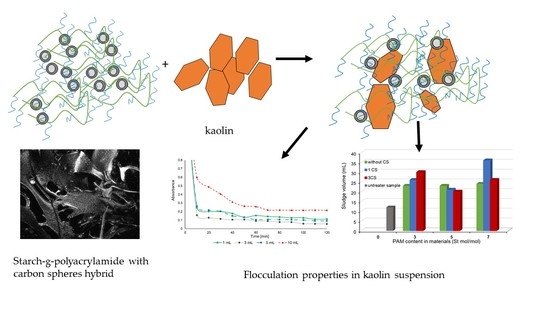
Synthesis and Characterization of Novel Hybrid Flocculants Based on Potato Starch Copolymers with Hollow Carbon Spheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Carbon Spheres
2.2.1. SiO2@m-SiO2_C18TMS Template Synthesis
2.2.2. Formation of the Carbon Spheres
2.3. Synthesis of Starch Grafted Polyacrylamide with Carbon Spheres
2.4. Characterization Methods
3. Results and Discussion
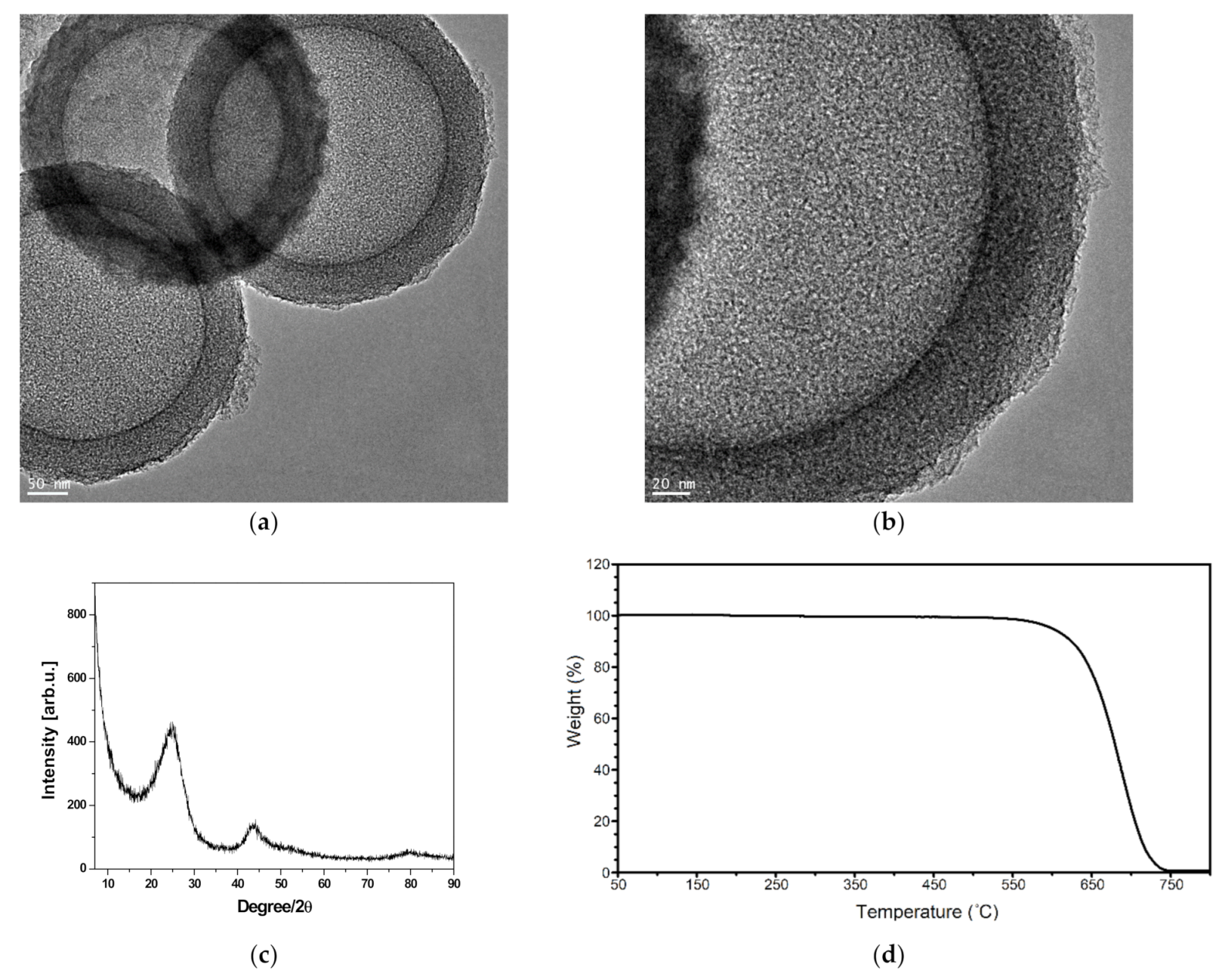
3.1. Properties of the Prepared Carbon Sphere
3.2. Starch Grafted Polyacrylamide with Carbon Spheres
3.3. Properties of Starch Grafted Polyacrylamide with CS Hybrids
3.3.1. Chemical Structure
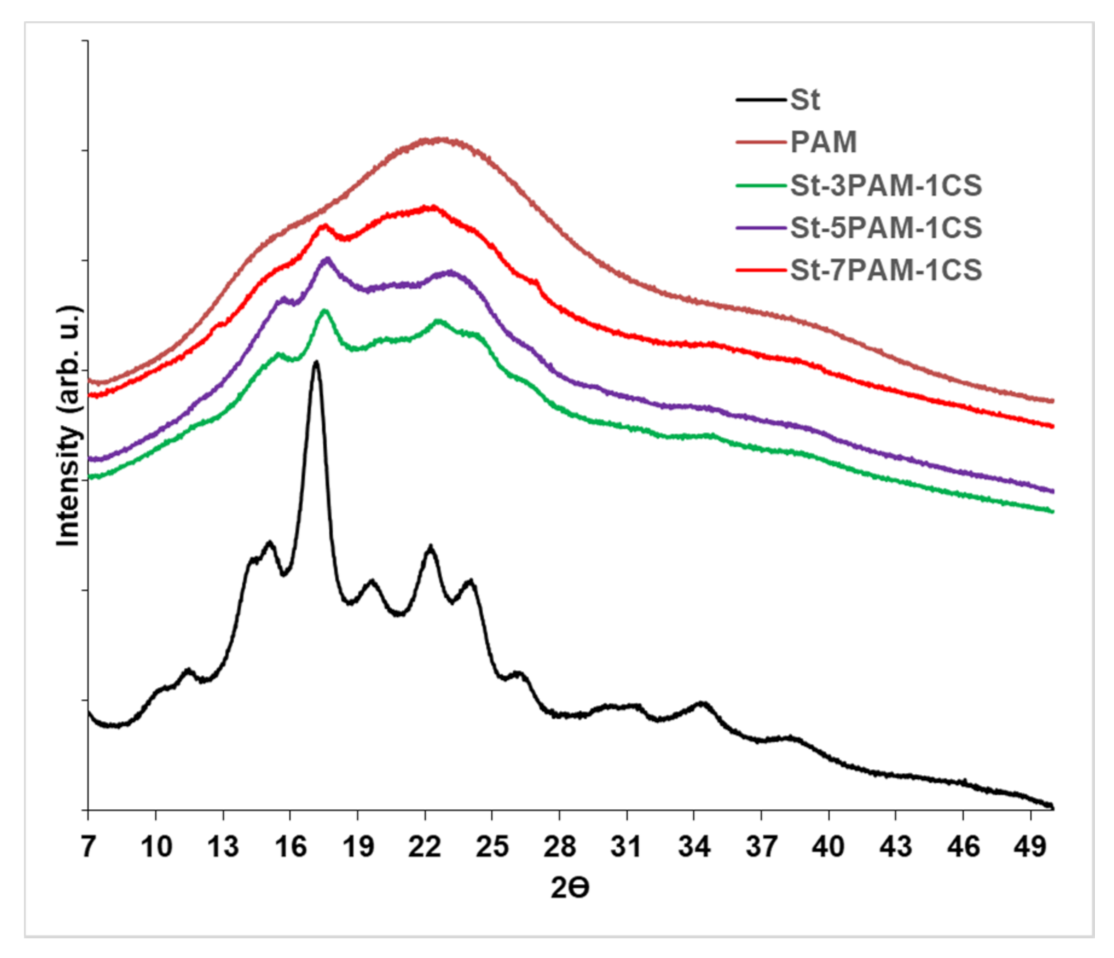
3.3.2. Crystallinity
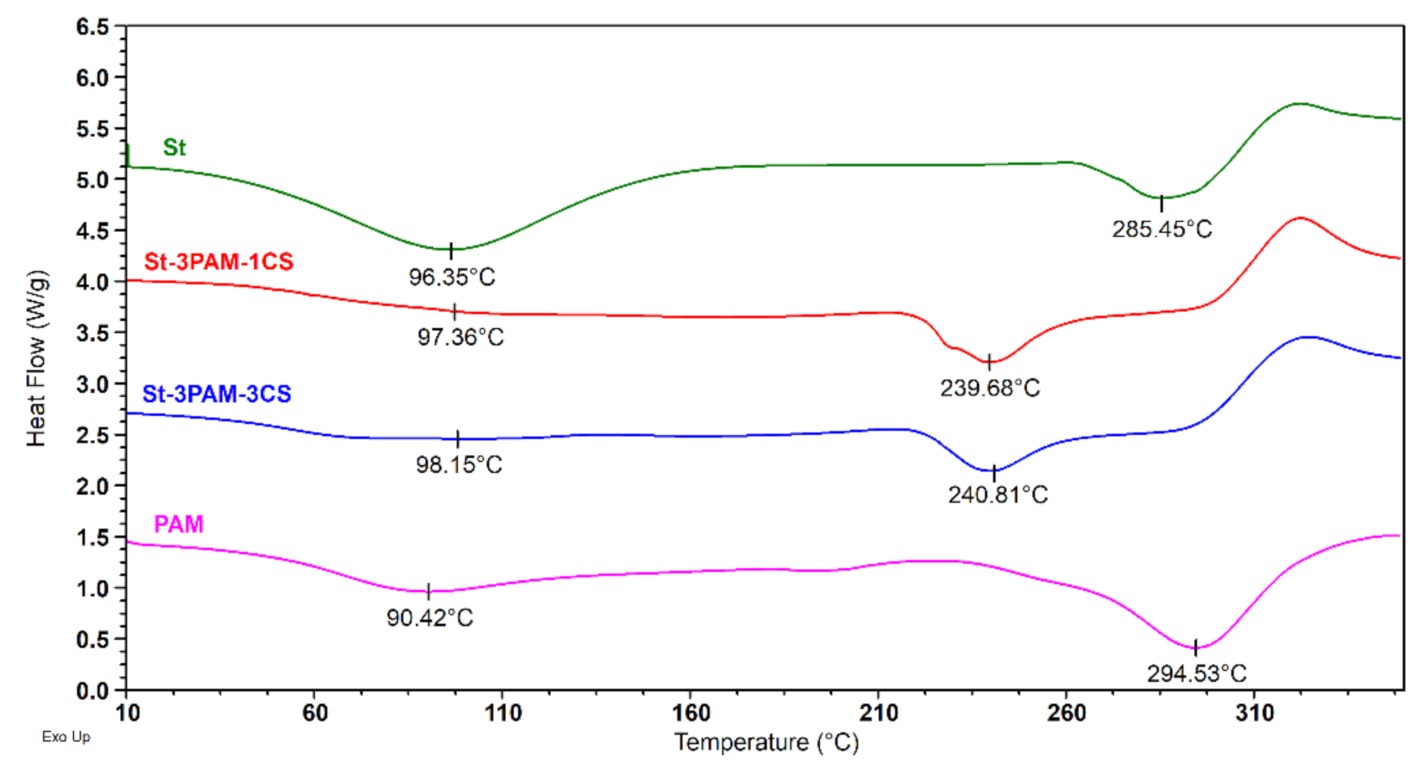
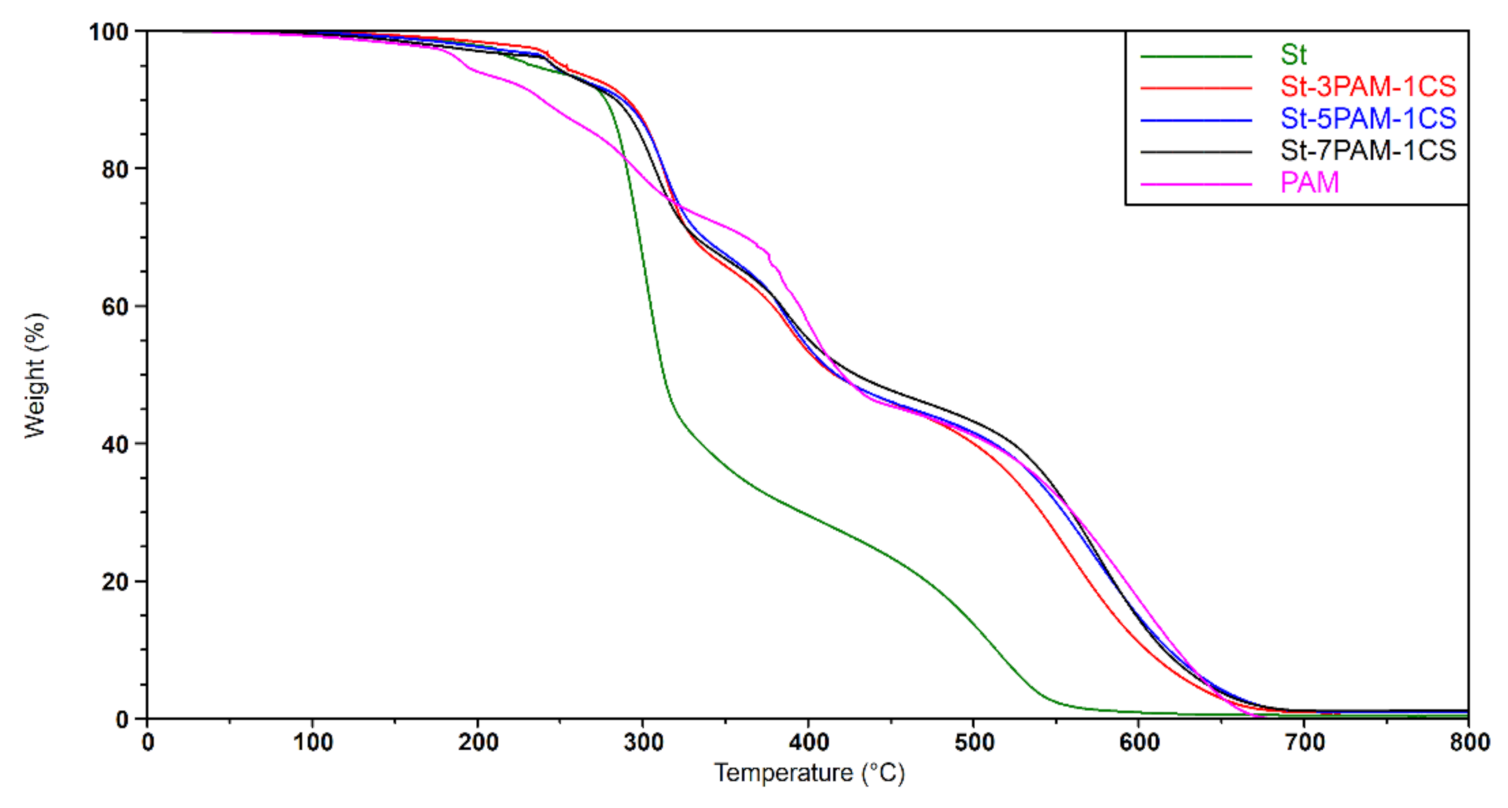
3.3.3. Thermal Features
3.3.4. Surface Morphology
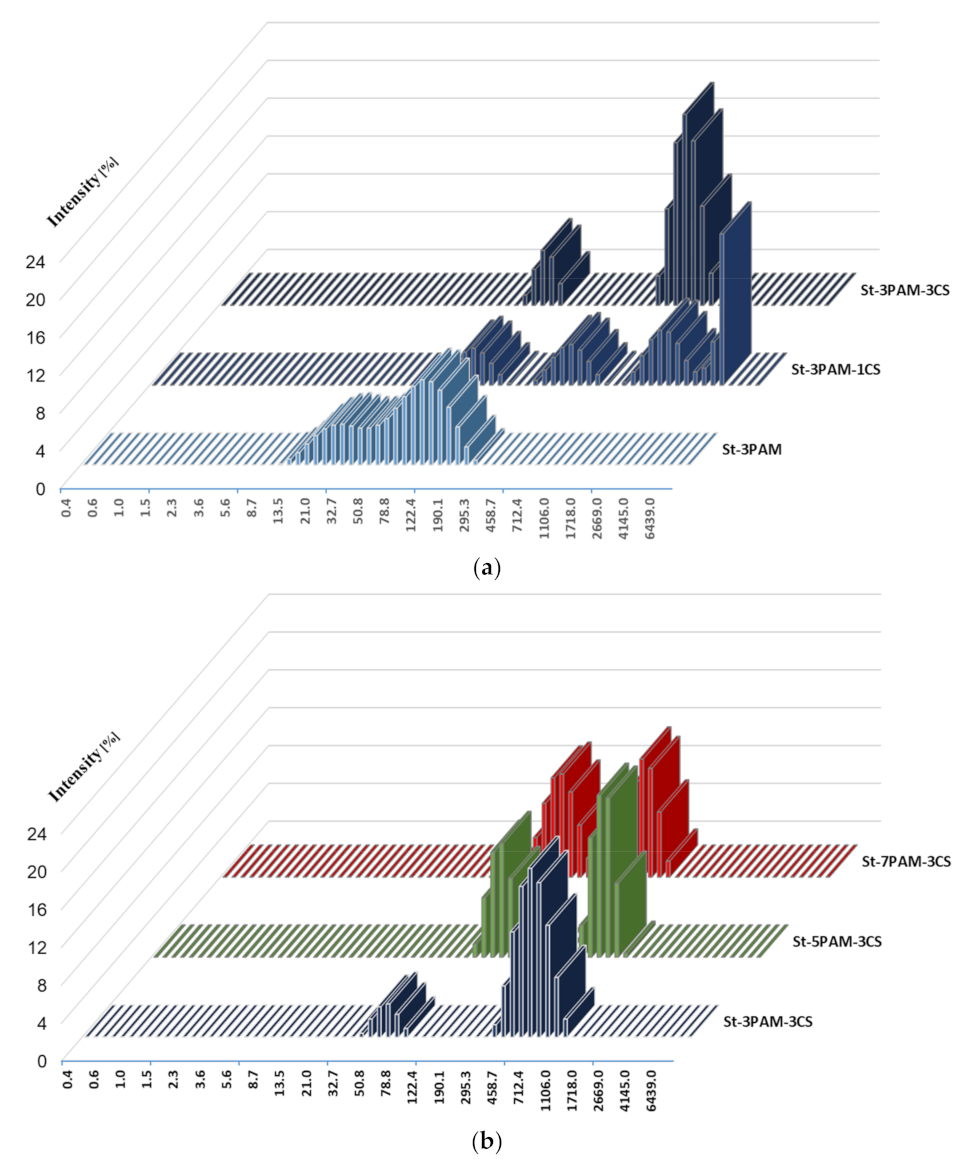
3.3.5. Particle Size Analysis of Polymer Hybrids
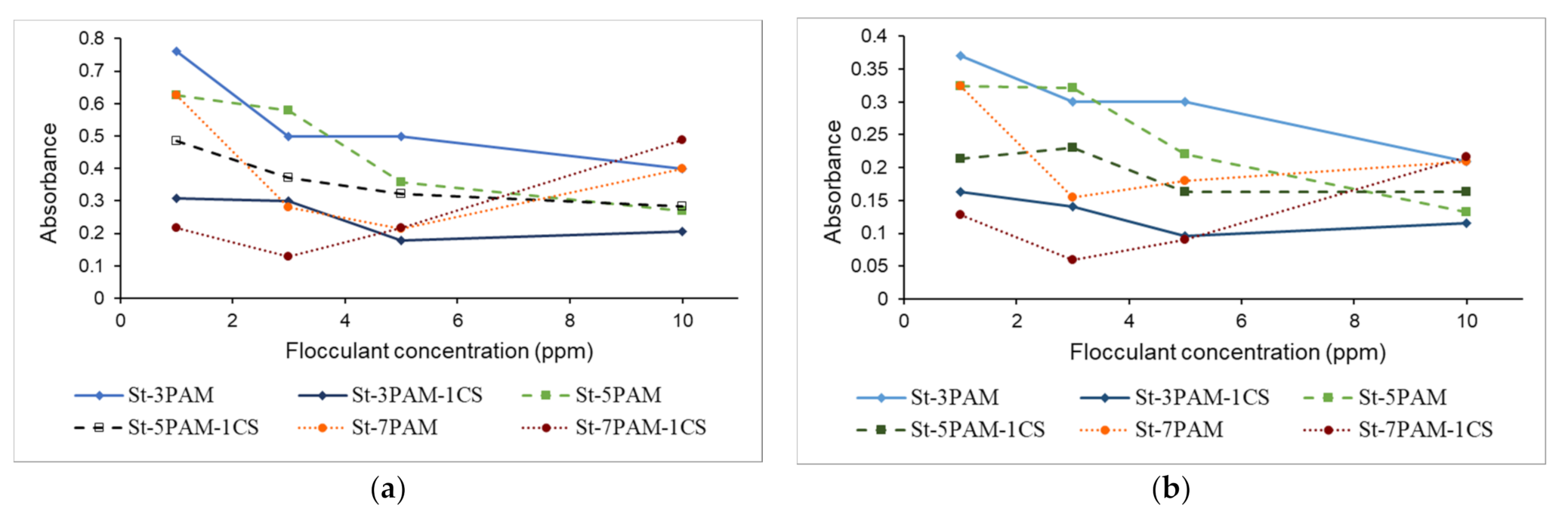
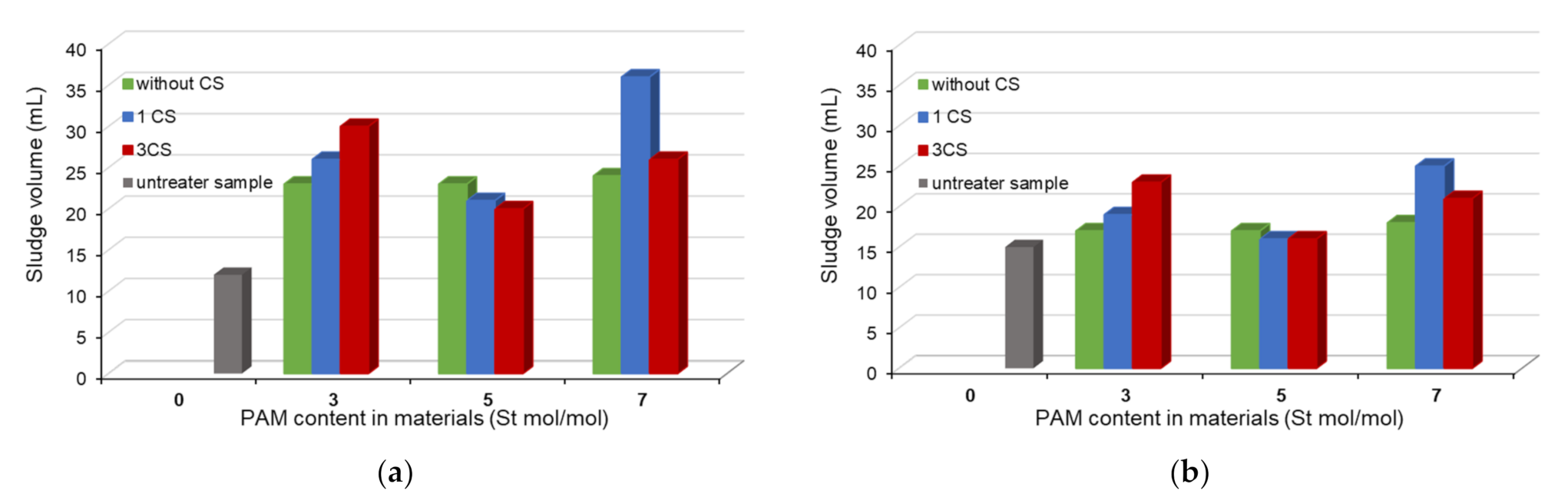
3.4. Flocculation Effectiveness
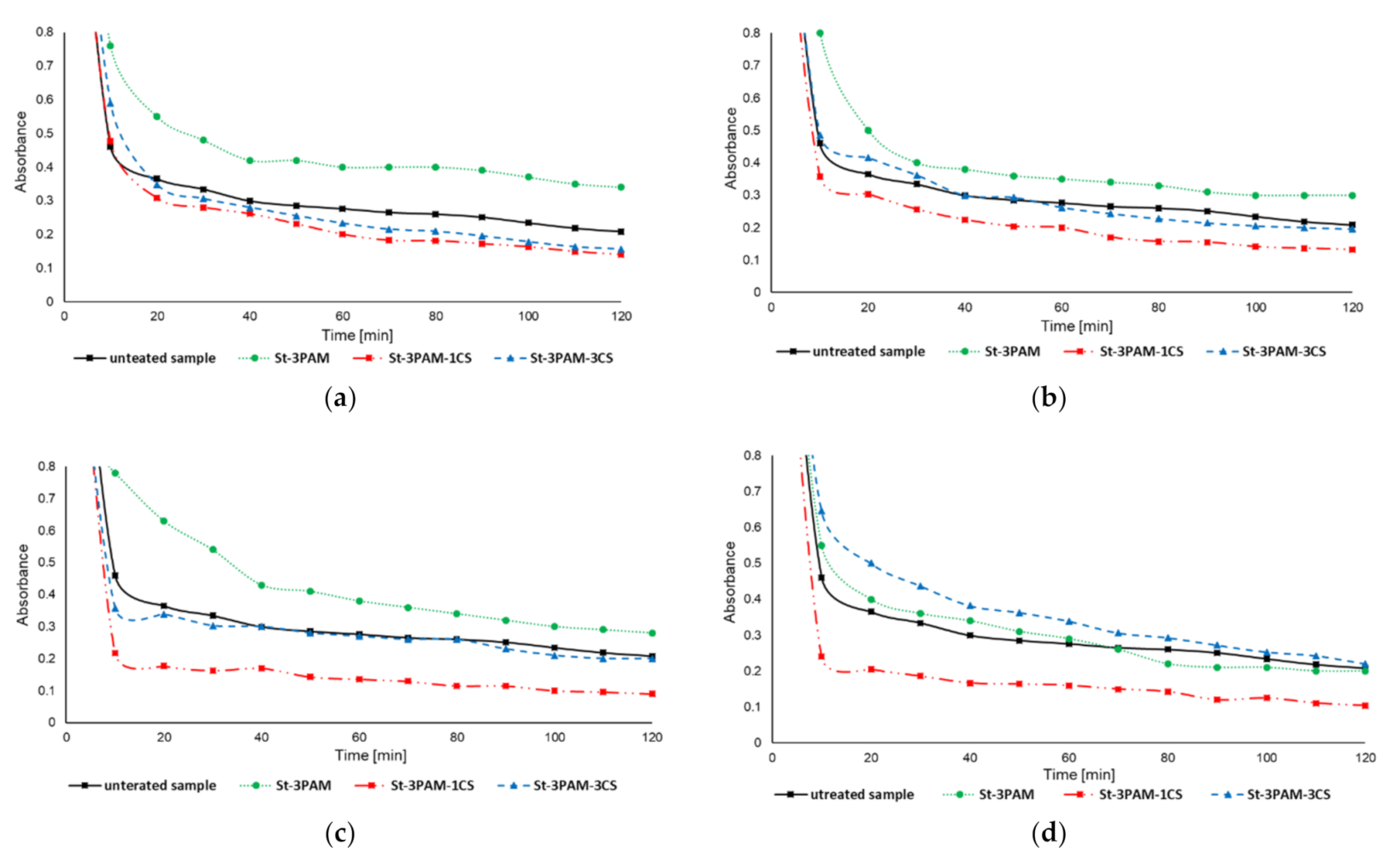
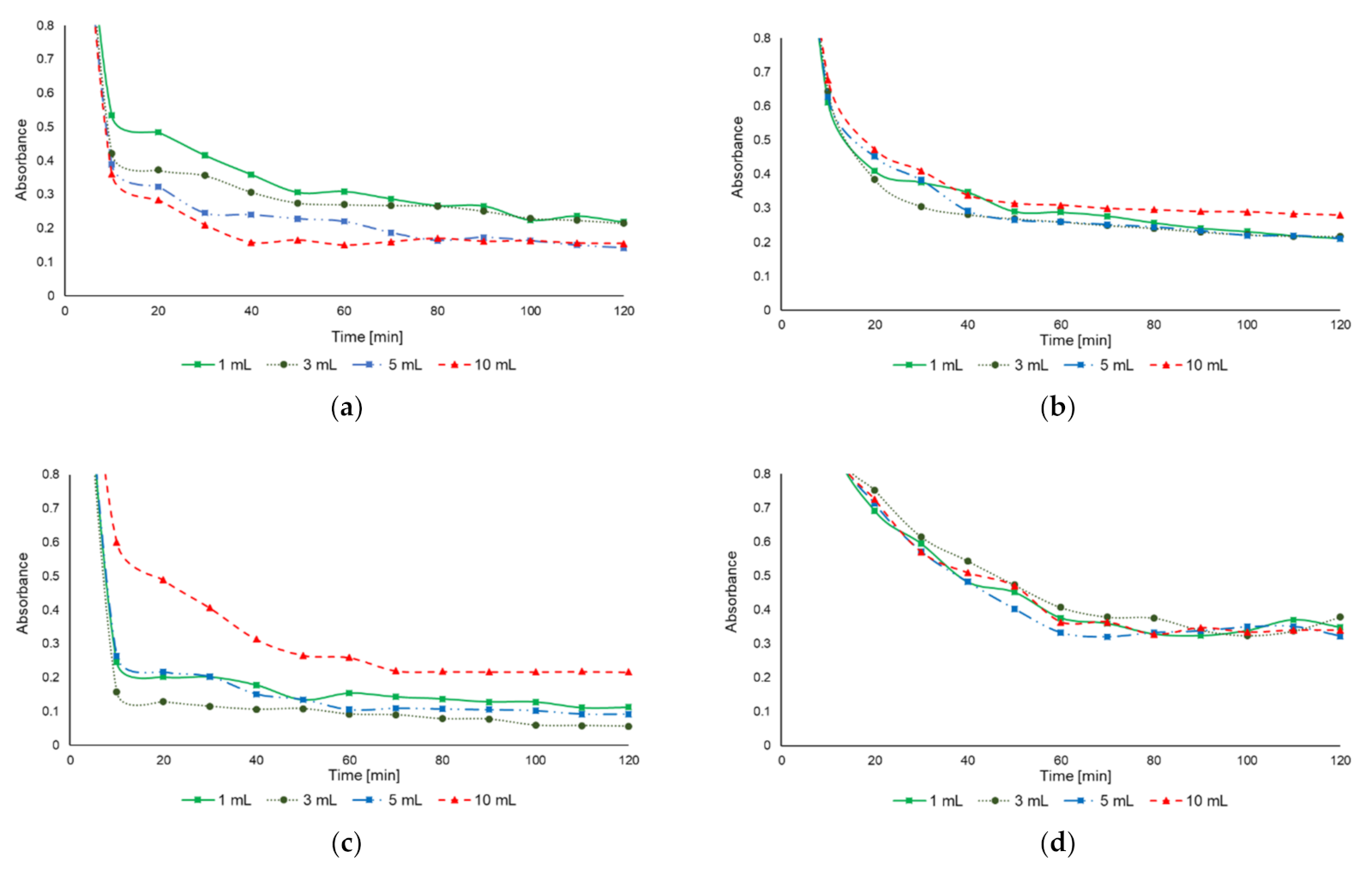
3.4.1. Jar Tests
3.4.2. Sludge Volume
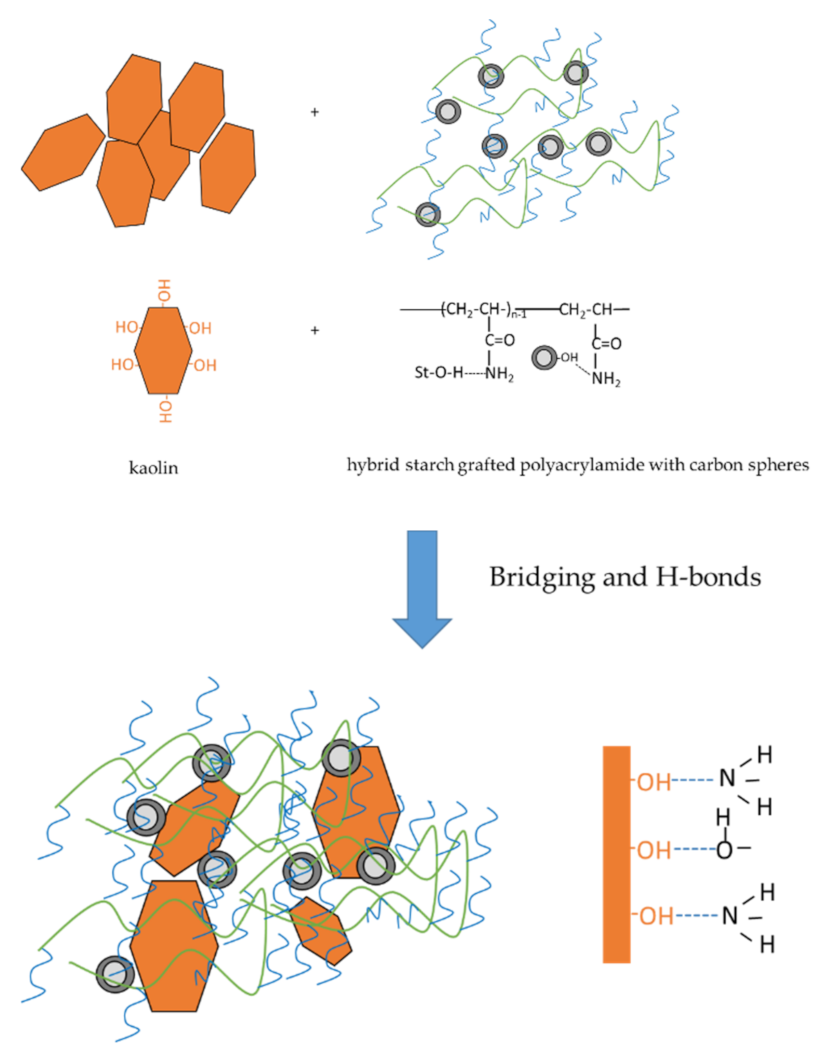
3.4.3. Flocculation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Xin, K.L. A sustainable plan for China’s drinking water. Nat. Cell Biol. 2014, 511, 527–528. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nat. Cell Biol. 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Jiang, J.Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Maćzak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent Achievements in Polymer Bio-Based Flocculants for Water Treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef]
