Preparation of a Fucoidan-Grafted Hyaluronan Composite Hydrogel for the Induction of Osteoblast Differentiation in Osteoblast-Like Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation MHA and MFu
2.3. Characterization of MHA and MFu Using Fourier Transform Infrared Spectroscopy (FTIR) and 1H NMR Spectroscopy
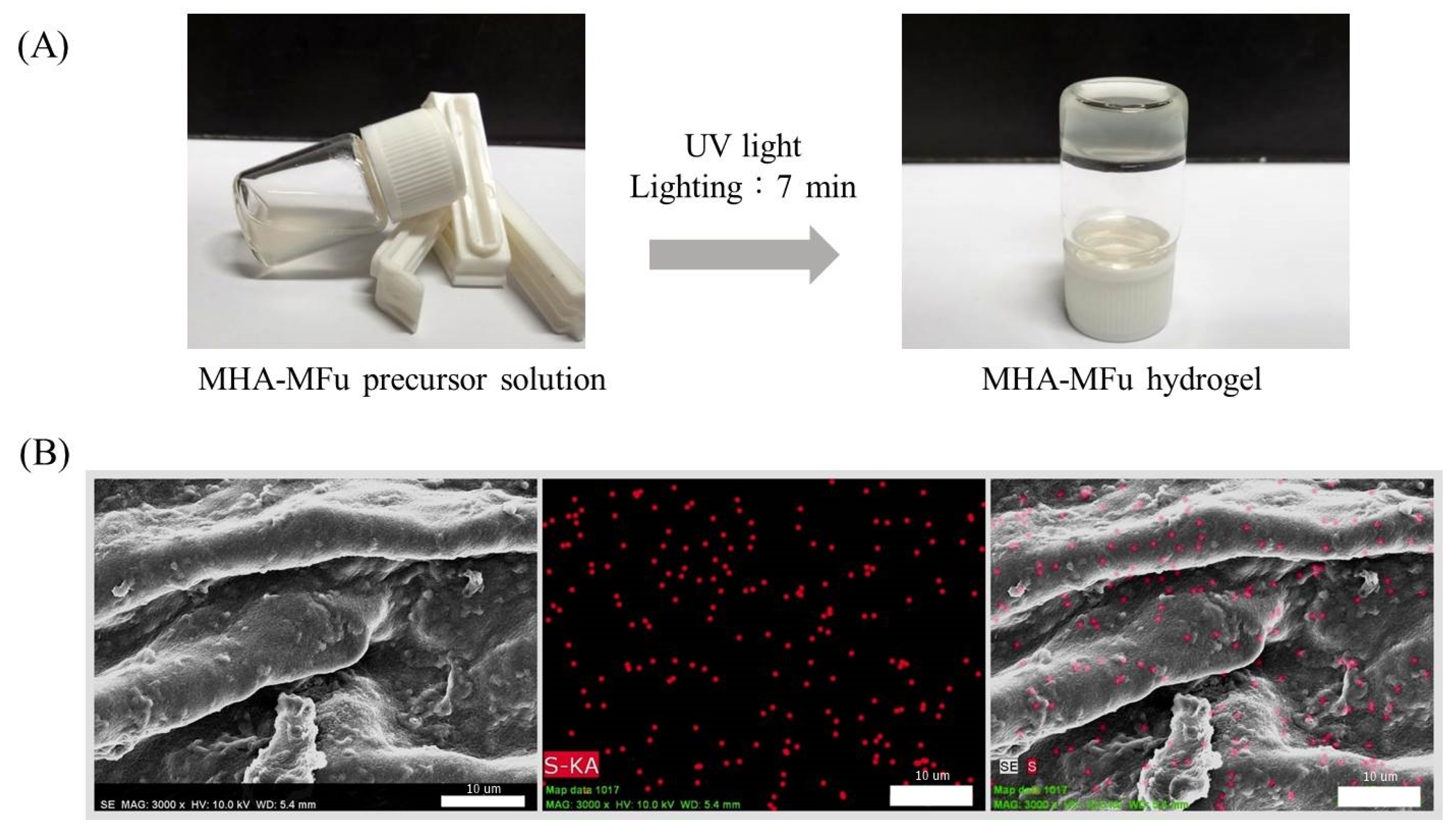
2.4. Preparation of the MHA and MHA-MFu Hydrogels
2.5. Mechanical Properties Assay
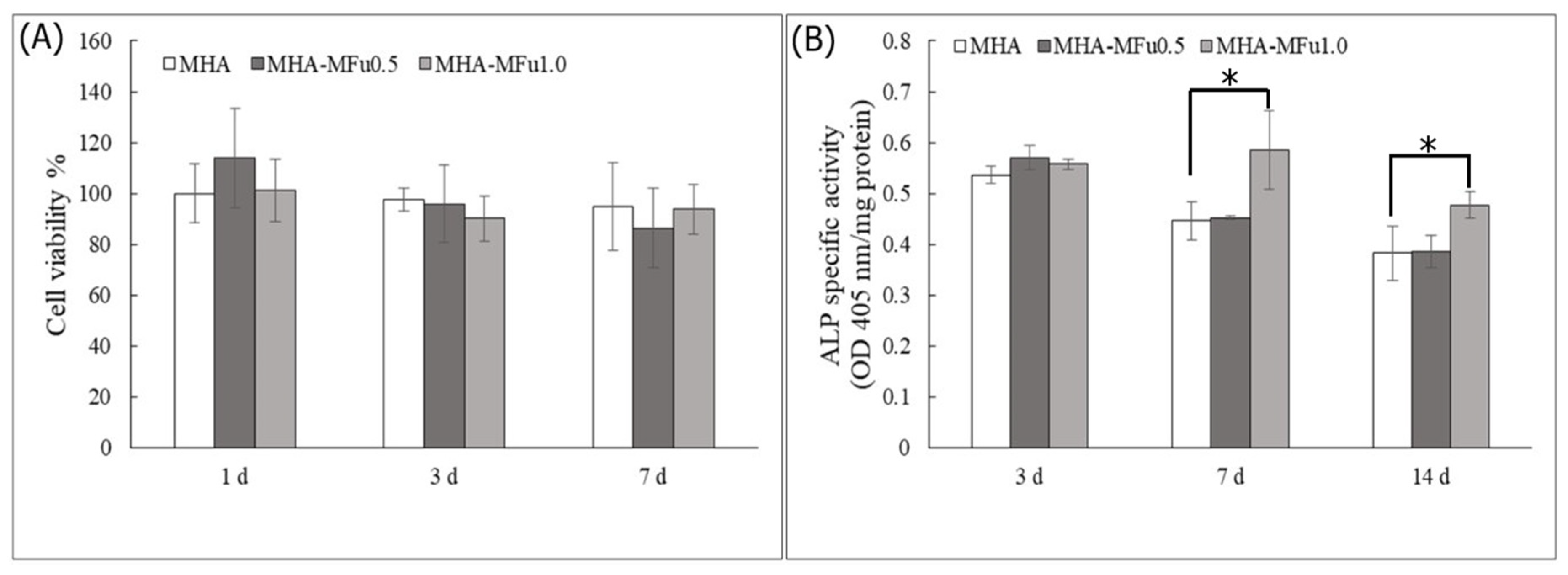
2.6. Culture Conditions and Proliferation of Preosteoblastic MG63 Cells on MHA and MHA-MFu Hydrogels
2.7. ALP-Specific Activity
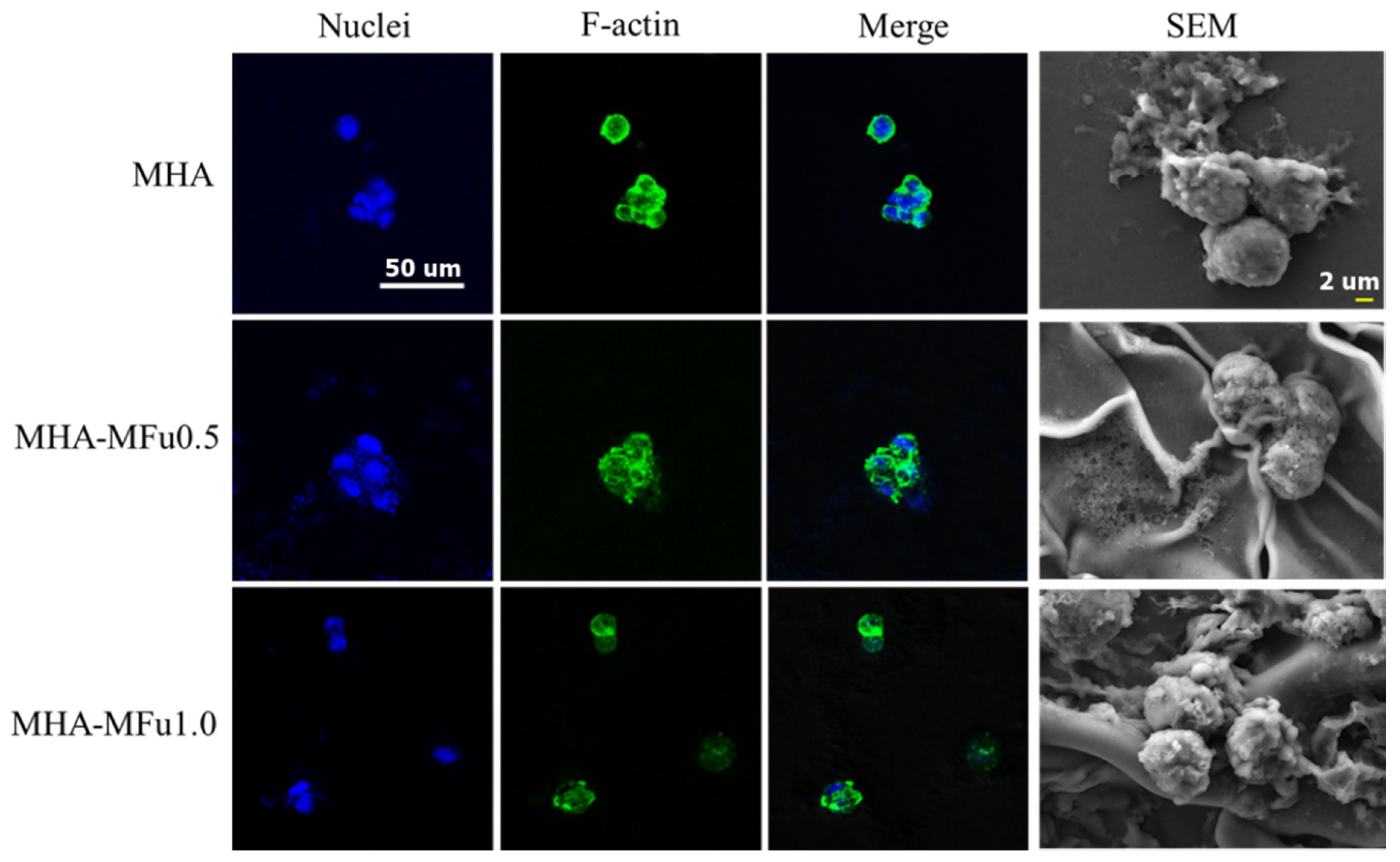
2.8. Cell Attachment Using Immunofluorescence and SEM
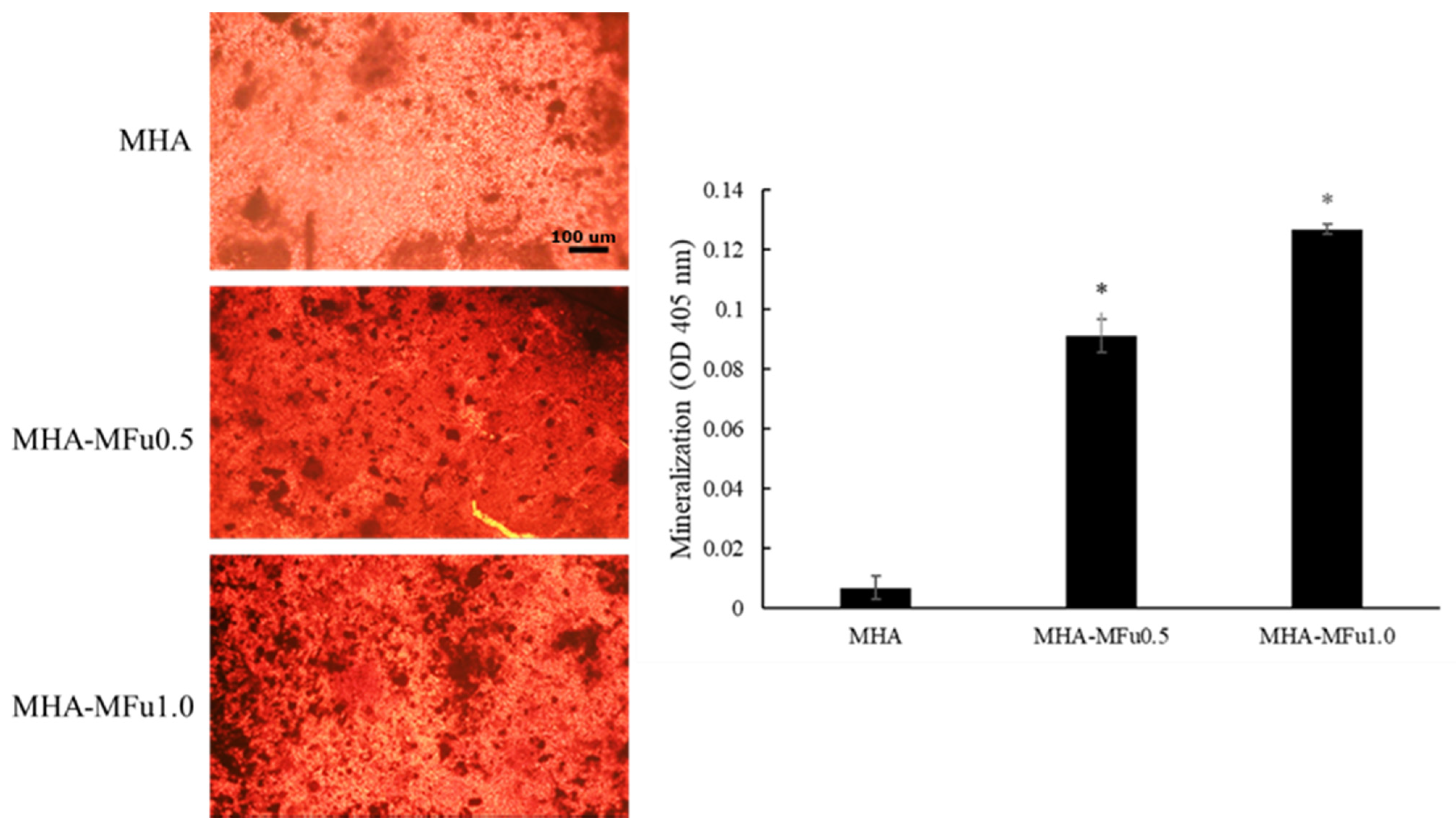
2.9. Mineralization Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of MHA and MFu
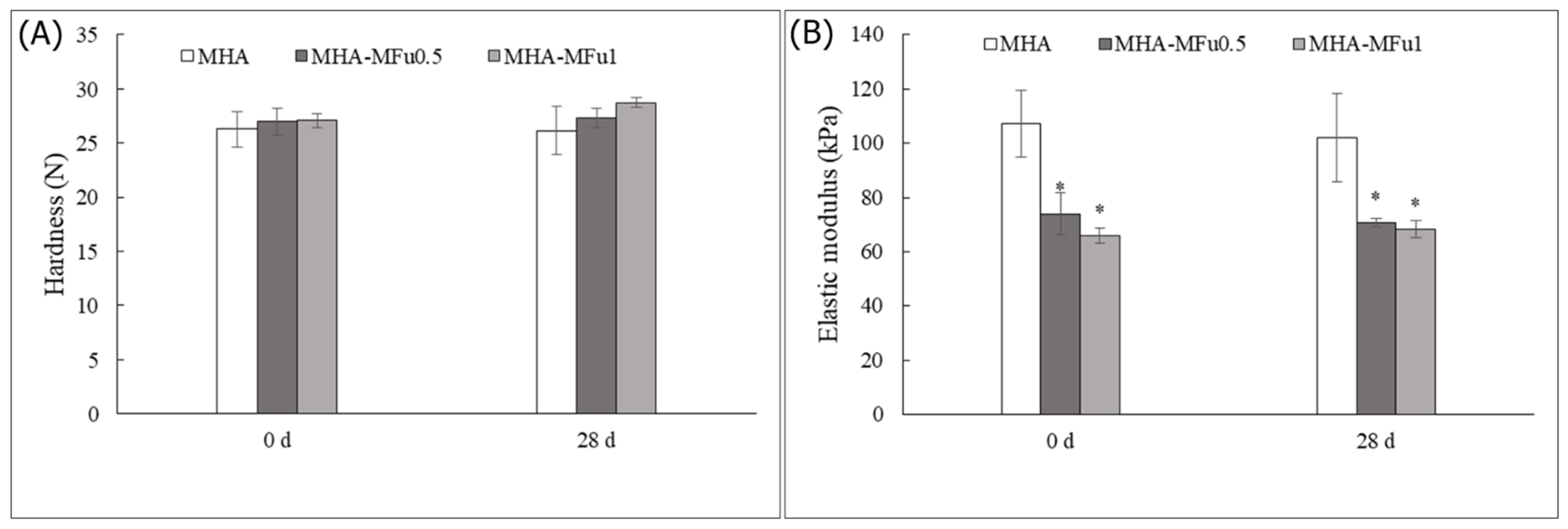
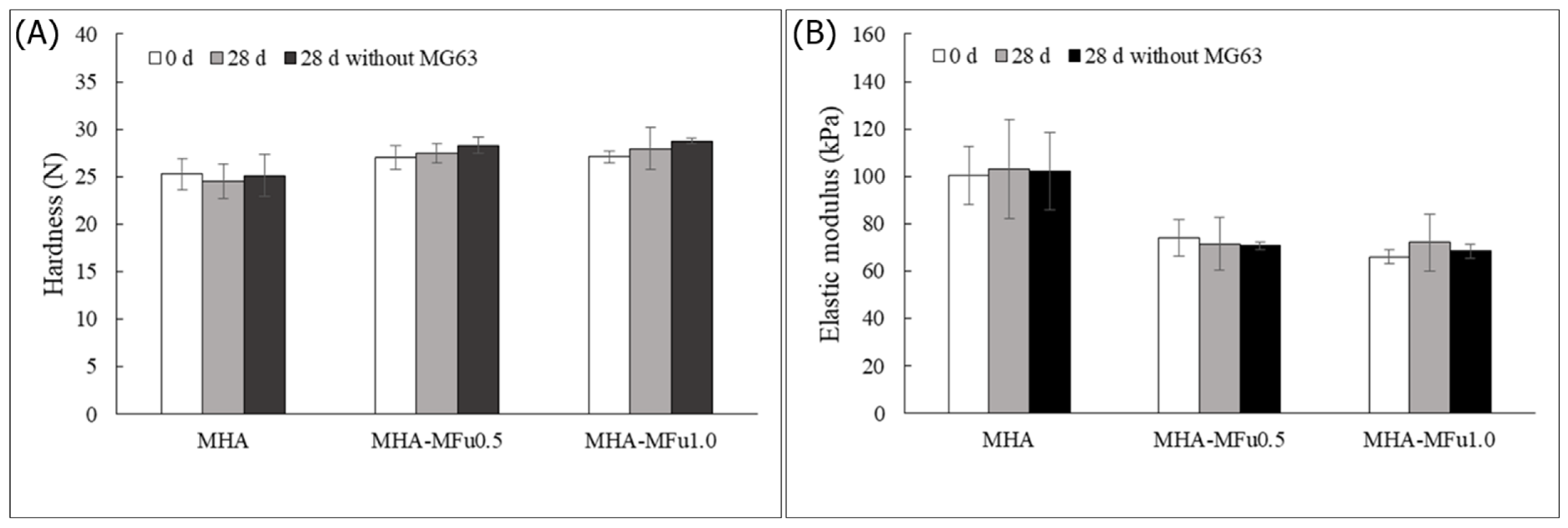
3.2. Effect of Osteoblast Cells on the Mechanical Properties of the MHA and MHA-MFu Hydrogels
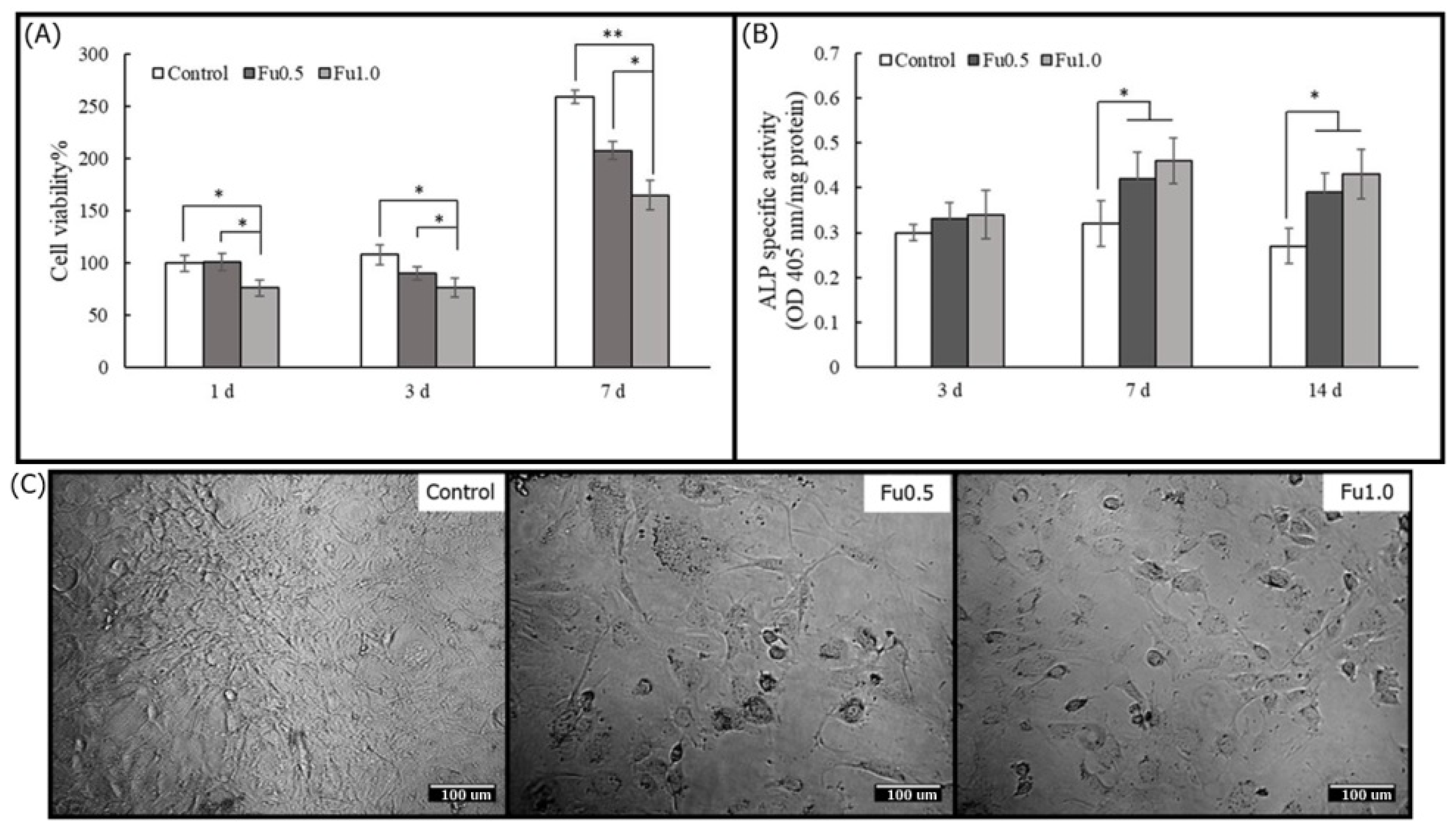
3.3. Effects of Fu on Osteoblast Cells in Plate Culture
3.4. Cell Behavior on MHA and MHA-MFu Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omary, R.; Chernoguz, D.; Lasri, V.; Leker, R. Decompressive hemicraniectomy reduces mortality in an animal model of intracerebral hemorrhage. J. Mol. Neurosci. 2013, 49, 157–161. [Google Scholar] [CrossRef]
