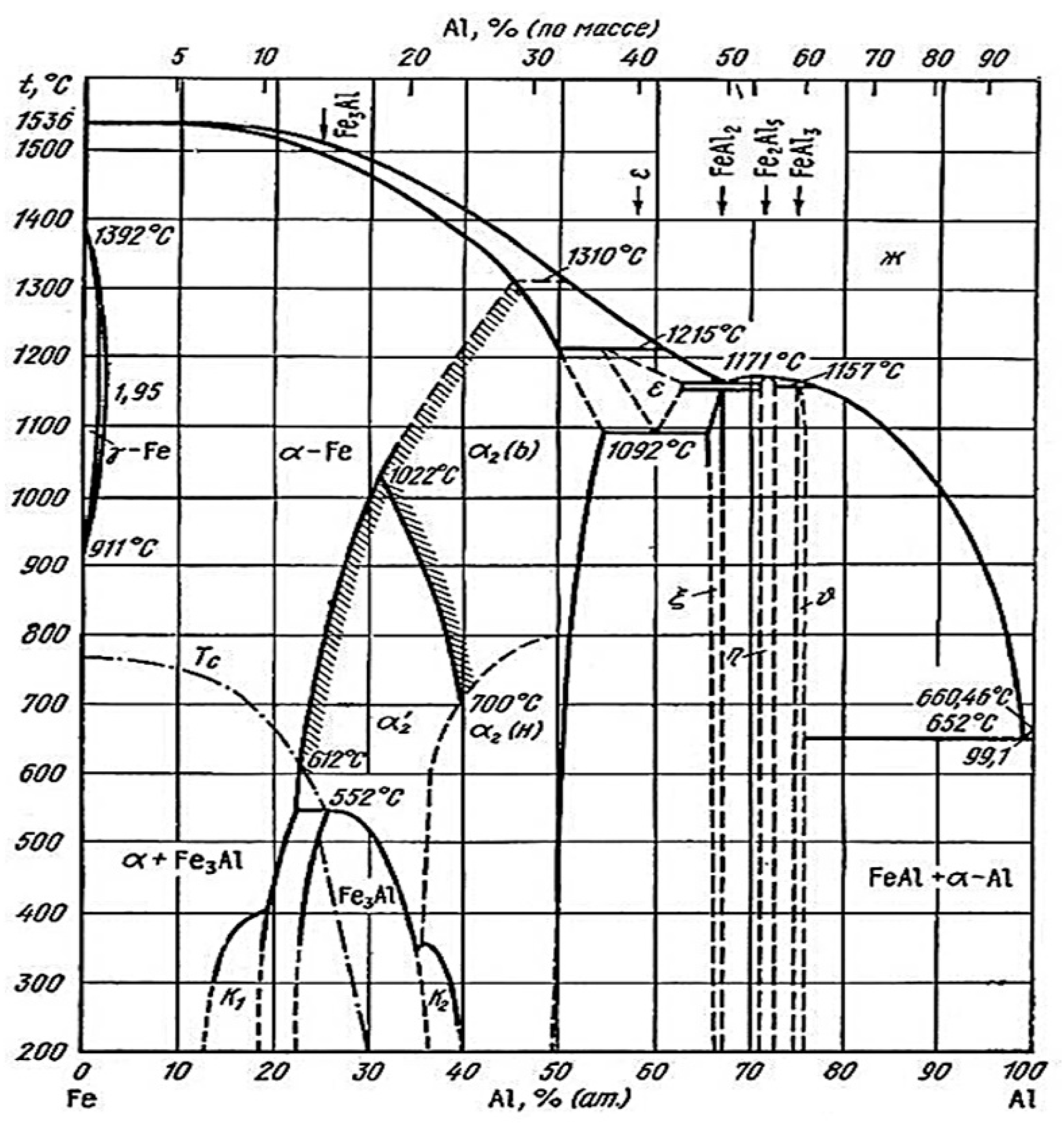

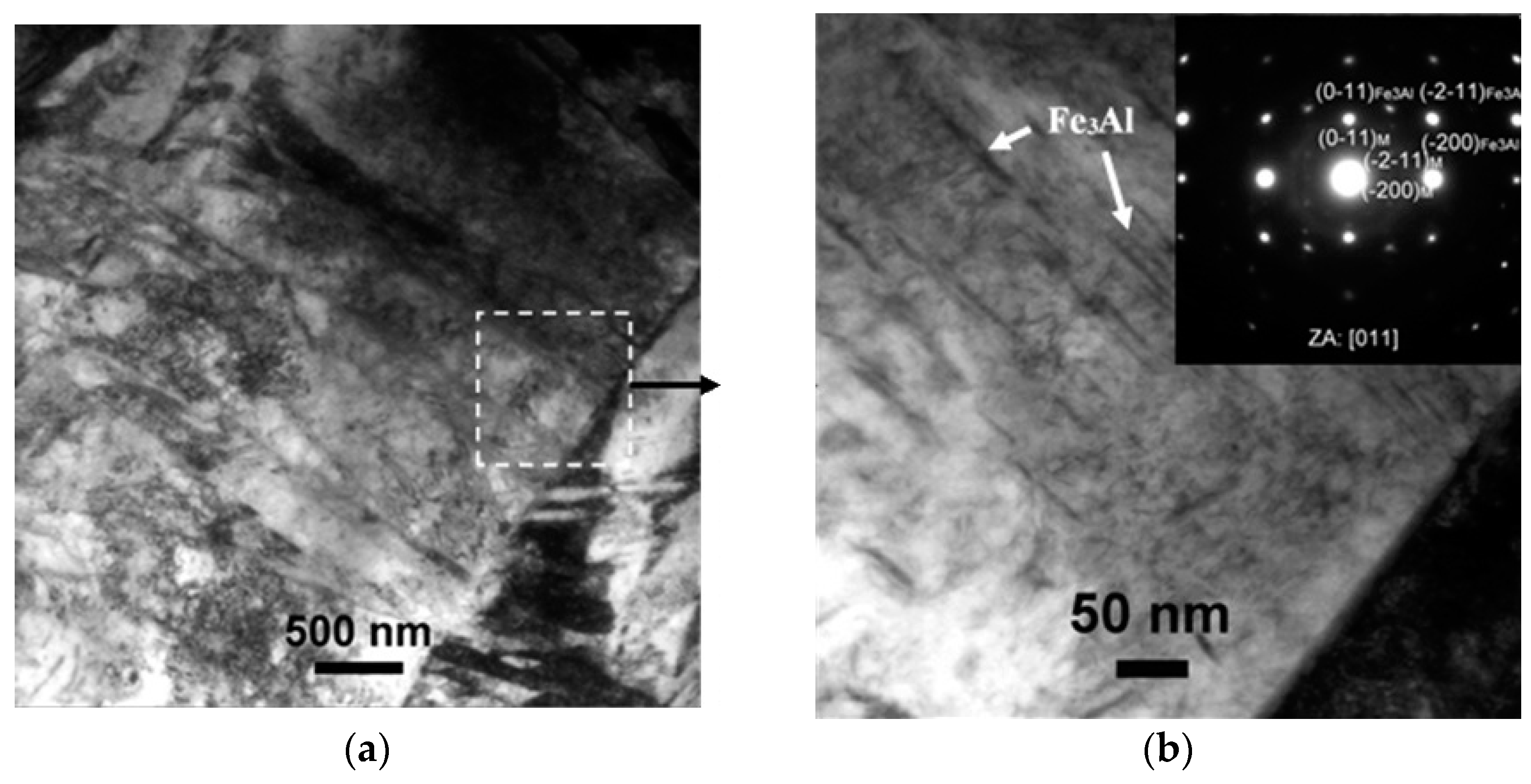
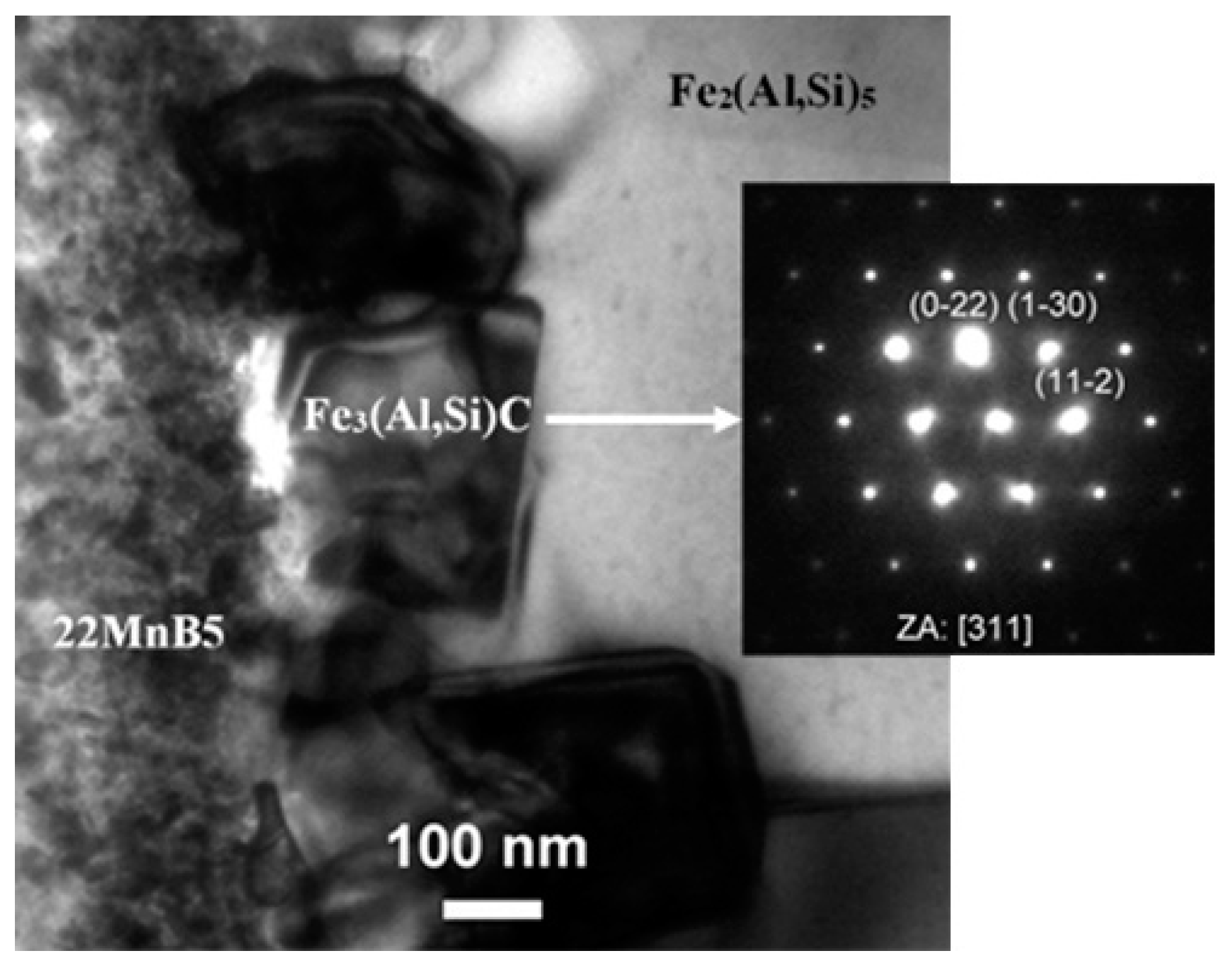

| Element | C | Si | Mn | P | S | Al | Cr | Ti | B |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.22 | 0.24 | 1.17 | 0.010 | 0.002 | 0.044 | 0.19 | 0.026 | 0.0027 |
| Element | O | Al | Si | Cr | Mn | Fe | Phase |
|---|---|---|---|---|---|---|---|
| point 1 | 1.9 | 79.4 | 17.6 | - | - | 1.1 | Al + Si, oxides |
| point 2 | - | 89.4 | 10.2 | - | - | 0.4 | α-Al + Si |
| point 3 | - | 71.5 | 11.3 | - | - | 17.2 | τ5-Fe2Al8Si |
| point 4 | - | 67.8 | 11.8 | - | 0.2 | 20.2 | τ5-Fe2Al8Si |
| point 5 | - | 58.9 | 5.7 | 0.1 | 0.4 | 34.9 | Fe2Al5 |