- Bolto, B.; Xie, Z. The Use of Polymers in the Flotation Treatment of Wastewater. Processes 2019, 7, 374. [Google Scholar] [CrossRef]
- Drzycimska, A.; Schmidt, B.; Spychaj, T. Modified acrylamide copolymers as flocculants for model aqueous suspensions. Pol. J. Chem. Technol. 2007, 9, 10–14. [Google Scholar] [CrossRef]
- Zou, W.; Fang, Z.; Huang, J.; Zhang, Z. Effect of salinity on adsorption of sodium hexametaphosphate and hydrophobically-modified polyacrylamide flocculant on kaolinite Al-OH surface. Colloids Surf. A Physiocochem. Eng. Asp. 2020, 585, 124055. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan: Derivatives, Composites and Applications, 1st ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2017. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef]
- Okuda, T.; Nishijima, W.; Sugimoto, M.; Saka, N.; Nakai, S.; Tanabe, K.; Ito, J.; Takenaka, K.; Okada, M. Removal of coagulant aluminum from water treatment residuals by acid. Water Res. 2014, 60, 75–81. [Google Scholar] [CrossRef]
- Spychaj, T.; Schmidt, B.; Ulfig, K.; Markowska--Szczupak, A. Starch-grafted-N-vinylformamide copolymers manufactured by reactive extrusion:Synthesis and characterization. Polimery 2012, 57, 95–100. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Barbarick, K.A.; Elliott, H.A. Drinking water treatment residuals: A review of recent uses. J. Environ. Qual. 2011, 40, 1–12. [Google Scholar] [CrossRef]
