2.1. Materials
In the synthesis and purification of polymeric microspheres, the following chemicals are used: styrene ≥99% (Fluka, Buchs, Switzerland), glycidyl methacrylate 99.5%, GMA (Sigma-Aldrich, St. Louis, MO, USA), ethylene glycol dimethylacrylate 98%, EGDMA (Sigma-Aldrich, St. Louis, MO, USA), 2,2′-azobisisobutyronitrile, AIBN (Fluka, Buchs, Switzerland), ethyl alcohol anhydrous 99.8% pure p.a. (POCh, Gliwice, Poland), polyvinylpyrrolidone, PVP-40 (Fluka, Buchs, Switzerland), sodium dodecyl sulfate ≥99%, SDS (Sigma-Aldrich, St. Louis, MO, USA) methanol 99.8% pure p.a. (POCh, Gliwice, Poland), tetrahydrofuran 99.5% pure (POCh, Gliwice, Poland), toluene 99.9% pure (Sigma-Aldrich, St. Louis, MO, USA), hexane 99.5% pure p.a. (POCh, Gliwice, Poland), dichloromethane 99.5% pure, (POCh, Gliwice, Poland), acetone 99.5% pure p.a. (POCh, Gliwice, Poland), hydrochloric acid 35–38% pure p.a. (POCh, Gliwice, Poland), sulfuric acid 30% pure p. a. (POCh, Gliwice, Poland), ammonium chloride (POCh, Gliwice, Poland).
In the functionalization of polymeric microspheres, the following chemicals are used: sodium cyclopentadienide, NaCp, 2 M in THF (Sigma-Aldrich, St. Louis, MO, USA), maleic anhydride 99% (Sigma-Aldrich, St. Louis, MO, USA), 4,4′-bis(maleimido)diphenylmethane 95%, BMI (Sigma-Aldrich, St. Louis, MO, USA), methacryloyl chloride 97% (Sigma-Aldrich, St. Louis, MO, USA), 2-mercaptopropionic acid 96%, 2-MPA (Sigma-Aldrich, St. Louis, MO, USA), trimethylamine anhydrous (POCh, Gliwice, Poland).
Mixtures of alkylbenzenes (toluene, ethylbenzene, propylbenzene, butylbenzene, pentylbenzene), alkyl aryl ketones (1-phenylethan-1-one, 1-phenylpropan-1-one, 1-phenylbutan-1-one, 1-phenylpentan-1-one, 1-phenylhexan-1-one, 1-phenylheptan-1-one), alkyl benzoates (methyl benzoate, ethyl benzoate, propyl benzoate, butyl benzoate, pentyl benzoate), N-alkylanilines (aniline, N-methylaniline, N-ethylaniline, N-propylaniline, N-butylaniline, N-pentylaniline) and alkyl aryl ethers (methoxybenzene, ethoxybenzene, propoxybenzene, butoxybenzene) obtained from the Department of Polymer Chemistry (Lublin, Poland) were used in the chromatographic studies.
For determination of the retention time of toluene, alkylphenones (1-phenylethan-1-one, 1-phenylbutan-1-one), phthalates (dimethyl phthalate, diethyl phthalate, dipropyl phthalate, dibutyl phthalate, dioctyl phthalate, dinonyl phthalate, didodecyl phthalate), and polystyrene standards with various molecular weights (580; 1050; 2450; 5100; 11,600; 30,300; 68,000; 120,000; 330,000; 750,000; 1,260,000; 2,750,000) from E. Merck (FRG, Darmstadt, Germany) were applied in the porous structure investigations by inverse size exclusion chromatography.
The solvents acetonitrile 99.9% (Merck, Darmstadt, Germany), methanol 99.8% (Merck, Darmstadt, Germany), and tetrahydrofuran 99.8% (Merck, Darmstadt, Germany) were used as mobile phases in HPLC and ISEC.
2.3. One-Step Swelling and Polymerization of Poly(GMA-co-EGDMA) Microspheres
One-stage swelling and polymerization was also conducted in a three-necked round-bottom flask equipped with a mechanical stirrer and a nitrogen gas inlet system.
In the first step, a 200 mL solution of 1% PVP and 0.25% SDS was prepared. The dried PS seeds (0.45 g) were redispersed into a 25 mL beaker containing 15 mL of PVP/SDS solution. The vessel was placed in the bath and the ultrasound was applied for about 1 min to “break up” any agglomerates.
A mixture of monomers (GMA and EGDMA, 13.4 mL), initiator (AIBN, 0.14 g) and porogenic solvent was prepared in a 50 mL beaker. The oil phase thus obtained was transferred to a 250 mL Erlenmeyer flask containing 150 mL of PVP/SDS solution. The whole was subjected to the homogenizer for about 5 min and then transferred to a 250 mL three-necked flask equipped with a mechanical stirrer and a thermometer. The solution was deoxygenated by bubbling the inert gas (nitrogen) through it for about 30 min. Then the rotation speed was set to 150 rpm.
