Micro-/Nano-Structured Ceramic Scaffolds That Mimic Natural Cancellous Bone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ceramic Scaffolds Synthesis
2.2. Scaffolds Characterization
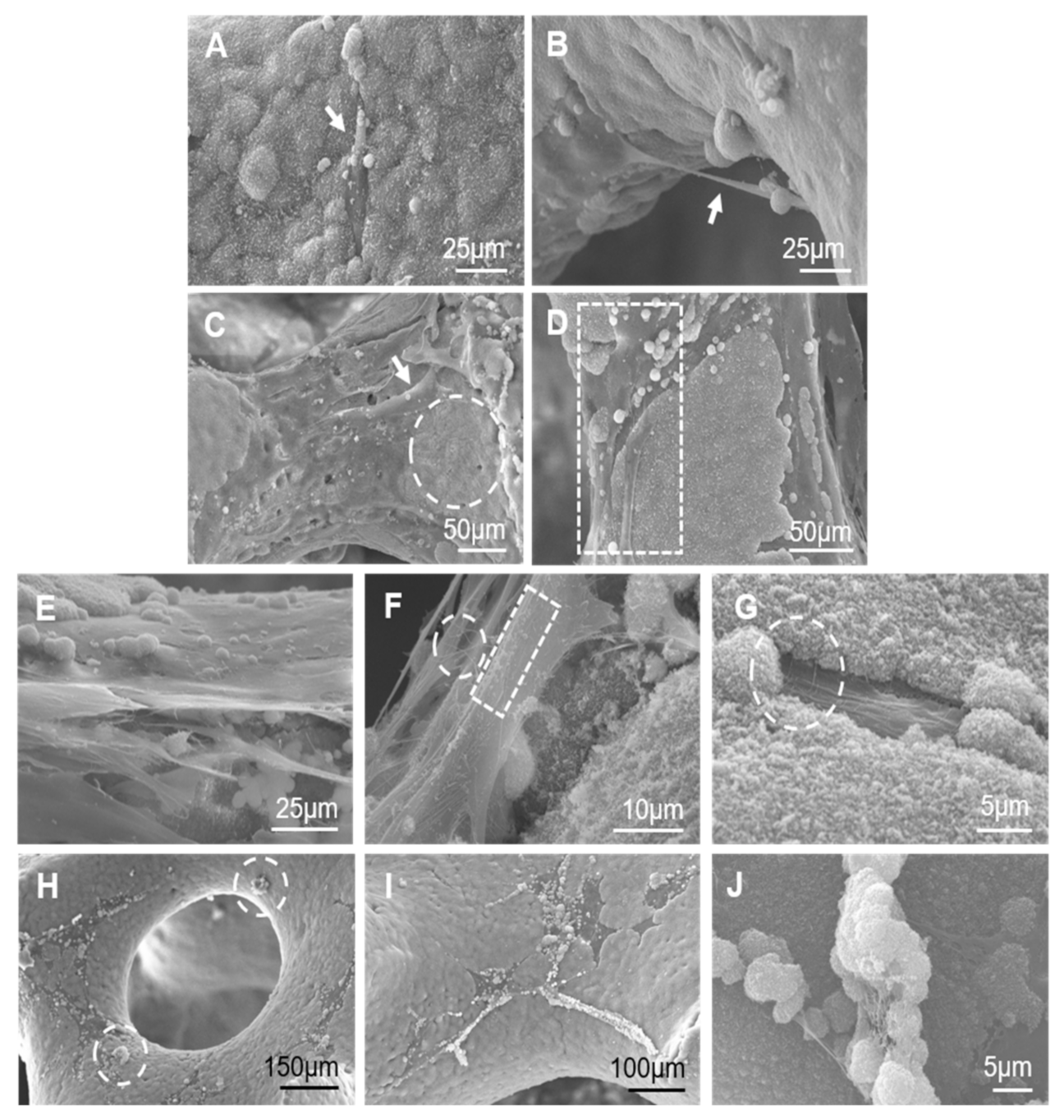
2.3. SEM Examination of Natural Cancellous Bone
- Fixation for 2.5 h in a McDowell’s and Trump’s 4F:1G fixative solution [16] that consists in a combination of 4% commercial formaldehyde and 1% glutaraldehyde in a buffer of 176 mOsm/liter. It is recommended as a primary fixative for the SEM analysis of samples.
- Washed in cacodylate buffer at 0.1 Μ and saccharose at 8% overnight at 4 °C.
- Fixation was completed with 1% osmium tetroxide and 0.1 Μ cacodylate for 1.5 h at 4 °C.
- Dehydration by gradient acetone series 30%, 50%, 70%, 90%, and 100% for 10 min each at room temperature
- Critical point drying in CO2.
- Finally, samples were gold-coated by the Bio-Rad Polaron coater (Bio-Rad Laboratories, Hercules, CA, USA).
2.4. Ah-MSCs: Isolation, Expansion and Characterization
2.5. Ah-MSCs Adhesion and Proliferation In Vitro Test
- (a)
- Basal growth medium (GM). It consists in 10% inactivated fetal bovine serum (FBS) and 1% antibiotics (penicillin (100 U/mL)/streptomycin (100 µg/mL)) supplemented in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA).
- (b)
- Osteogenic-inducing medium (OM). It consists in GM with an osteogenic supplement composed of L-ascorbic acid 2-phosphate (0.2 mM; mSigma, St. Louis, MO, USA), dexamethasone (10 nM; Sigma) and β-glycerolphosphate (10 mM; Merck, Darmstadt, Germany).