- Huang, M.; Liu, Z.; Li, A.; Yang, H. Dual functionality of a graft starch flocculant: Flocculation and antibacterial performance. J. Environ. Manag. 2017, 196, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, M.; Li, A.; Yang, H. Flocculation and antimicrobial properties of a cationized starch. Water Res. 2017, 119, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Sun, D.X.; Zhu, Y.C.; Yang, T.R.; Jin, X.H.; Wang, F. Preparation of carboxymethyl-quaternized oligochitosan and its scale inhibition and antibacterial activity. J. Water Reuse. Desalination 2014, 4, 65–75. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.W.; Cai, J.; Bai, J.F.; Yang, H.; Li, A.M. Preparation of dual-function starch-based flocculants for the simultaneous removal of turbidity and inhibition of Escherichia coli in water. Water Res. 2016, 98, 128–137. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Park, D.R.; Khan, S.; Uddin, A.; Yeom, I.T. Complexation of Antimony with Natural Organic Matter: Performance Evaluation during Coagulation-Flocculation Process. Int. J. Environ. Res. Public Health 2019, 16, 1092. [Google Scholar] [CrossRef] [PubMed]
- Inam, M.A.; Khan, R.; Arkam, M.; Khan, S.; Park, D.R.; Yeom, I.T. Interaction of Arsenic Species with Organic Ligands: Competitive Removal from Water by Coagulation-Flocculation-Sedimentation (C/F/S). Molecules 2019, 24, 1619. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Li, A.; Yang, H. Evaluation of structural effects on the flocculation performance of a co-graft starch-based flocculant. Water Res. 2017, 118, 160–166. [Google Scholar] [CrossRef]
- Wang, M.; Feng, L.; Fan, X.; Li, D.; Qu, W.; Jiang, S.; Li, S. Fabrication of Bifunctional Chitosan-Based Flocculants: Characterization, Assessment of Flocculation, and Sterilization Performance. Materials 2018, 11, 2009. [Google Scholar] [CrossRef]