- Stiver, S.I. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg. Focus 2009, 26, E7. [Google Scholar] [CrossRef]
- Viviek, J.; Reilly, P. Syndrome of the trephined. J. Neurosurg. 2009, 111, 650–652. [Google Scholar]
- Herold, T.J.; Taylor, S.; Abbrescia, K.; Hunter, C. Post-traumatic subdural hygroma: Case report. J. Emerg. Med. 2004, 27, 361–366. [Google Scholar] [CrossRef]
- Morselli, C.; Zaed, I.; Tropeano, M.P.; Cataletti, G.; Iaccarino, C.; Rossini, Z.; Servadei, F. Comparison between the different types of heterologous materials used in cranioplasty: A systematic review of the literature. J. Neurosurg. Sci. 2019, 63, 723–736. [Google Scholar] [CrossRef]
- Townsend, J.M.; Andrews, B.T.; Feng, Y.; Wang, J.; Nudo, R.J.; Van Kampen, E.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Superior calvarial bone regeneration using pentenoate-functionalized hyaluronic acid hydrogels with devitalized tendon particles. Acta Biomater. 2018, 71, 148–155. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular matrix mimics using hyaluronan-based biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Welzel, P.B.; Prokoph, S.; Zieris, A.; Grimmer, M.; Zschoche, S.; Freudenberg, U.; Werner, C. Modulating