The suspension of PS seeds was dropped into the flask using a plastic Pasteur pipette. The swelling process ran for 24 h at the temperature of 30 °C. The temperature was then increased to 70 °C to initiate the polymerization reaction and the process continued for another 24 h.
The resulting GE microspheres were filtered at the reduced pressure in a Büchner funnel. To rinse the surfactant and residual unreacted monomers from the system, the material was washed on the filter with large amounts of hot water, 20 mL of tetrahydrofuran, and 20 mL of acetone.
Due to the proposed use of polymer microspheres as the stationary phases in liquid chromatography, the polystyrene present in the material should be removed. The non-crosslinked linear polymer is soluble in organic samples and may distort the results of the chromatographic separation. For this purpose, the obtained GE microspheres were placed in a 250 mL single neck round bottom flask containing 100 mL of tetrahydrofuran. The polystyrene seeds are dissolved in this solvent which is also used as a mobile phase in chromatography. A flask equipped with a water jacketed reflux condenser was placed on a heating mantle. After about 4 h the microspheres were filtered in vacuum in a Büchner funnel and washed through the filter with pure tetrahydrofuran.
After deaerating with nitrogen, the polymerization was carried out at 70 °C for 24 h. The particles were dried in vacuum at ambient temperature.
2.4. Surface Modification
Scheme 1 presents the routes of functionalization of GE polymeric microspheres. The glycidyl-based microspheres with carboxylic groups were synthesized by the Diels-Alder reaction with sodium cyclopentadienide and maleic anhydride in accordance with the procedure described earlier [
38].
2.4.1. Microspheres GE-Cp
Poly(GMA-co-EGDMA) microspheres (4 g, epoxide value, EV = 1.8 mmol g−1, 7.2 mmol of epoxide groups) were suspended in 100 mL of dry tetrahydrofuran in a round bottom flask closed with a silicone septum. After using a vacuum pump, nitrogen was added to obtain an anaerobic environment and then the flask was cooled in an ice-salt bath to −7 °C. A double excess solution of sodium cyclopentadienide in THF (2 M, 7.2 mL, 14.4 mmol) in 25 mL of dry tetrahydrofuran was added dropwise to the microspheres using a syringe. After 1 h, the ice-salt bath was removed, and the mixture was stirred slightly for another 24 h at ambient temperature. The reaction was quenched by pouring the reaction mixture into 100 mL of saturated NH4Cl solution. Subsequently, the microspheres were filtrated off and washed successively by 100 mL of acetone, ethyl alcohol-water (1/1), 3% HCl in water, water, tetrahydrofuran, and hexane. The obtained GE-Cp microspheres were dried in an oven overnight at 50 °C.
2.4.2. Microspheres GE-COOH
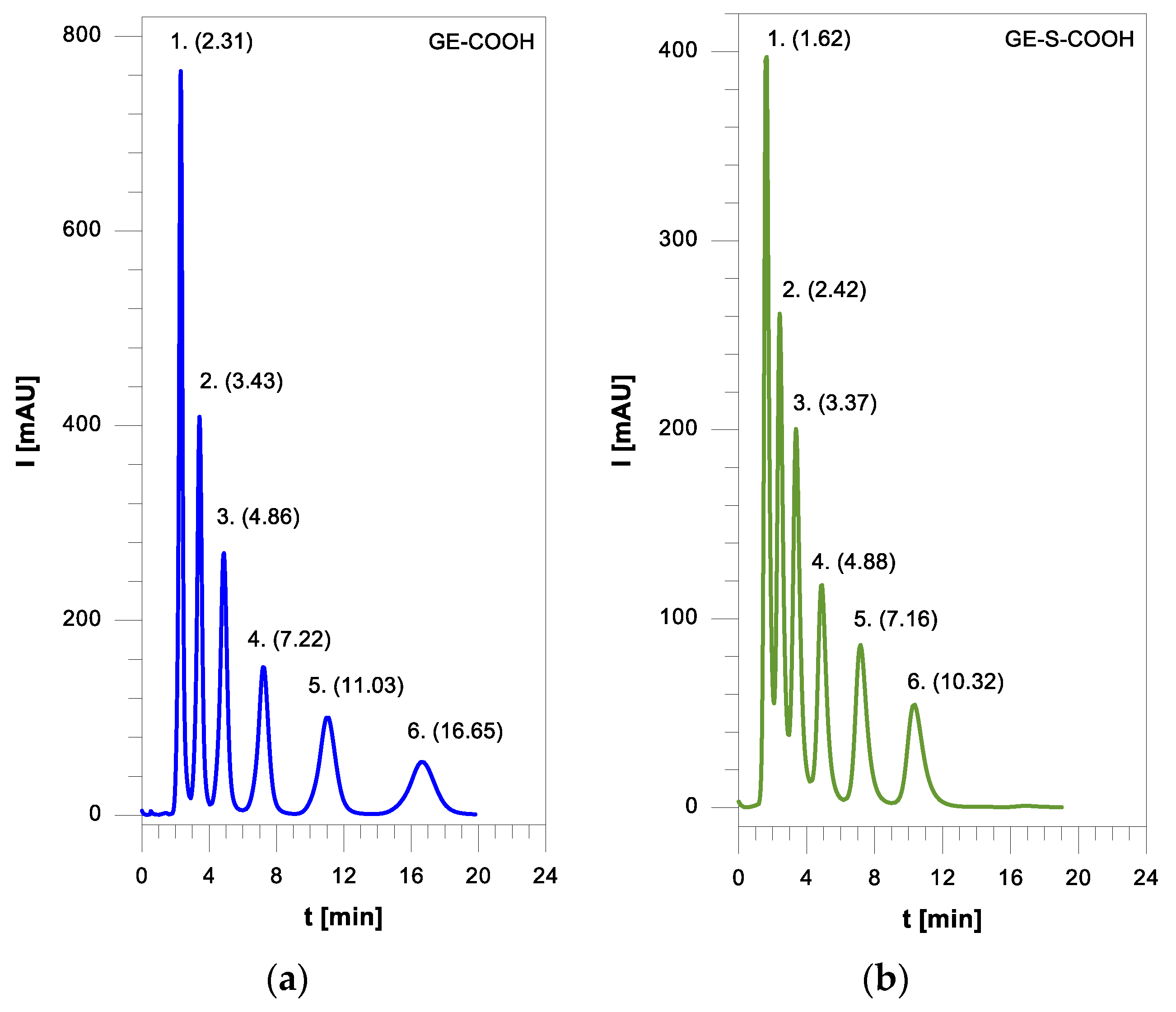