- Shi, C.; Sun, W.; Sun, Y.; Chen, L.; Xu, Y.; Tang, M. Synthesis, Characterization, and Sludge Dewaterability Evaluation of the Chitosan-Based Flocculant CCPAD. Polymers 2019, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Al Bitar, H.A.; Zhu, Y.; Xu, Y.; Shi, Z. Synthesis of Polymer Grafted Starches and Their Flocculation Properties in Clay Suspension. Minerals 2020, 10, 1054. [Google Scholar] [CrossRef]
- Yue, T.; Wu, X.; Chen, X.; Liu, T. A Study on the Flocculation and Sedimentation of Iron Tailings Slurry Based on the Regulating Behavior of Fe3+. Minerals 2018, 8, 421. [Google Scholar] [CrossRef]
- Schmidt, B. Nanocomposite starch graft copolymers with carbon nanotubes-synthesis and flocculation efficiency. Polimery 2020, 65, 226–231. [Google Scholar] [CrossRef]
- Schmidt, B. Flocculation efficiency of hybrid polymers with trivalent metal cations. Pol. J. Chem. Technol. 2018, 20, 96–101. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, Q.; Cao, T.; Chang, J.; Xu, S.; Xu, Z. Effect of electrolytes on interactions between a novel organic-inorganic hybrid polymer flocculant and kaolinite particles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590. [Google Scholar] [CrossRef]
- Eutamene, M.; Benbakhti, A.; Khodja, M.; Jada, A. Preparation and Aqueous Properties of Starch-grafted Polyacrylamide Copolymers. Starch Stärke 2009, 61, 81–91. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Fan, Y.; Liu, X.; Ren, S.; Wen, Y.; Shen, B. Synthesis and characterization of multi-active site grafting starch copolymer initiated by KMnO4 and HIO4/H2SO4 systems. Carbohydr. Polym. 2015, 117, 247–254. [Google Scholar] [CrossRef]