biofunctional starPEG heparin hydrogels by varying size and ratio of the constituents. Polymers 2011, 3, 602–620. [Google Scholar] [CrossRef] [Green Version]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Oommen, O.P.; Wang, S.; Kisiel, M.; Sloff, M.; Hilborn, J.; Varghese, O.P. Smart design of stable extracellular matrix mimetic hydrogel: Synthesis, characterization, and in vitro and in vivo evaluation for tissue engineering. Adv. Funct. Mater. 2013, 23, 1273–1280. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; De Ruijter, M.; Abbadessa, A.; Melchels, F.P.; Öner, F.C.; Dhert, W.J.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [Green Version]
- Prestwich, G.D.; Kuo, J.W. Chemically-modified HA for therapy and regenerative medicine. Curr. Pharm. Biotechnol. 2008, 9, 242–245. [Google Scholar] [CrossRef]
- Mamada, A.; Tanaka, T.; Kungwatchakun, D.; Irie, M. Photoinduced phase transition of gels. Macromolecules 1990, 23, 1517–1519. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanaka, T. Phase transition in polymer gels induced by visible light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Cui, N.; Qian, J.; Liu, T.; Zhao, N.; Wang, H. Hyaluronic acid hydrogel scaffolds with a triple degradation behavior for bone tissue engineering. Carbohydr Polym. 