Improving of Sensitivity of PbS Quantum Dot Based SWIR Photodetector Using P3HT
Abstract
:1. Introduction
2. Materials and Methods
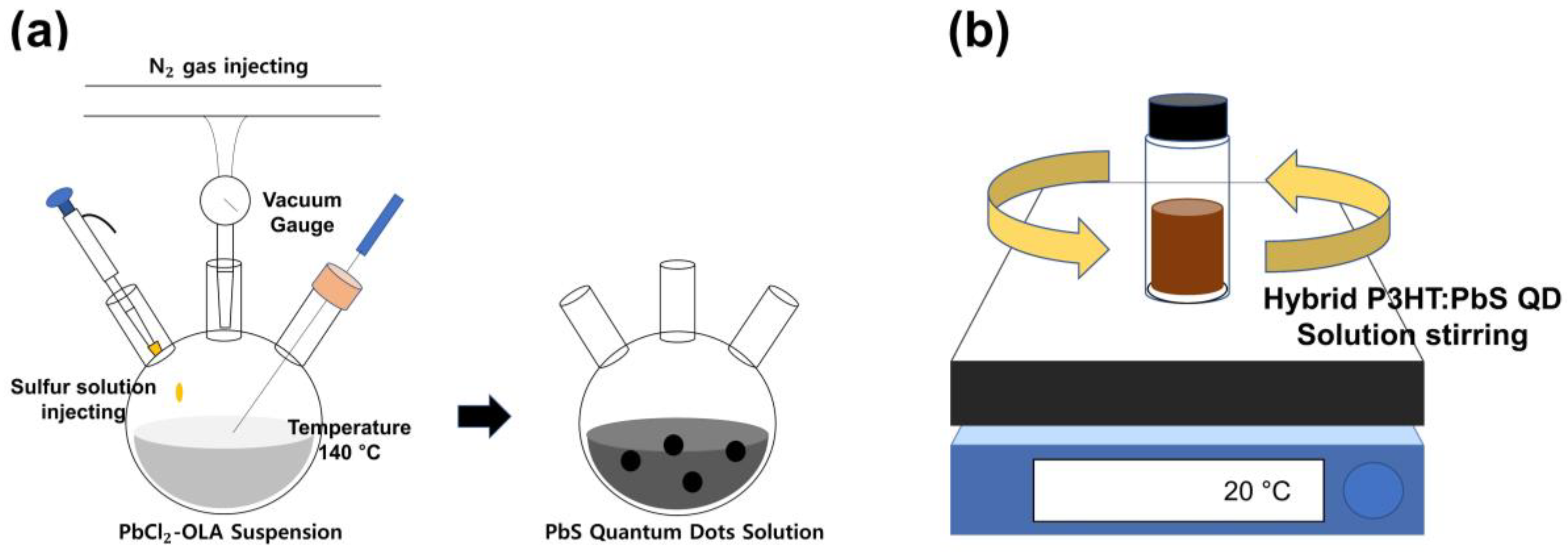
2.1. Fabrication of the Hybrid P3HT:PbS QD Solution
2.2. Synthesis of ZnO NPs
2.3. Fabrication of the SWIR Photodetector
2.4. Measurements
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, X.; Wang, Z.; Hou, X.; Yu, X.; Yang, D. Visible-blind short-wavelength infrared photodetector with high responsivity based on hyperdoped silicon. Photon. Res. 2019, 7, 351–358. [Google Scholar] [CrossRef]
- Usamentiaga, R.; Venegas, P.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F.G. Infrared Thermography for Temperature Measure ment and Non-Destructive Testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehzangi, A.; McClintock, R.; Haddadi, A.; Wu, D.; Chevallier, R.; Razeghi, M. Type–II superlattices base visible/extended short–wavelength infrared photodetectors with a bandstructure–engineered photo–generated carrier extractor. Sci. Rep. 2019, 9, 5003. [Google Scholar] [CrossRef] [Green Version]
- Zaini, N.; Van der Meer, F.; Van Ruitenbeek, F.; De Smeth, B.; Amri, F.; Lievens, C. An Alternative Quality Control Technique for Mineral Chemistry Analysis of Portland Cement-Grade Limestone Using Shortwave Infrared Spectroscopy. Remote Sens. 2016, 8, 950. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.W.; Patil, P.; Marko, I.P.; Clarke, E.; Sweeney, S.J.; Ng, J.S.; David, J.P.R.; Tan, C.H. Electrical and optical characterization of low temperature grown InGaAs for photodiode applications. Semicond. Sci. Technol. 2020, 35, 095031. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Zhao, Y.; Teng, Y.; Hao, X.; Li, X.; Zhu, H.; Zhu, H.; Wu, Q.; Huang, Y. MOCVD growth of InAs/GaSb type-Ⅱ superlattices on InAs substrates for short wavelength infrared detection. Infrared Phys. Technol. 2020, 105, 103209. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.Y.; So, F. Unraveling the Gain Mechanism in High Performance Solution-Processed PbS infrared PIN Photodiodes. Adv. Funct. Mater. 2015, 25, 1233–1238. [Google Scholar] [CrossRef]
- Moreels, I.; Justo, Y.; Geyter, B.D.; Haustraete, K.; Martins, J.C.; Hens, Z. Size-Tunable, Bright, and Stable PbS Quantum Dots: A Surface Chemistry Study. ACS Nano 2011, 5, 2004–2012. [Google Scholar] [CrossRef]
- Zhao, H.; Chaker, M.; Wu, N.; Ma, D. Towards controlled synthesis and better understanding of highly luminescent PbS/CdS core/shell quantum dots. J. Mater. Chem. 2011, 21, 8898. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Gharahcheshmeh, M.H.; Moody, N.; Bawendi, M.G.; Gleason, K.K.; Kong, J. Efficient, Flexible, and Ultra-Lightweight Inverted PbS Quantum Dots Solar Cells on All-CVD-Growth of Parylene/Graphene/oCVD PEDOT Substrate with High Power-per-Weight. Adv. Mater. Interfaces 2020, 7, 2000498. [Google Scholar] [CrossRef]