- Al-Karawi, A.J.M.; Al-Daraji, R. Preparation and using of acrylamide grafted starch as polymer drug carrier. Carbohydr. Polym. 2010, 79, 769–774. [Google Scholar] [CrossRef]
- Chang, Q.; Hao, X.; Duan, L. Synthesis of crosslinked starch-graft-polyacrylamide-co-sodium xanthate and its performances in wastewater treatment. J. Hazard. Mater. 2008, 159, 548–553. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, H.; Yuan, B.; Huang, M.; Yang, H.; Li, A.; Bai, J.; Cheng, R. Synthesis of amphoteric starch- based grafting flocculants for flocculation of both positively and negatively charged colloidal contaminants from water. Chem. Eng. J. 2014, 224, 209–217. [Google Scholar] [CrossRef]
- Ji, J.; Li, J.; Qiu, J.; Li, X. Polyacrylamide–starch composite flocculant as a membrane fouling reducer: Key factors of fouling reduction. Sep. Purif. Technol. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Ren, N.; Wang, Y.; Huang, J.; Liu, W.; Su, Z.; Yang, J. Synthesis and properties of polyacrylamide/hollow coal gangue spheres superabsorbent composites. J. Appl. Polym. Sci. 2013, 2184–2187. [Google Scholar] [CrossRef]
- Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L. Starch-based nano-biocomposites. Prog. Polym. Sci. 2013, 38, 1590–1628. [Google Scholar] [CrossRef]
- Yang, S.; Hu, J.; Chen, C.; Shao, D.; Wang, W. Mutual Effects of Pb(II) and Humic Acid Adsorption on Multiwalled Carbon Nanotubes/Polyacrylamide Composites from Aqueous Solutions. Environ. Sci. Technol. 2011, 45, 3621–3627. [Google Scholar] [CrossRef]
- Huang, Y.; Xue, X.; Fu, K. Application of Spherical Polyelectrolyte Brushes Microparticle System in Flocculation and Retention. Polymers 2020, 12, 746. [Google Scholar] [CrossRef]