| point 6 | - | - | 0.5 | 0.3 | 1.3 | 97.9 | Steel |
| Element | Al | Si | Cr | Mn | Fe | Phase |
|---|---|---|---|---|---|---|
| point 1 | 52.1 | 5.9 | - | 0.5 | 41.5 | FeAl(Si) |
| point 2 | 46.2 | 10.0 | 0.2 | 0.8 | 42.8 | FeAl(Si) |
| point 3 | 68.0 | 1.9 | - | 0.3 | 29.8 | Fe2Al5 |
| point 4 | 41.4 | 11.1 | - | 0.9 | 46.6 | FeAl(Si) |
| point 5 | 39.7 | 10.6 | 0.1 | 0.7 | 48.9 | FeAl(Si) |
| point 6 | 16.5 | 6.6 | 0.2 | 0.8 | 75.9 | α-Fe(Al,Si) |
| point 7 | 7.9 | - | 0.2 | 1.1 | 90.8 | α-Fe(Al,Si) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucera, V.; Cabibbo, M.; Prusa, F.; Fojt, J.; Petr-Soini, J.; Pilvousek, T.; Kolarikova, M.; Vojtech, D. Phase Composition of Al-Si Coating from the Initial State to the Hot-Stamped Condition. Materials 2021, 14, 1125. https://doi.org/10.3390/ma14051125
Kucera V, Cabibbo M, Prusa F, Fojt J, Petr-Soini J, Pilvousek T, Kolarikova M, Vojtech D. Phase Composition of Al-Si Coating from the Initial State to the Hot-Stamped Condition. Materials. 2021; 14(5):1125. https://doi.org/10.3390/ma14051125
Chicago/Turabian StyleKucera, Vojtech, Marcello Cabibbo, Filip Prusa, Jaroslav Fojt, Jaroslav Petr-Soini, Tomas Pilvousek, Marie Kolarikova, and Dalibor Vojtech. 2021. "Phase Composition of Al-Si Coating from the Initial State to the Hot-Stamped Condition" Materials 14, no. 5: 1125. https://doi.org/10.3390/ma14051125