2.6. Testing Alkaline Phosphatase Specific Activity in Cells
2.7. Alkaline Phosphatase Grafting to Scaffolds
2.8. Enzymatic Activity Test
2.9. Statistical Analysis
3. Results
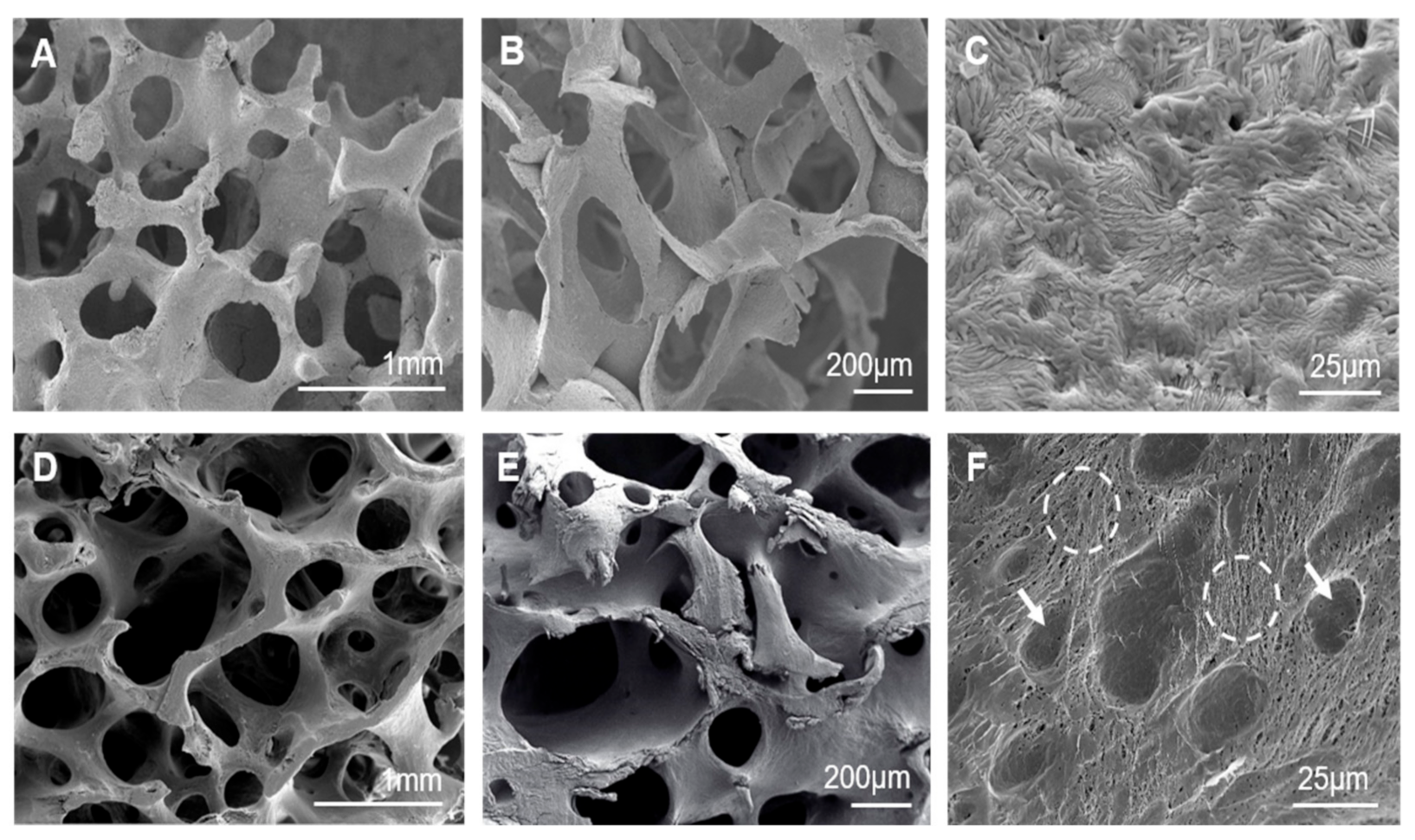
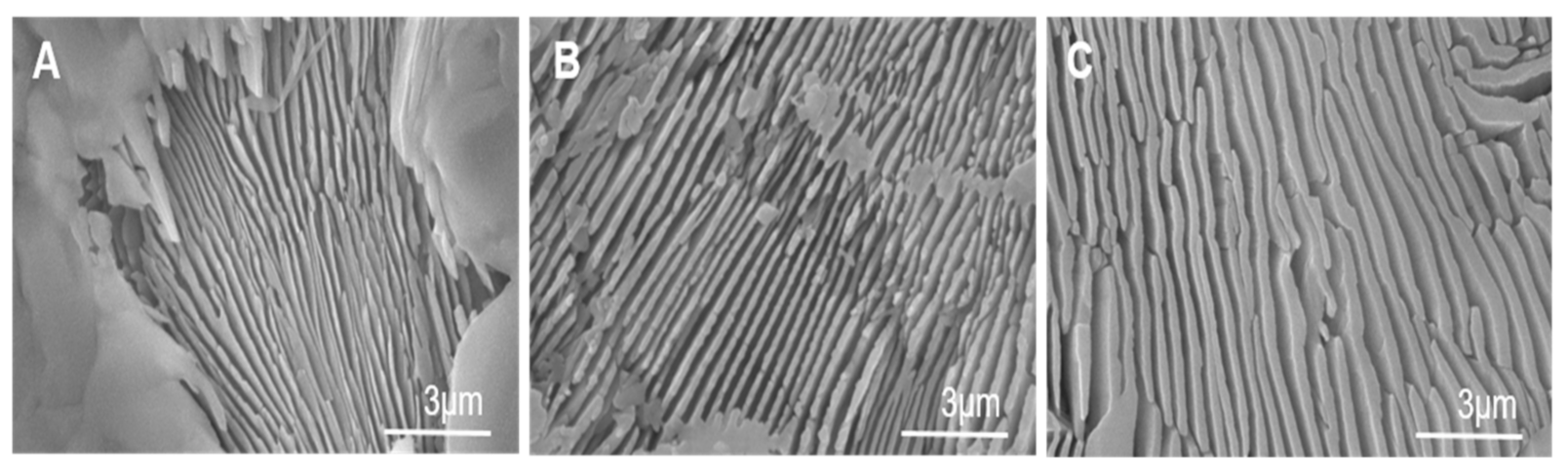
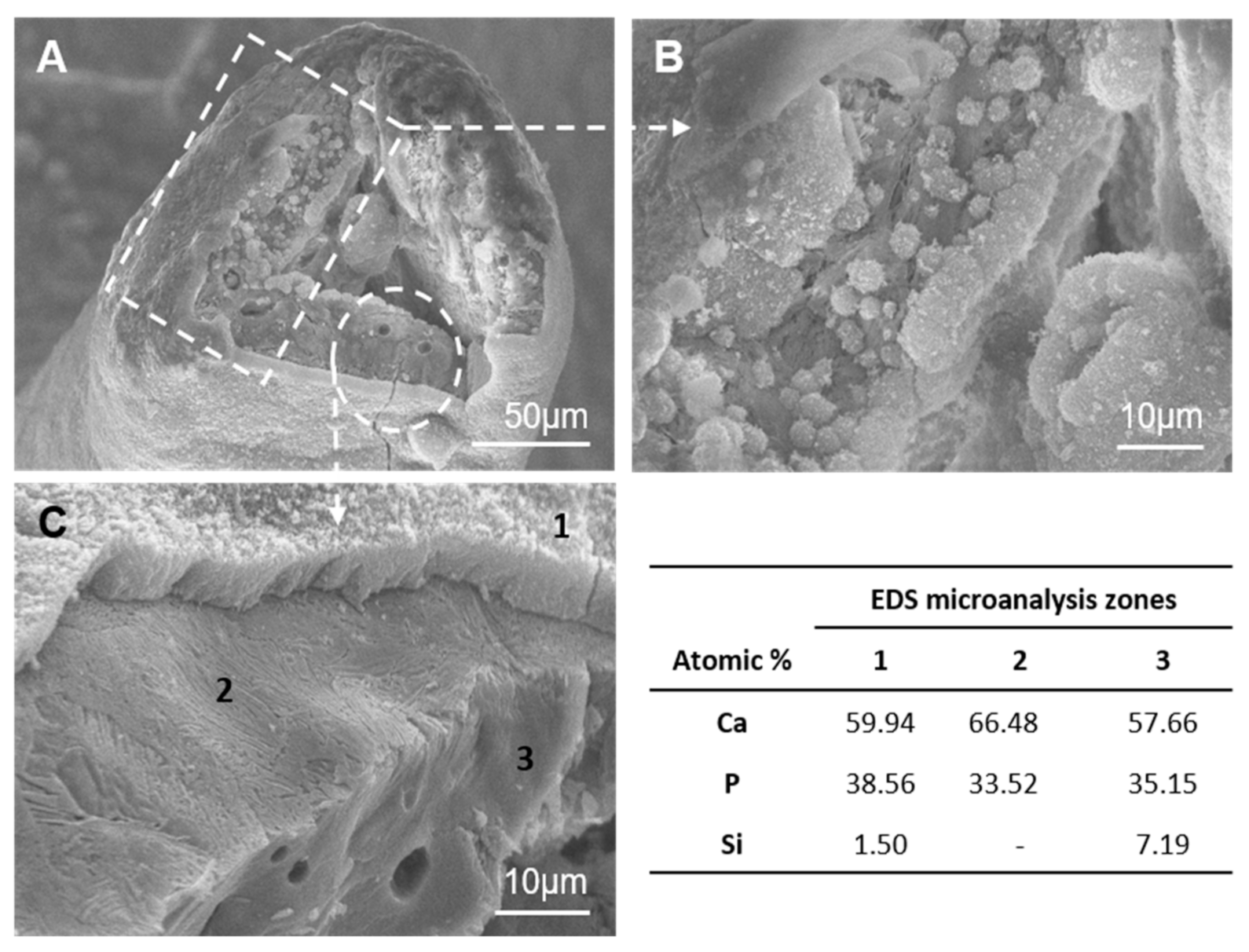
3.1. Scaffolds’ Characterization and Cancellous Bone Examination