2015, 126, 192–198. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, Y.; Koo, P.; Lee, K.; Qin, L.; Cheng, J.; Kumta, S. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J. Biomed. Mater. Res. A 2003, 66, 880–884. [Google Scholar] [CrossRef]
- Quade, M.; Knaack, S.; Weber, D.; König, U.; Paul, B.; Simon, P.; Rösen-Wolff, A.; Schwartz-Albiez, R.; Gelinsky, M.; Lode, A. Heparin modification of a biomimetic bone matrix modulates osteogenic and angiogenic cell response in vitro. Eur. Cell. Mater. 2017, 33, 105–120. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing cell proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts by BMP-2 delivery in graphene oxide-incorporated PLGA/HA biodegradable microcarriers. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, T.; Xiao, Z.; Tang, P.; Xiao, Y.; Fan, Y.; Zhang, X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2267–2279. [Google Scholar] [CrossRef]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef]
- Kylin, H. Zur Biochemie der Meeresalgen. Physiol Chem. 1913, 83, 171–197. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK-and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef]
- Hwang, P.A.; Hung, Y.L.; Phan, N.N.; Chang, P.M.; Li, K.L.; Lin, Y.C. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 2016, 68, 1349–1359. [Google Scholar] [CrossRef]
- Pajovich, H.T.; Banerjee, I.A. Biomineralization of fucoidan-peptide blends and their potential applications in bone tissue regeneration. J. Funct. Biomater. 2017, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunanithi, P.; Murali, M.R.; Samuel, S.; Raghavendran, H.R.B.; Abbas, A.A.; Kamarul, T. Three dimensional alginate-fucoidan composite hydrogel augments the chondrogenic differentiation of mesenchymal stromal cells. Carbohydr. Polym. 2016, 147, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Reys, L.L.; Silva, S.S.; Soares da Costa, D.; Oliveira, N.M.; Mano, J.F.; Reis, R.L.; Silva, T.H. Fucoidan hydrogels photo-cross-linked with visible radiation as matrices for cell culture. ACS Biomater. Sci. Eng. 2016, 2, 1151–1161. [Google Scholar] [CrossRef]
- Hwang, P.A.; Lin, H.T.V.; Lin, H.Y.; Lo, S.K. Dietary supplementation with low-molecular-weight fucoidan enhances innate and adaptive immune responses and protects against Mycoplasma pneumoniae antigen stimulation. Mar. Drugs 2019, 17, 175. [Google Scholar] [CrossRef] [Green Version]
- Hwang, P.A.; Yan, M.D.; Kuo, K.L.; Phan, N.N.; Lin, Y.C. A mechanism of low molecular weight fucoidans degraded by enzymatic and acidic hydrolysis for the prevention of UVB damage. J. Appl. Phycol. 2017, 29, 521–529. [Google Scholar] [CrossRef]