- Kang, X.; Xia, Z.; Chen, R.; Sun, H.; Yang, W. Effects of inorganic ions, organic polymers, and fly ashes on the sedimentation characteristics of kaolinite suspensions. Appl. Clay Sci. 2019, 181. [Google Scholar] [CrossRef]
- Wenelska, K.; Michalkiewicz, B.; Gong, J.; Tang, T.; Kaleńczuk, R.J.; Chen, X.; Mijowska, E. In situ deposition of Pd nanoparticles with controllable diameters in hollow carbon spheres for hydrogen storage. Int. J. Hydrog. Energy 2013, 38, 16179–16184. [Google Scholar] [CrossRef]
- Schmidt, B.; Zielińska, B.; Chen, X. Nanocomposite Flocculant of Starch Graft Copolymers and the Method of Obtaining Nanocomposite Flocculant of Starch Graft Copolymers. Patent Application PL Z424396, 2018. under review (In Polish). [Google Scholar]
- Kim, B.J.; Lee, Y.S.; Park, S.J. Preparation of platinum-decorated porous graphite nanofibers, and their hydrogen storage behaviors. J. Colloid Interface Sci. 2008, 318, 530–533. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, M.; Yan, W.; Liu, X.; Jiang, B.; Qian, Z.; Gao, Y.; Lu, X.; Chen, X.; Wang, Q. Delivery of a chemotherapeutic drug using novel hollow carbon spheres for esophageal cancer treatment. Int. J. Nanomed. 2017, 12, 6759–6769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mao, H.; Tang, J.; Chen, J.; Wan, J.; Hou, K.; Peng, Y.; Halat, D.M.; Xiao, L.; Zhang, R.; Lv, X.; et al. Designing hierarchical nanoporous membranes for highly efficient gas adsorption and storage. Sci. Adv. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Rokicka, J.; Janik, J.; Wilpiszewska, K. Preparation and characterization of potato starch copolymers with a high. natural polymer content for the removal of Cu(II) and Fe(III) from solutions. Polymers 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yua, L.; Shenb, S.; Simonc, G.P.; Liua, H.; Chena, L. How rheological behaviors of concentrated starch affect graft copolymerization of acrylamide and resultant hydrogel. Carbohydr. Polym. 2019, 219, 395–404. [Google Scholar] [CrossRef]
- Díaz, A.P.; Lourdin, D.; Della Valle, G.; Quintero, A.F.; Ceballos, H.; Tran, T.; Dufour, D. Thermomechanical characterization of an amylose-free starch extracted from cassava (Manihot esculenta, Crantz). Carbohydr. Polym. 2017, 157, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Biswal, D.R.; Singh, R.P. Characterisation of carboxymethyl celluloseand polyacrylamide graft copolymer. Carbohydr. Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Gaabour, L.H. Spectroscopic and thermal analysis of polyacrylamide/chitosan (PAM/CS) blend loaded by gold nanoparticles. Results Phys. 2017, 7, 2153–2158. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Z.; Jia, H.; He, W.; Ge, X. Formation of Monodisperse Polyacrylamide Particles by Radiation- InducedDispersion Polymerization: Particle Size and Size Distribution. J. Colloid Interface Sci. 2002, 253, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zheng, H.; Sun, Y.; Zhang, S.; Liang, J.; Liu, Y.; An, Y. Evaluation of a novel dextran-based flocculant on treatment of dye wastewater: Effect of kaolin particles. Sci. Total Environ. 2018, 640–641, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, J.; Ding, H.; Zhu, G.; Fu, K.; Fu, X. Synthesis of cationic polyacrylamide by low-pressure UV initiation for turbidity water flocculation. Chem. Eng. J. 2017, 312, 20–29. [Google Scholar] [CrossRef]