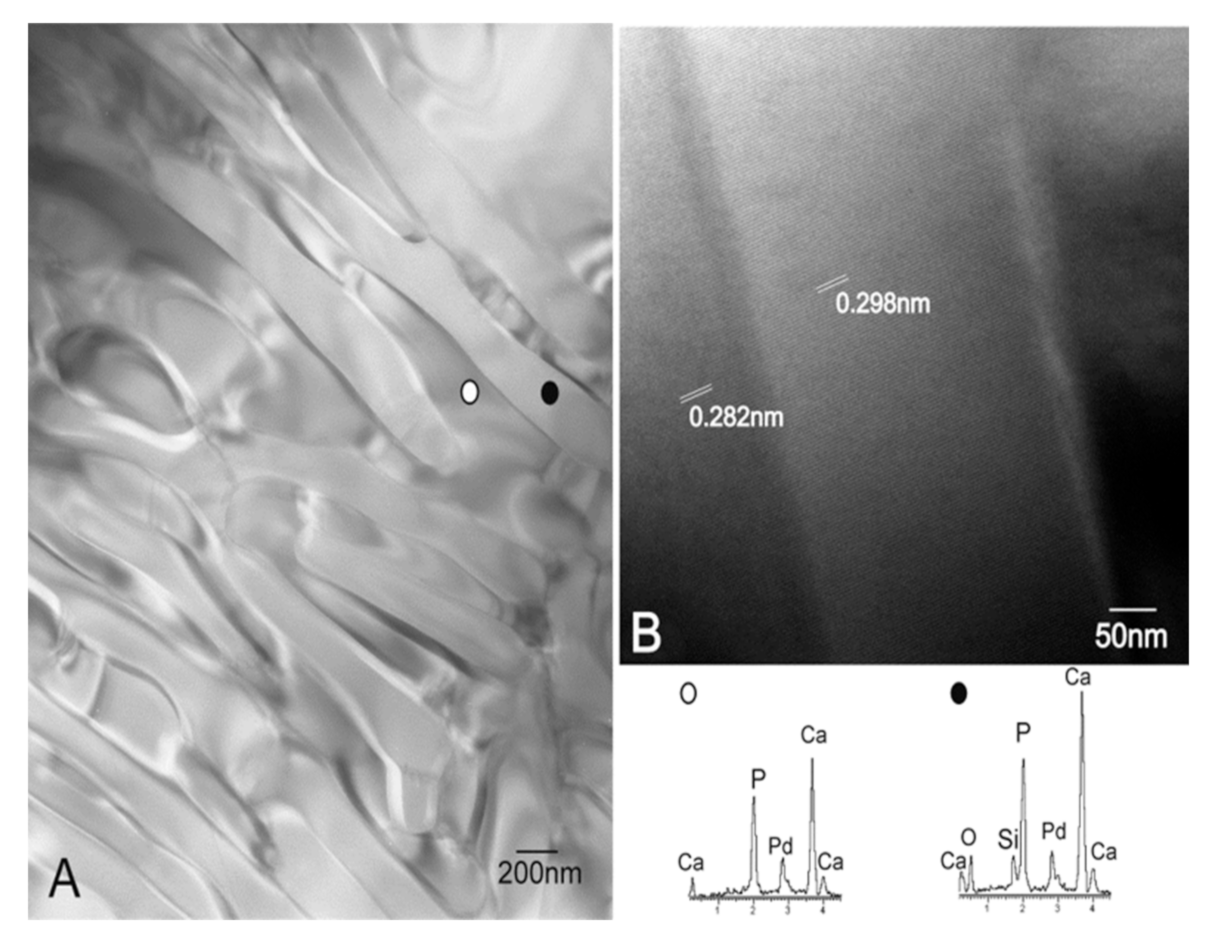
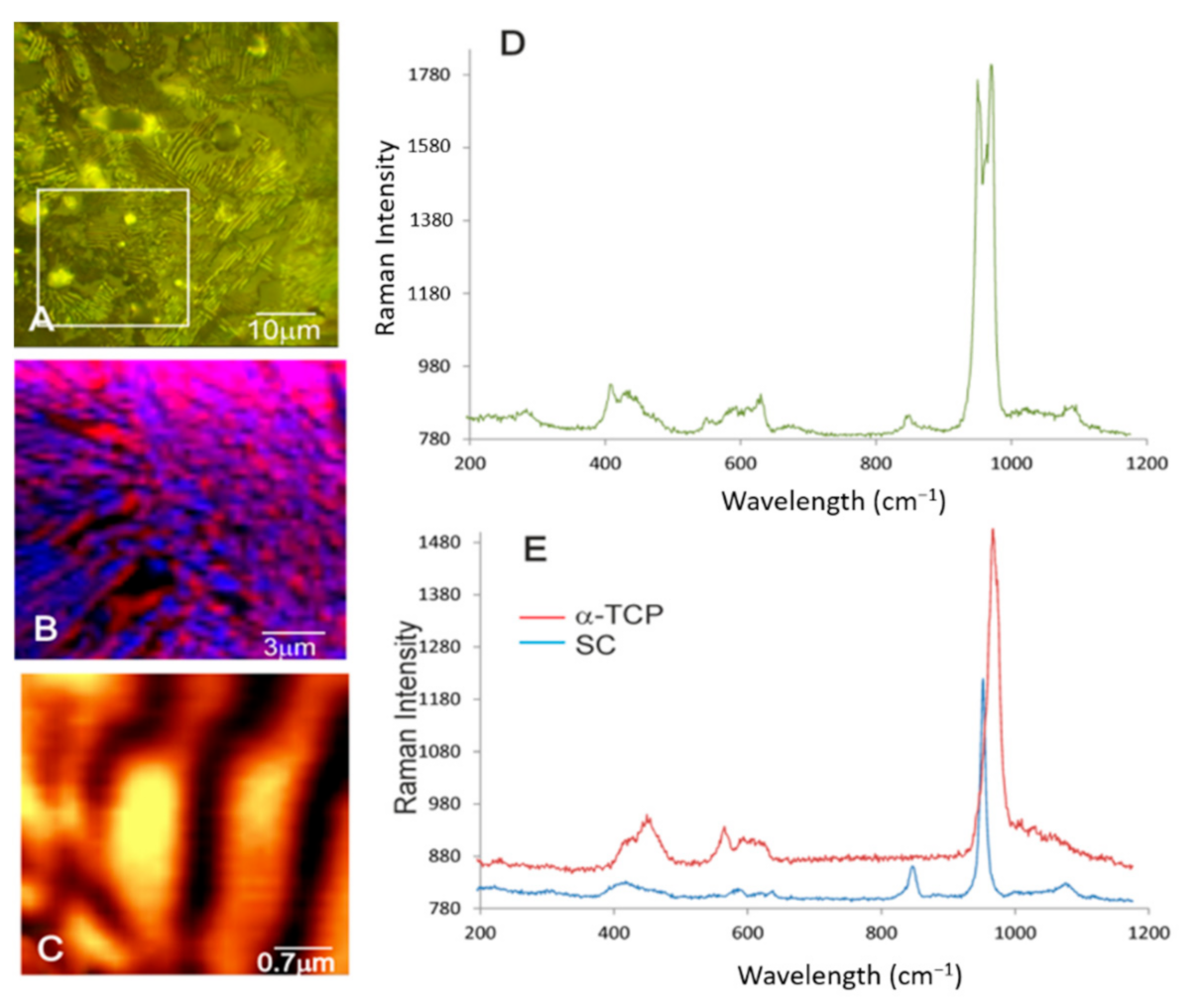
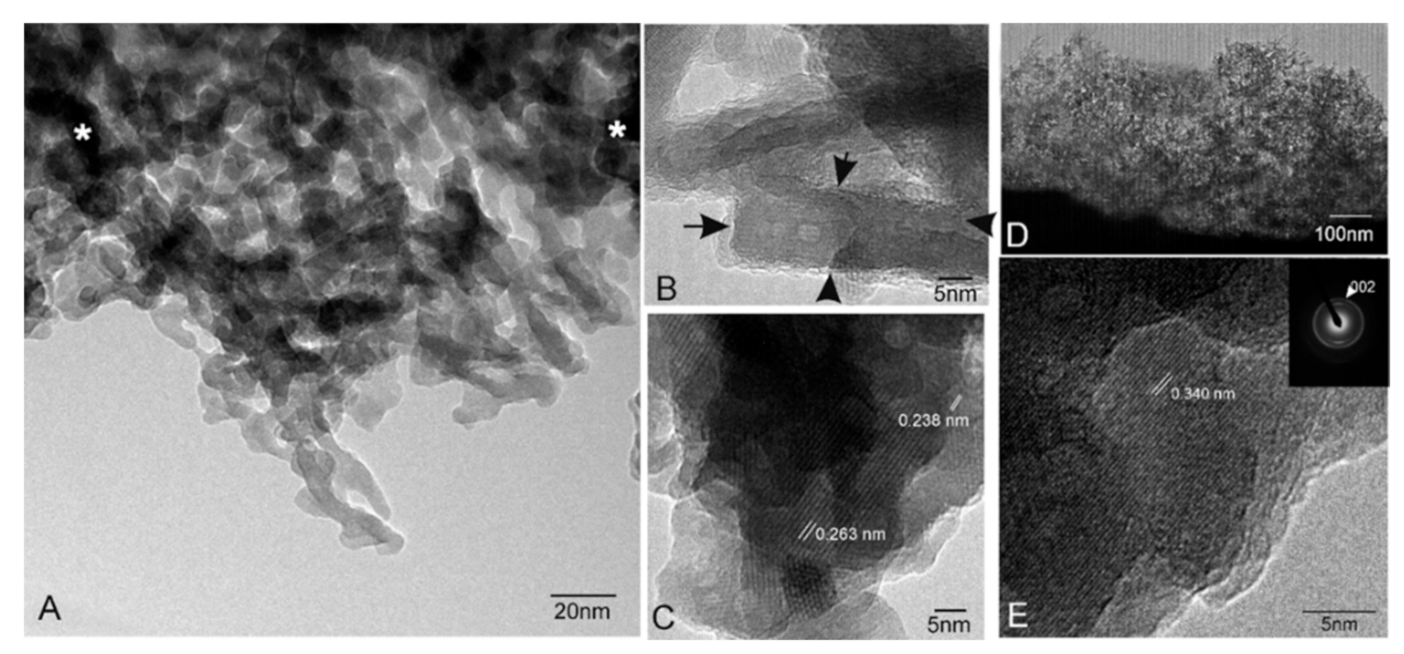
3.2. Characterization of the Nanostructure of Scaffold Surfaces
3.3. Cell adhesion and Proliferation In Vitro Test
- (1)
- A dense dune-like layer on the GM-scaffold boundary with an average Ca/P ratio of 1.55. EDS indicated less silicon than the original sample (3). This layer was about 9 ± 0.05 µm thick. The crystalline nature of this dune-like layer and the Ca/P ratio corresponded to the precipitated calcium-deficient nonstoichiometric hydroxyapatite.
- (2)
- A lamellar microstructure lay beneath the precipitated hydroxyapatite layer with an average Ca/P ratio of 1.98. EDS confirmed that the silicon was not present in the lamellar microstructure.
- (3)
- The original scaffold material remained inside the structure with an average Ca/P ratio of 1.64. EDS indicated higher silicon content than the precipitated hydroxyapatite layer.