- Moreels, I.; Lambert, K.; Smeets, D.; Muynck, D.D.; Nollet, T.; Martins, J.C.; Vanhaecke, F.; Vantomme, A.; Delerue, C.; Allan, G.; et al. Size-Dependent Optical Properties of Colloidal PbS Quantum Dots. ACS Nano 2009, 3, 3023–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saran, R.; Curry, R.J. Lead sulphide nanocrystal photodetector technologies. Nat. Photonics 2016, 10, 81–92. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, Y.; Huang, J.; Xu, X.; Cui, S.; Lu, Z. Hole transport layer selection toward efficient colloidal PbS quantum dot solar cells. Opt. Express 2019, 27, A1338–A1349. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.H.; Speirs, M.J.; Chang, F.K.; Piveteau, L.; Kovalenk, M.V.; Chen, J.S.; Wu, J.J.; Loi, M.A. Increasing photon absorption and stability of PbS quantum dot solar cells using a ZnO interlayer. Appl. Phys. Lett. 2015, 107, 183901. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Brzozowski, L.; Barkhouse, D.A.R.; Debnath, R.; Levina, L.; Sargent, E.H. Schottky Quantum Dot Solar Cells Stable in Air under Solar Illumination. Adv. Mater. 2010, 22, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, S.; Lee, J.; Park, C.; Kim, O.; Xu, B.; Bae, J.; Kang, S. Uncooled Short-Wave Infrared Sensor Based on PbS Quantum Dots Using ZnO NPs. Nanomaterials 2019, 9, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.S.; Venkateswarlu, P.; Rao, V.R.; Rao, G.N. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano. Lett. 2013, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.J.; Chen, J.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W.; Nathan, A. Size Tunable ZnO Nanoparticles to Enhance Electron Injection in Solution Processed QLEDs. ACS Photonics 2016, 3, 215–222. [Google Scholar] [CrossRef]
- Mammeri, F.; Bourhis, E.L.; Rozes, L.; Sanchez, C. Mechanical properties of hybrid organic-inorganic materials. J. Mater. Chem. 2005, 15, 3787–3811. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Mohiuddin, S.; Kandemirli, F.; Malik, M.I. Synthesis and characterization of poly(3-hexylthiophene):improvement of regioregularity and energy band gap. RSC Adv. 2018, 8, 8319–8328. [Google Scholar] [CrossRef] [Green Version]