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Hachet, E.; Van Den Berghe, H.; Bayma, E.; Block, M.R.; Auzély-Velty, R. Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules 2012, 13, 1818–1827. [Google Scholar] [CrossRef] [Green Version]
- Chandra, M.; Shamasundar, B. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Farsi, S.A. Instrumental texture profile analysis (TPA) of date flesh as a function of moisture content. J. Food Eng. 2005, 66, 505–511. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, e12338. [Google Scholar] [CrossRef] [Green Version]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, H.; Xu, C.; Yu, D.; Xu, H.; Hu, Y. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether. RSC Adv. 2020, 10, 7206–7213. [Google Scholar] [CrossRef]
- Lampe, K.J.; Bjugstad, K.B.; Mahoney, M.J. Impact of degradable macromer content in a poly(ethylene glycol) hydrogel on neural cell metabolic activity, redox state, proliferation, and differentiation. Tissue Eng. Part A 2010, 16, 1857–1866. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, A.; Jung, W.K.; Jeon, T.J. Effects of fucoidan on cell morphology and migration in osteoblasts. Food Sci. Biotechnol. 2015, 24, 699–704. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Tan, Y.; Fan, H.; Zhang, X. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen. Biomater. 2018, 5, 43–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.S.; Jung, W.K.; Kim, J.A.; Choi, I.W.; Kim, S.K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Cho, T.M.; Kim, W.J.; Moon, S.K. AKT signaling is involved in fucoidan-induced inhibition of growth and migration of human bladder cancer cells. Food Chem. Toxicol. 2014, 64, 344–352. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.S.; Kim, E. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Lin, S.; Zhu, M.; Deng, Y.; Chen, X.; Wei, K.; Xu, J.; Li, G.; Bian, L. Synthetic presentation of noncanonical Wnt5a motif promotes mechanosensing-dependent differentiation of stem cells and regeneration. Sci. Adv. 2019, 5, eaaw3896. [Google Scholar] [CrossRef] [Green Version]
- Chou, L.; Firth, J.D.; Uitto, V.J.; Brunette, D.M. Substratum surface topography alters cell shape and regulates fibronectin mRNA level, mRNA stability, secretion and assembly in human fibroblasts. J. Cell Sci. 1995, 108, 1563–1573. [Google Scholar]