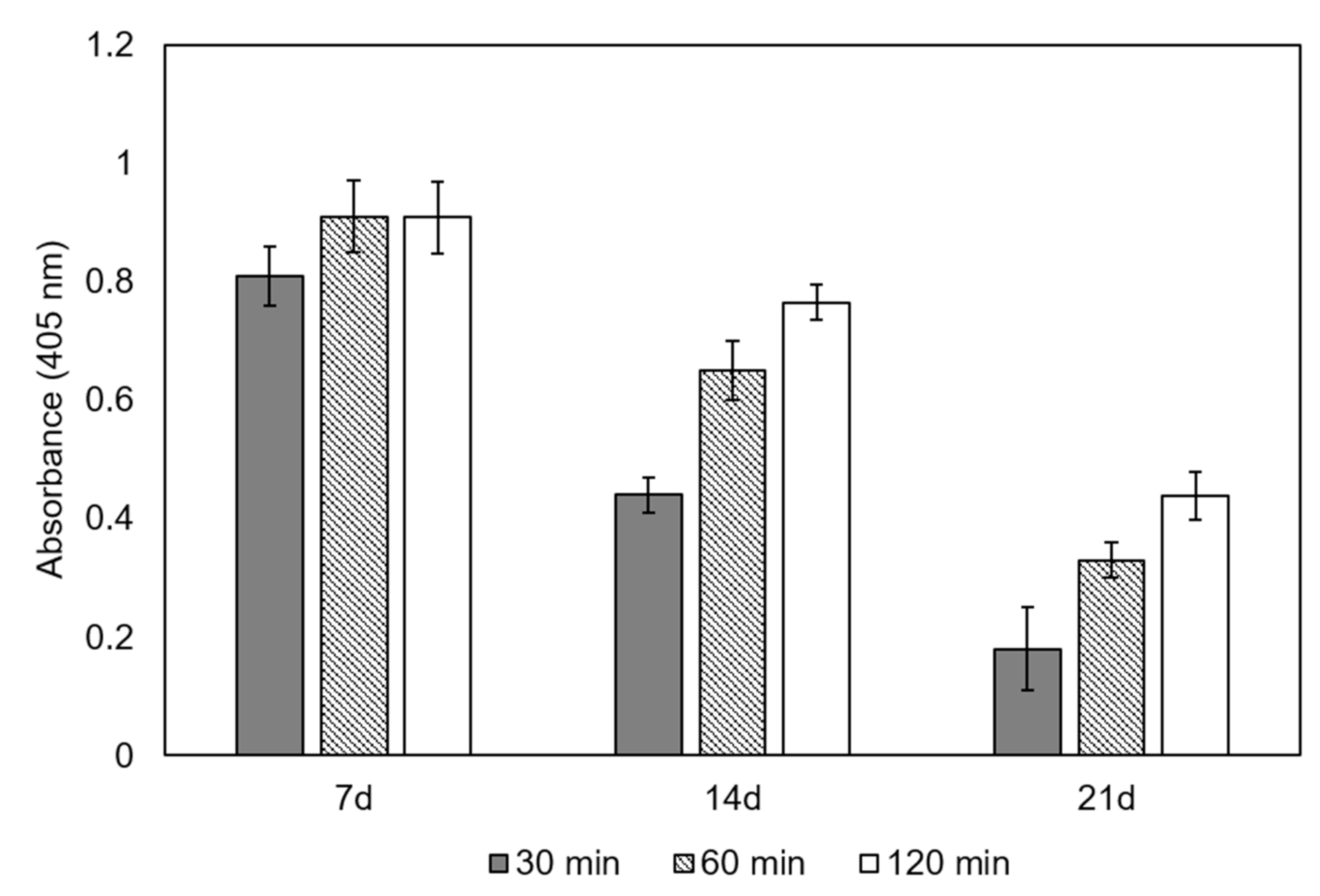
3.4. Specific Alkaline Phosphatase Activity in Cells
3.5. Alkaline Phosphatase Grafting to Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Jones, J.R. New trends in bioactive scaffolds: The importance of nanostructure. J. Eur. Ceram. Soc. 2009, 29, 1275–1281. [Google Scholar] [CrossRef]
- García, J.R.; García, A.J. Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Deliv. Transl. Res. 2016, 6, 77–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes, B.; Santana, A.; Arribas, M.I.; Vicente-Salar, N.; de Aza, P.N.; Roche, E.; Such, J.; Reig, J.A. Phenotypic differences during the osteogenic differentiation of single cell-derived clones isolated from human lipoaspirates. J. Tissue Eng. Regen. Med. 2011, 5, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Liu, K.; Zhang, F.; Wei, W.; Chen, C.; Huang, Q. Microstructure and in vitro bioactivity of silicon-substituted hydroxyapatite. Silicon 2017, 9, 543–553. [Google Scholar] [CrossRef]
- Serena, S.; Caballero, A.; de Aza, P.N.; Sainz, M.A. New evaluation of the in vitro response of silicocarnotite monophasic material. Ceram. Int. 2015, 41, 9412–9419. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef] [Green Version]
- Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramirez-Fernandez, M.P.; Negri, B.; Abboud, M.; Martinez, I.M.; de Aza, P.N. Physical Properties, Mechanical Behavior, and Electron Microscopy Study of a New α-TCP Block Graft with Silicon in an Animal Model. J. Biomed. Mater. Res. A 2012, 12, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. A Review of Bioactive Silicate Ceramics. Biomed. Mater. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.M.; Velasquez, P.A.; de Aza, P.N. The sub-system α-TCPss-silicocarnotite within the binary system Ca3(PO4)2–Ca2SiO4. J. Am. Ceram. Soc. 2012, 95, 1112–1117. [Google Scholar] [CrossRef]
- De Aza, P.N.; Serena, S.; Luklinska, Z.B. Manufacture and characterization of a new Si-Ca-P biphasic ceramic. Ceram. Int. 2018, 44, 13623–13629. [Google Scholar] [CrossRef]
- Díaz-Arca, A.; Mazón, P.; de Aza, P.N. Eutectoid scaffold as potential tissue engineer guide. J. Am. Ceram. Soc. 2019, 102, 7168–7177. [Google Scholar] [CrossRef]
- McDowell, E.M.; Trump, B.F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch. Pathol. Lab. Med. 1976, 100, 405–414. [Google Scholar]
- Meseguer-Olmo, L.; Aznar-Cervantes, S.; Mazón, P.; De Aza, P.N. “In vitro” behavior of adult mesenchymal stem cells of human bone marrow origin seeded on a novel bioactive ceramic in the Ca2SiO4–Ca3(PO4)2 system. J. Mater. Sci. Mater. Med. 2012, 23, 3003–3014. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- De Aza, P.; García-Bernal, D.; Cragnolini, F.; Velasquez, P.; Meseguer-Olmo, L. The effects of Ca2SiO4–Ca3(PO4)2 ceramics on adult human mesenchymal stem cell viability, adhesion, proliferation, differentiation and function. Mater. Sci. Eng. C. 2013, 33, 4009–4020. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- De Jonge, L.T.; Leuwemburg, S.C.G.; van den Beucken, J.J.J.P.; Wolke, J.G.C.; Jansen, A. Electrosprayed enzyme coatings as bioinspired alternative to bioceramic coatings for orthopedic and oral implants. Adv. Funct. Mater. 2009, 19, 1–8. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Cerm. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Boyde, A.; Hobdell, M.H. Scanning Electron Microscopy of Lamellar Bone. Z. Zellforsch. 1968, 93, 213–231. [Google Scholar] [CrossRef]
- Boyde, A.; Hobdell, M.H. Scanning Electron Microscopy of primary membrane bone. Z. Zellforsch. 1969, 99, 98–108. [Google Scholar] [CrossRef]
