A Comparative Study of Electron Radiation Responses of Pu2Zr2O7 and La2Zr2O7: An abinitio Molecular Dynamics Study
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussions
3.1. Ground State Properties of Pu2Zr2O7 and La2Zr2O7
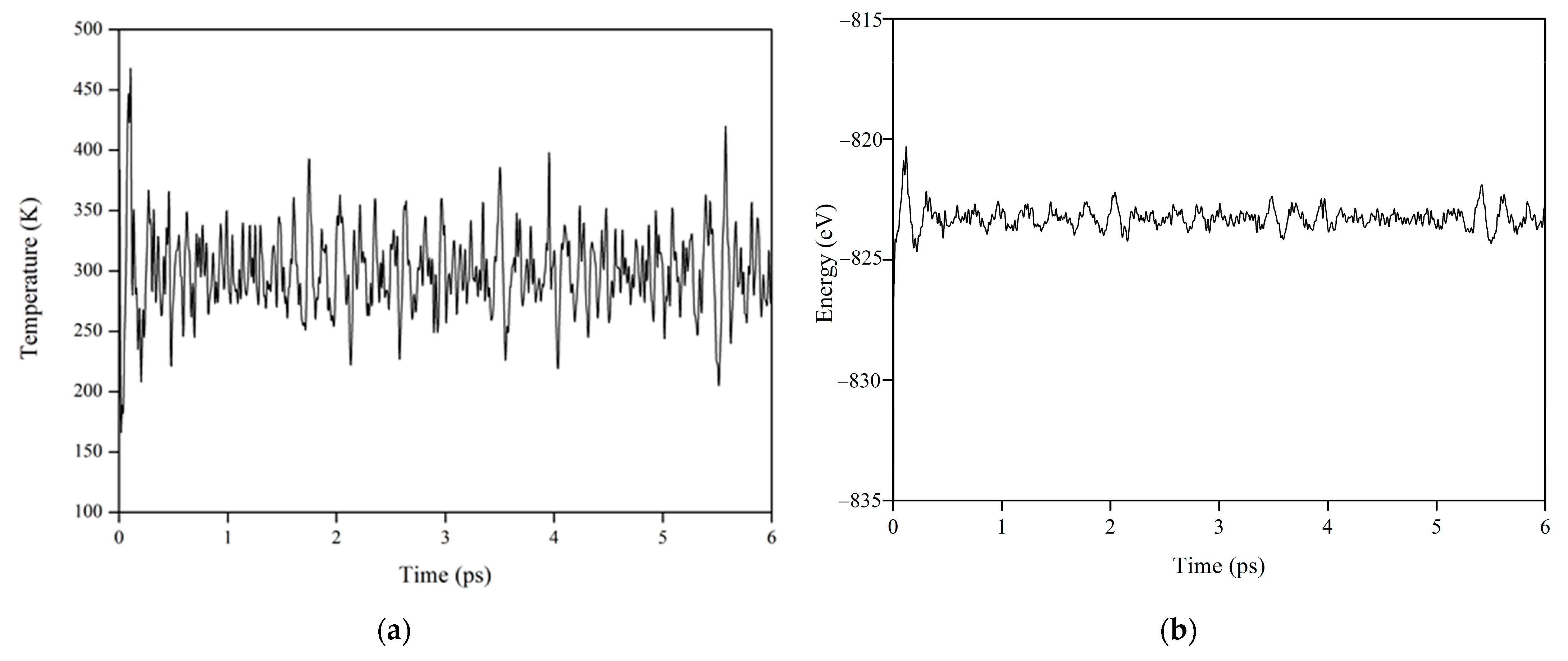
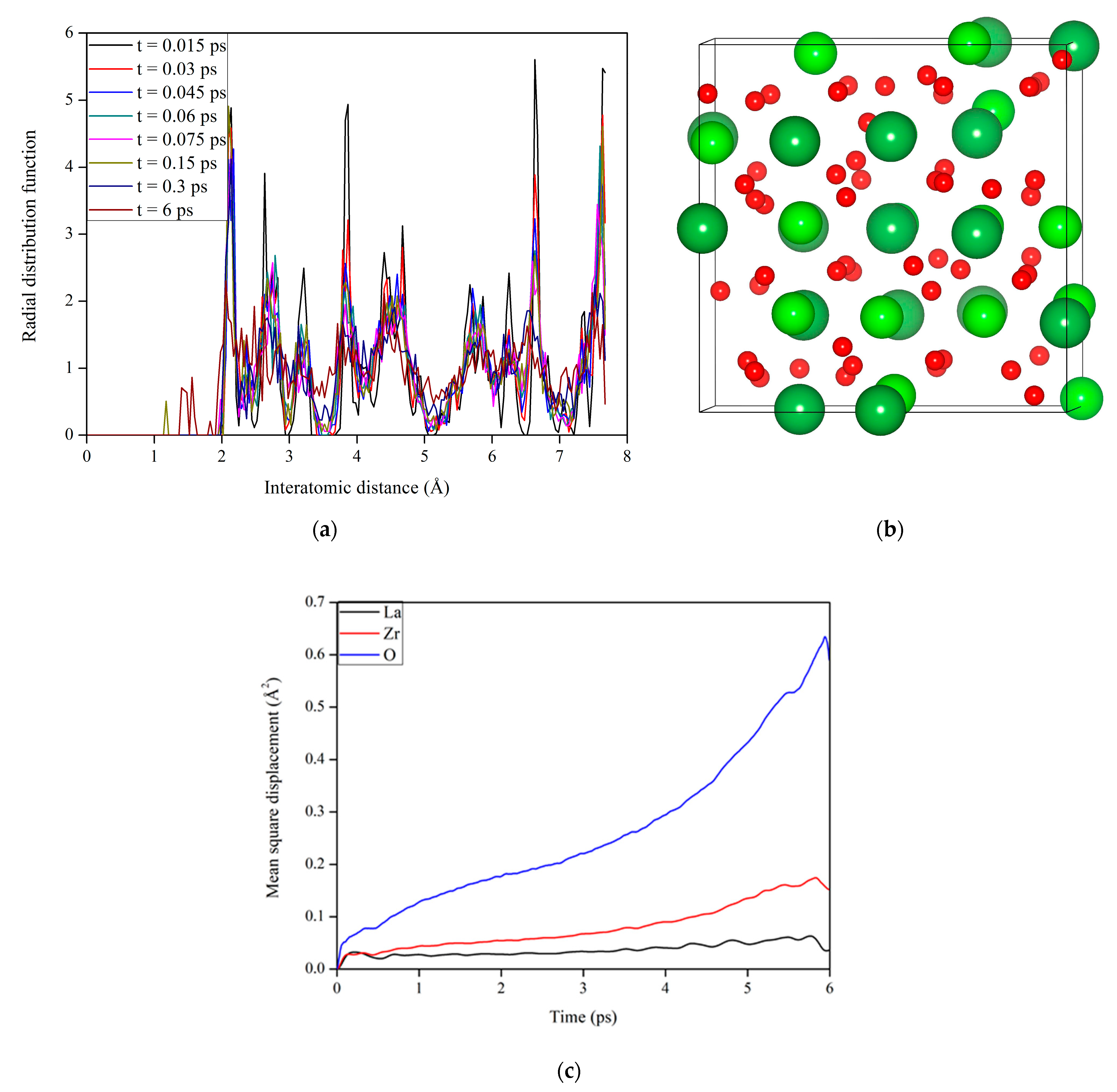
3.2. Microstructural Evolution in La2Zr2O7 under Electronic Excitation
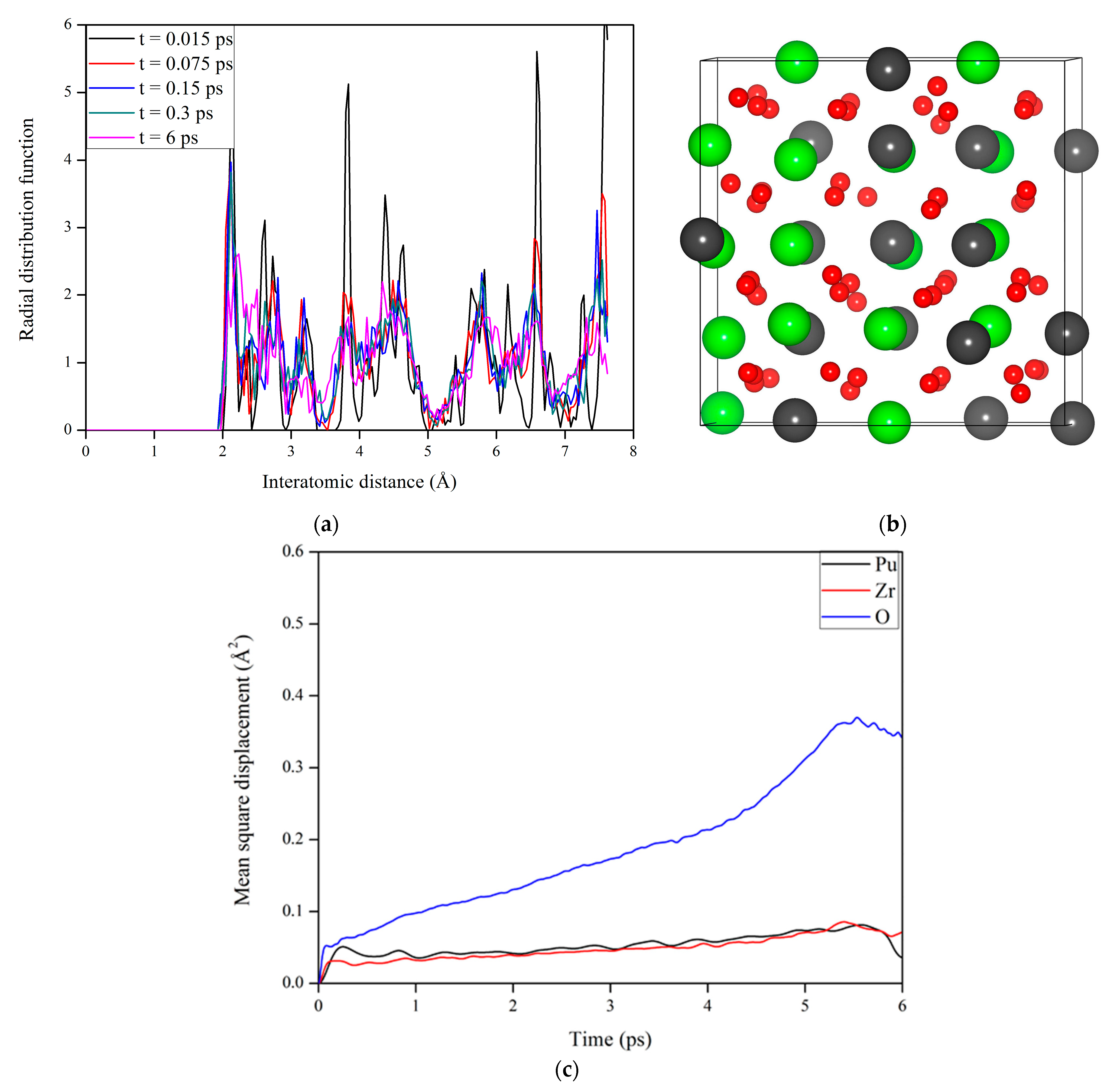
3.3. Microstructural Evolution in Pu2Zr2O7 under Electronic Excitation
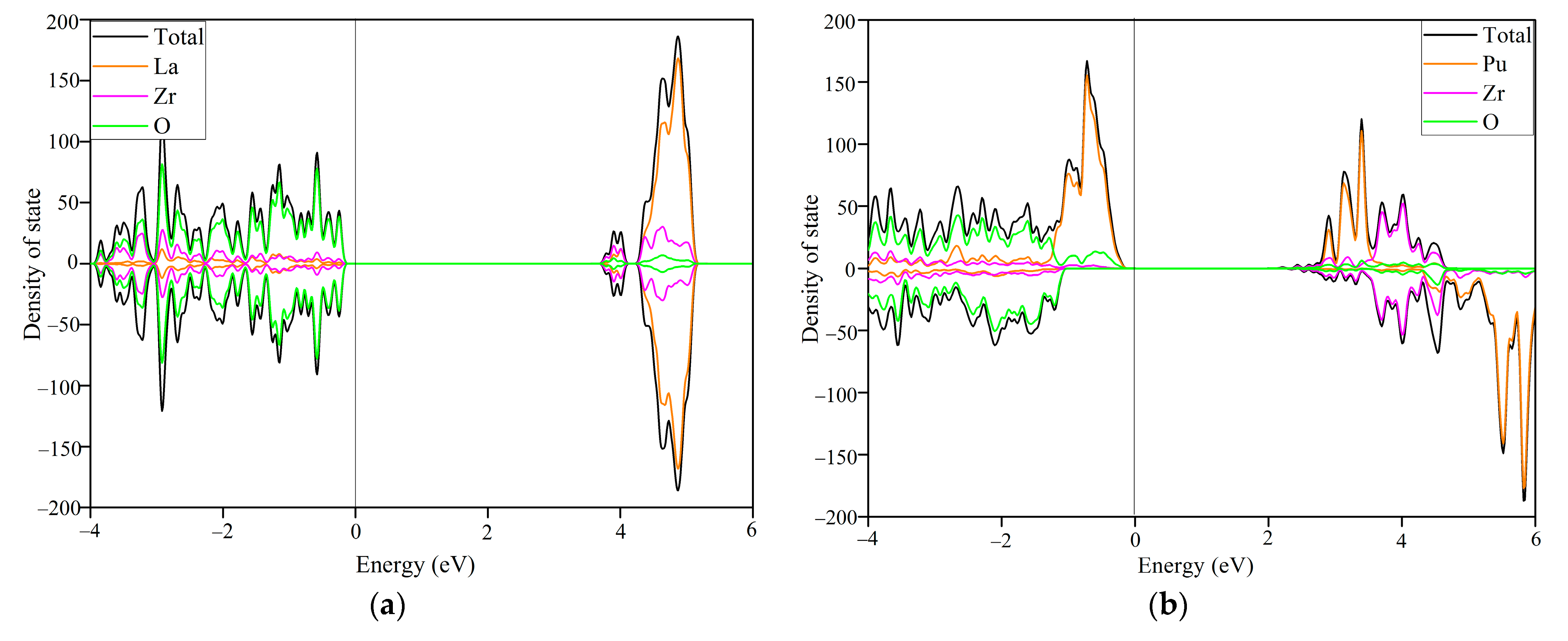
3.4. Origin of the Different Electronic Radiation Responses between La2Zr2O7 and Pu2Zr2O7
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ewing, R.C. Nuclear waste forms for actinides. Proc. Natl. Acad. Sci. USA 1999, 96, 3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.Y.; Jiang, M.; Zhao, F.A.; Liu, Z.J.; Zu, X.T. Thermal and mechanical stability, electronic structure and energetic properties of Pu-containing pyrochlores: La2−yPuyZr2O7 and La2Zr2−yPuyO7 (0 <= y <= 2) . J. Nucl. Mater. 2015, 466, 162–171. [Google Scholar] [CrossRef]
- Ewing, R.C.; Weber, W.J.; Lian, J. Nuclear waste disposal—Pyrochlore (A2B2O7): Nuclear waste form for the immobilization of plutonium and “minor” actinides. J. Appl. Phys. 2004, 95, 5949–5971. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.Y.; Gao, F.; Weber, W.J. Ab initio investigation of phase stability of Y2Ti2O7 and Y2Zr2O7 under high pressure. Phys. Rev. B 2009, 80, 4. [Google Scholar] [CrossRef]