- Holliday, S.; Ashraf, R.S.; Wadsworth, A.; Baran, D.; Yousaf, S.A.; Nielsen, C.B.; Tan, C.H.; Dimitrov, S.D.; Shang, Z.; Gasparini, N.; et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 2016, 7, 11585. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.J.; Song, J.H.; Jeong, S.; Baik, S.J. PbS Colloidal Quantum Dot Solar Cells With Organic Hole Transport Layers for Enhanced Carrier Separation and Ambient Stability. IEEE J. Photovolt. 2018, 8, 2784766. [Google Scholar] [CrossRef]
- Xu, W.; Peng, H.; Zhu, T.; Yi, C.; Gong, X. A solution-processed near-infrared polymer:PbS quantum dot photodetectors. RSC Adv. 2017, 7, 34633. [Google Scholar] [CrossRef] [Green Version]
- Firdaus, Y.; Vandenplas, E.; Khetubol, A.; Cheyns, D.; Gehlhaar, R.; Van der Auweraer, M. Charge transport and recombination in P3HT:PbS solar cells. J. Appl. Phys. 2015, 117, 095503. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Li, J.; Yan, F. Highly sensitive organic near-infrared phototransistors based on poly(3-hexylthiophene) and PbS quantum dots. J. Mater. Chem. 2012, 22, 21673–21678. [Google Scholar] [CrossRef]
- Yu, G.; Heeger, A.J. Charge separation and photovoltaic conversion in polymer composites with internal donor/acceptor heterojunctions. J. Appl. Phys. 1995, 78, 4510–4515. [Google Scholar] [CrossRef]
- Thomas, A.; Vinayakan, R.; Ison, V.V. An inverted ZnO/P3HT:PbS bulk-heterojunction hybrid solar cell with a CdSe quantum dot interface buffer layer. RSC Adv. 2020, 10, 16693–16699. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, Q. Solution-processed P3HT:PbS based NIR Photodetector with FET Configuration. IEEE Photonics Technol. Lett. 2020, 32, 19260716. [Google Scholar] [CrossRef]





| Properties | PbS QD From −3 V to −1 V | Hybrid P3HT:PbS QD From −3 V to −1 V |
|---|---|---|
| Dark current (mA) | From −13.3 to −1.6 | From −9.9 to −1.2 |
| Light current (mA) | From −17.6 to −2.1 | From −28.5 to −4.1 |
| On/off ratio | From 1.3 to 1.3 | From 2.9 to 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, K.-H.; Jang, J.; Kang, I.M.; Bae, J.-H. Improving of Sensitivity of PbS Quantum Dot Based SWIR Photodetector Using P3HT. Materials 2021, 14, 1488. https://doi.org/10.3390/ma14061488
Seo K-H, Jang J, Kang IM, Bae J-H. Improving of Sensitivity of PbS Quantum Dot Based SWIR Photodetector Using P3HT. Materials. 2021; 14(6):1488. https://doi.org/10.3390/ma14061488
Chicago/Turabian StyleSeo, Kyeong-Ho, Jaewon Jang, In Man Kang, and Jin-Hyuk Bae. 2021. "Improving of Sensitivity of PbS Quantum Dot Based SWIR Photodetector Using P3HT" Materials 14, no. 6: 1488. https://doi.org/10.3390/ma14061488
APA StyleSeo, K.-H., Jang, J., Kang, I. M., & Bae, J.-H. (2021). Improving of Sensitivity of PbS Quantum Dot Based SWIR Photodetector Using P3HT. Materials, 14(6), 1488. https://doi.org/10.3390/ma14061488