- Jayasinghe, J.A.P.; Jones, S.J.; Boyde, A. Scanning electron microscopy of human lumbar vertebral trabecular bone surfaces. Virchows Archiv. A Pathol. Anat. 1993, 422, 25–34. [Google Scholar] [CrossRef]
- Martínez, I.M.; Velasquez, P.A.; de Aza, P.N. Synthesis and stability of α-tricalcium phosphate doped with dicalcium silicate in the system Ca3(PO4)2–Ca2SiO4. Mater. Charact. 2010, 61, 761–767. [Google Scholar] [CrossRef]
- Ibáñez, J.; Artús, L.; Cuscó, R.; López, A.; Menéndez, E.; Andrade, M.C. Hydration and carbonation of monoclinic C2S and C3S studied by Raman spectroscopy. J. Raman Spectrosc. 2001, 38, 61–67. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Jillavenkatesa, A.; Condrate, R.A. The infrared and Raman spectra of β-and α tricalcium phosphate (Ca3(PO4)2). Spectrosc. Lett. 1998, 31, 1619–1634. [Google Scholar] [CrossRef]
- De Bruijn, J.D.; van Blitterswijk, C.A.; Davies, J.E. Initial bone matrix formation at the hydroxyapatite interface in vivo. J. Biomed. Mater. Res. 1995, 29, 89–99. [Google Scholar] [CrossRef]
- Goonoo, N.; Fahmi, A.; Jonas, U.; Gimié, F.; Arsa, I.A.; Bénard, S.; Schönherr, H.; Bhaw-Luximon, A. Improved Multicellular Response, Biomimetic Mineralization, Angiogenesis, and Reduced Foreign Body Response of Modified Polydioxanone Scaffolds for Skeletal Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 5834–5850. [Google Scholar] [CrossRef]
- Liao, S.; Watari, F.; Xu, G.; Ngiam, M.; Ramakrishna, S.; Chan, C.K. Morphological Effects of Variant Carbonates in Biomimetic Hydroxyapatite. Mater. Lett. 2007, 61, 3624–3628. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.H.H.A.; Müller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The Role of Prenucleation Clusters in Surface-Induced Calcium Phosphate Crystallization. Nat. Mater. 2010, 12, 1010–1014. [Google Scholar] [CrossRef]
- Porter, A.E.; Botelho, C.M.; Lopes, M.A.; Santos, J.D.; Best, S.M.; Bonfield, W. Ultrastructural comparison of dissolution and apatite precipitation on hydroxyapatite and silicon-substituted hydroxyapatite in vitro and in vivo. J. Biomed. Mater. Res. A 2004, 69, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Freyman, T.M.; Yannas, I.V.; Gibson, L.J. Cellular materials as porous scaffolds for tissue engineering. Prog. Mater. Sci. 2001, 46, 273–282. [Google Scholar] [CrossRef]
- Lu, J.X.; Flautre, B.; Anselme, K.; Hardoiun, P.; Gallur, A.; Descamps, M.; Thierry, B. Role of interconnections in porous bioceramics on bone recolonisation in vitro and in vivo. J. Mater. Sci. Mater. Med. 1999, 10, 111–120. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Glimcher, M.J.; Cooper, R.R.; Recker, R. Bone Biology. Part I: Structure, Blood Supply, Cells, Matrix, and Mineralization. J. Bone Jt. Surg. Am. 1995, 77, 1256–1275. [Google Scholar] [CrossRef]
- Stefanescu, D.M. Science and Engineering of Casting Solidification, 3rd ed.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Bang, L.T.; Ishikawa, K.; Othman, R. Effect of silicon and heat-treatment temperature on the morphology and mechanical properties of silicon—Substituted hydroxyapatite. Ceram. Int. 2011, 37, 3637–3642. [Google Scholar] [CrossRef]
- Kim, Y.U.; Lee, B.H.; Kim, M.C.; Kim, K.N.; Kim, K.M.; Choi, S.H.; Kim, C.K.; LeGeros, R.Z.; Lee, Y.K. Effect of Cooling Rate and Particle Size on Compressive Strength of Macroporous Hydroxyapatite. Key Eng. Mater. 2006, 309–311, 1047–1050. [Google Scholar] [CrossRef]
- Kanatani, M.; Sugimoto, T.; Kano, J.; Chihara, K. IGF-I mediates the stimulatory effect of high phosphate concentration on osteoblastic cell proliferation. J. Cell. Phys. 2002, 190, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.J. Secretory territories and rate of matrix production of osteoblasts. Calcif. Tissue Res. 1974, 14, 309–315. [Google Scholar] [CrossRef]