- Moon, P.K.; Tuller, H.L. Evaluation of the Gd2(ZrxTi1−x)2O7 pyrochlore system as an oxygen gas sensor. Sens. Actuators B: Chem. 1990, 1, 199–202. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Xiao, H.Y.; Zu, X.T.; Gao, F.; Weber, W.J. First-principles calculation of structural and energetic properties for A2Ti2O7 (A = Lu, Er, Y, Gd, Sm, Nd, La). J. Mater. Res. 2009, 24, 1335–1341. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Zu, X.T.; Gao, F.; Weber, W.J. First-principles study of energetic and electronic properties of A2Ti2O7 (A=Sm, Gd, Er) pyrochlore. J. Appl. Phys. 2008, 104, 073503. [Google Scholar] [CrossRef]
- Weber, W.J.; Ewing, R.C. Plutonium immobilization and radiation effects. Science 2000, 289, 2051–2052. [Google Scholar] [CrossRef]
- Weber, W.J.; Wald, J.W.; Matzke, H. Self-radiation damage in Gd2Ti2O7. Mater. Lett. 1985, 3, 173–180. [Google Scholar] [CrossRef]
- Hayakawa, I.; Kamizono, H. Durability of an La2Zr2O7 waste form in water. J. Mater. Sci. 1993, 28, 513–517. [Google Scholar] [CrossRef]
- Kamizono, H.; Hayakawa, I.; Muraoka, S. Durability of Zirconium-Containing Ceramic Waste Forms in Water. J. Am. Ceram. Soc. 1991, 74, 863–864. [Google Scholar] [CrossRef]