- Folkman, J.; Moscona, A. Role of cell shape in growth control. Nature 1978, 273, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Werb, Z.; Hembry, R.M.; Murphy, G.; Aggeler, J. Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: Relationship of inducing events to changes in cytoskeletal architecture. J. Cell Biol. 1986, 102, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.Y.; Lu, M.R.; Weng, R.C.; Lin, H.M. Hierarchically biomimetic scaffold of a collagen-mesoporous bioactive glass nanofiber composite for bone tissue engineering. Biomed. Mater. 2015, 10, 025007. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, F.-Y.; Chen, J.-J.; Sung, W.-C.; Hwang, P.-A. Preparation of a Fucoidan-Grafted Hyaluronan Composite Hydrogel for the Induction of Osteoblast Differentiation in Osteoblast-Like Cells. Materials 2021, 14, 1168. https://doi.org/10.3390/ma14051168
Hsu F-Y, Chen J-J, Sung W-C, Hwang P-A. Preparation of a Fucoidan-Grafted Hyaluronan Composite Hydrogel for the Induction of Osteoblast Differentiation in Osteoblast-Like Cells. Materials. 2021; 14(5):1168. https://doi.org/10.3390/ma14051168
Chicago/Turabian StyleHsu, Fu-Yin, Jheng-Jie Chen, Wen-Chieh Sung, and Pai-An Hwang. 2021. "Preparation of a Fucoidan-Grafted Hyaluronan Composite Hydrogel for the Induction of Osteoblast Differentiation in Osteoblast-Like Cells" Materials 14, no. 5: 1168. https://doi.org/10.3390/ma14051168