- Pazzaglia, U.E.; Congiu, T.; Marchese, M.; Dell’Orbo, C. The shape modulation of osteoblast-osteocyte transformation and its correlation with the fibrillar organization in secondary osteons. Cell Tissue Res. 2010, 340, 533–554. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mat. Sci. Eng. R. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Pietak, A.M.; Reid, J.W.; Stott, M.J.; Sayer, M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials 2007, 28, 4023–4032. [Google Scholar] [CrossRef]
- Fielding, G.; Feuerstein, J.; Bandyopadhyay, A.; Bose, S. SiO2 and SrO Doped β-TCP: Influence of Dopants on Mechanical and Biological Properties. In Biomaterials Science: Processing, Properties and Applications II; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012; Volume 237, pp. 171–181. [Google Scholar] [CrossRef]
- Díaz-Arca, A.; Velasquez, P.; Mazon, P.; De Aza, P.N. Mechanism of in vitro reaction of a new scaffold ceramic similar to porous bone. J. Eur. Ceram. Soc. 2020, 40, 2200–2206. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Sfihi, H.; Barroug, A. Physico-Chemical Properties of Nanocrystalline Apatites: Implications for Biominerals and Biomaterials. Mater. Sci. Eng. C 2007, 27, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [Green Version]
- Lutisanova, G.; Palou, M.T.; Kozankova, J. Comparison of bioactivity in vitro of glass and glass ceramic materials during soaking in SBF and DMEM medium. Ceram. Silik. 2011, 55, 199–207. [Google Scholar]
- Theodorou, G.; Goudouri, O.M.; Kontonasaki, E.; Chatzistavrou, X.; Papadopoulou, L.; Kantiranis, N.; Paraskevopoulos, K.M. Comparative Bioactivity Study of 45S5 and 58S Bioglasses in Organic and Inorganic Environment. Bioceram. Dev. Appl. 2011, 1, 1–4. [Google Scholar] [CrossRef]
- Verné, E.; Ferraris, S.; Vitale-Brovarone, C.; Spriano, S.; Bianchi, C.L.; Naldoni, A.; Morra, M.; Cassinelli, C. Alkaline phosphatase grafting on bioactive glasses and glass ceramics. Acta Biomater. 2010, 6, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verné, E.; Ferraris, S.; Vitale-Brovarone, C.; Cochis, A.; Rimondini, L. Bioactive glass fuctionalized with alkaline phosphatase stimulates bone extracellular matrix deposition and calcification in vitro. Appl. Surf. Sci. 2014, 313, 372–381. [Google Scholar] [CrossRef]


| Cooling Rate | 50 °C/h | 16.5 °C/h | 5.5 °C/h |
|---|---|---|---|
| Volumetric Shrinkage (%) * | 30 ± 0.5 | 30 ± 0.5 | 30 ± 0.5 |
| Compressive strength (MPa) * | 0.62 ± 0.07 | 1.67 ± 0.05 | 3.38 ± 0.06 |
| Lamellar width range (nm) | 100–250 | 300–560 | 600–940 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Arca, A.; Ros-Tárraga, P.; Tomé, M.J.M.; De Aza, A.H.; Meseguer-Olmo, L.; Mazón, P.; De Aza, P.N. Micro-/Nano-Structured Ceramic Scaffolds That Mimic Natural Cancellous Bone. Materials 2021, 14, 1439. https://doi.org/10.3390/ma14061439
Díaz-Arca A, Ros-Tárraga P, Tomé MJM, De Aza AH, Meseguer-Olmo L, Mazón P, De Aza PN. Micro-/Nano-Structured Ceramic Scaffolds That Mimic Natural Cancellous Bone. Materials. 2021; 14(6):1439. https://doi.org/10.3390/ma14061439
Chicago/Turabian StyleDíaz-Arca, Anabel, Patricia Ros-Tárraga, María J. Martínez Tomé, Antonio H. De Aza, Luis Meseguer-Olmo, Patricia Mazón, and Piedad N. De Aza. 2021. "Micro-/Nano-Structured Ceramic Scaffolds That Mimic Natural Cancellous Bone" Materials 14, no. 6: 1439. https://doi.org/10.3390/ma14061439
APA StyleDíaz-Arca, A., Ros-Tárraga, P., Tomé, M. J. M., De Aza, A. H., Meseguer-Olmo, L., Mazón, P., & De Aza, P. N. (2021). Micro-/Nano-Structured Ceramic Scaffolds That Mimic Natural Cancellous Bone. Materials, 14(6), 1439. https://doi.org/10.3390/ma14061439