- Hayakawa, I.; Kamizono, H. Durability of an La2Zr2O7 waste form containing various amounts of simulated HLW elements. J. Nucl. Mater. 1993, 202, 163–168. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Minervini, L.; Grimes, R.W.; Valdez, J.A.; Ishimaru, M.; Li, F.; McClellan, K.J.; Hartmann, T. Radiation tolerance of complex oxides. Science 2000, 289, 748–751. [Google Scholar] [CrossRef]
- Wang, S.X.; Begg, B.D.; Wang, L.M.; Ewing, R.C.; Weber, W.J.; Kutty, K.V.G. Radiation stability of gadolinium zirconate: A waste form for plutonium disposition. J. Mater. Res. 1999, 14, 4470–4473. [Google Scholar] [CrossRef]
- Lian, J.; Zu, X.T.; Kutty, K.V.G.; Chen, J.; Wang, L.M.; Ewing, R.C. Ion-irradiation-induced amorphization of La2Zr2O7 pyrochlore. Phys. Rev. B 2002, 66, 5. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Minervini, L.; Grimes, R.W.; Valdez, J.A.; Hartmann, T. A comparison between radiation damage accumulation in oxides with pyrochlore and florite structures. Radiat. Eff. Defects Solids 2001, 155, 133–137. [Google Scholar] [CrossRef]