GE-Cp microspheres (2 g, 3.0 mmol of Cp moieties) and maleic anhydride (588 mg, 6.0 mmol excess) were suspended in 50 mL of acetone in a 100 mL round bottom flask and were stirred for 2.5 h at 50 °C. The microspheres were washed thoroughly (2 × 25 mL of ethyl alcohol and 2 × 25 mL of acetone) to remove the physically absorbed anhydride moiety and dried in vacuum at ambient temperature. Then their cyclic anhydride groups were hydrolyzed using water with a few drops of sulfuric acid for 4 h at 80 °C. Under this mild condition, no ester hydrolysis occurs. The obtained GE-COOH microspheres were vacuum filtered on a Büchner funnel and washed on the filter with acetone and then oven dried overnight at 50 °C.
2.4.3. Microspheres GE-BMI
To a 100 mL round-bottom flask containing 50 mL of acetone, 1 g of GE10-Cp microspheres (1.5 mmol of Cp moieties) and 1.075 g of 4,4′-bis(maleimido)diphenylmethane (3.0 mmol excess) were added. This was heated at 50 °C for 2.5 h with constant stirring.
The resulting GE-BMI microspheres were vacuum filtered on a Büchner funnel, rinsed with acetone then oven dried overnight at 50 °C.
2.4.4. Microspheres GE-OH
This simple modification is based on the epoxide ring opening by the hydrolysis of GE materials with water and a few drops of sulfuric acid. The process was carried out for 3 h at 100 °C. Under such mild conditions, no ester hydrolysis takes place. The obtained GE-OH microspheres were vacuum filtered on a Büchner funnel, washed with acetone and then oven dried overnight at 50 °C.
2.4.5. Microspheres GE-MAC
To a 250 mL round-bottom flask containing 100 mL of dichloromethane, 4 g of GE-OH microspheres with hydroxyl value, HV = 3.0 mmol g−1 (12.0 mmol) and 2.9 g of the catalyst—triethylamine (TEA, 28.8 mmol excess)—were added. This was placed in an ice bath with constant stirring. Once the temperature was about 0 °C, the dropwise step addition step of 2.5 mL methacryloyl chloride (28.8 mmol, excess) started. The process was carried out for about 1 h at a temperature not below 0 °C, then for 3 h at room temperature with constant stirring.
The resulting GE-MAC microspheres were vacuum filtered on a Büchner funnel and washed with dichloromethane and acetone and then oven dried overnight at 50 °C.
2.4.6. Microspheres GE-S-COOH
First, 1.5 g of GE-MAC microspheres and 7 g of triethylamine, as catalyst, were added to a 250 mL round bottom flask containing 100 mL of dichloromethane and placed in an ice bath with constant stirring. The step of adding 2-mercaptopropionic acid in dichloromethane started dropwise (3.75 g of 2-MPA was added to a 25 mL addition funnel and made up with dichloromethane). After 1 h at a temperature of not lower than 0 °C, the process was carried out at room temperature for 3 h and next the flask was placed in a water bath for 72 h at 40 °C while stirring. The resulting GE-S-COOH microspheres were vacuum filtered on a Büchner funnel and washed with dichloromethane, methanol, tetrahydrofuran and acetone and then oven dried overnight at 50 °C.