| Molar Ratio St:AM | Label of Samples | Mass of CSs (g) | Yield (%) | ||||
|---|---|---|---|---|---|---|---|
| Concentration of CSs (wt.%) | 1 | 3 | 1 | 3 | 1 | 3 | |
| Hybrid-Starch Grafted Polyacrylamide with CSs | 1:03 | St-3PAM-1CS | St-3PAM-3CS | 0.032 | 0.096 | 84 | 83 |
| 1:05 | St-5PAM-1CS | St-5PAM-3CS | 0.053 | 0.159 | 85 | 86 | |
| 1:07 | St-7PAM-1CS | St-7PAM-3CS | 0.075 | 0.225 | 82 | 84 | |
| Copolymer- Starch Grafted Polyacrylamide | 1:03 | St-3PAM | 0 | 88 | |||
| 1:05 | St-5PAM | 0 | 87 | ||||
| 1:07 | St-7PAM | 0 | 85 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, B.; Kowalczyk, K.; Zielinska, B. Synthesis and Characterization of Novel Hybrid Flocculants Based on Potato Starch Copolymers with Hollow Carbon Spheres. Materials 2021, 14, 1498. https://doi.org/10.3390/ma14061498
Schmidt B, Kowalczyk K, Zielinska B. Synthesis and Characterization of Novel Hybrid Flocculants Based on Potato Starch Copolymers with Hollow Carbon Spheres. Materials. 2021; 14(6):1498. https://doi.org/10.3390/ma14061498
Chicago/Turabian StyleSchmidt, Beata, Krzysztof Kowalczyk, and Beata Zielinska. 2021. "Synthesis and Characterization of Novel Hybrid Flocculants Based on Potato Starch Copolymers with Hollow Carbon Spheres" Materials 14, no. 6: 1498. https://doi.org/10.3390/ma14061498
APA StyleSchmidt, B., Kowalczyk, K., & Zielinska, B. (2021). Synthesis and Characterization of Novel Hybrid Flocculants Based on Potato Starch Copolymers with Hollow Carbon Spheres. Materials, 14(6), 1498. https://doi.org/10.3390/ma14061498