- Mafi, E.; Soudi, A.; Gu, Y. Electronically Driven Amorphization in Phase-Change In2Se3 Nanowires. J. Phys. Chem. C 2012, 116, 22539–22544. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Krbal, M.; Fons, P.; Tominaga, J.; Uruga, T. Distortion-triggered loss of long-range order in solids with bonding energy hierarchy. Nat. Chem. 2011, 3, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J. Models and mechanisms of irradiation-induced amorphization in ceramics. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2000, 166, 98–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Weber, W.J.; Shutthanandan, V.; Devanathan, R.; Thevuthasan, S.; Balakrishnan, G.; Paul, D.M. Damage evolution on Sm and O sublattices in Au-implanted samarium titanate pyrochlore. J. Appl. Phys. 2004, 95, 2866–2872. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Stanek, C.R.; Sickafus, K.E.; Uberuaga, B.P. First-principles prediction of disordering tendencies in pyrochlore oxides. Phys. Rev. B 2009, 79, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Bae, I.-T.; Weber, W.J. Atomic collision and ionization effects in oxides. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 2828–2833. [Google Scholar] [CrossRef]
- Sattonnay, G.; Moll, S.; Thomé, L.; Legros, C.; Calvo, A.; Herbst-Ghysel, M.; Decorse, C.; Monnet, I. Effect of composition on the behavior of pyrochlores irradiated with swift heavy ions. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 272, 261–265. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Weber, W.J.; Zhang, Y.; Zu, X.T.; Li, S. Electronic excitation induced amorphization in titanate pyrochlores: An ab initio molecular dynamics study. Sci. Rep. 2015, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Sassi, M.; Kaspar, T.; Rosso, K.M.; Spurgeon, S.R. Effect of structure and composition on the electronic excitation induced amorphization of La2Ti2-xZrxO7 ceramics. Sci. Rep. 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.A.; Xiao, H.Y.; Jiang, M.; Liu, Z.J.; Zu, X.T. A DFT plus U study of Pu immobilization in Gd2Zr2O7. J. Nucl. Mater. 2015, 467, 937–948. [Google Scholar] [CrossRef]
- Williford, R.E.; Weber, W.J. Computer simulation of Pu3+ and Pu4+ substitutions in gadolinium zirconate. J. Nucl. Mater. 2001, 299, 140–147. [Google Scholar] [CrossRef]
- Cleave, A.; Grimes, R.W.; Sickafus, K. Plutonium and uranium accommodation in pyrochlore oxides. Philos. Mag. 2005, 85, 967–980. [Google Scholar] [CrossRef]
- Kulkarni, N.K.; Sampath, S.; Venugopal, V. Preparation and characterisation of Pu-pyrochlore: [La1−xPux]2 Zr2O7 (x=0–1). J. Nucl. Mater. 2000, 281, 248–250. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Wang, Y.; Perdew, J.P. Correlation hole of the spin-polarized electron-gas, with exact small-wave-vector and high-density scaling. Phys. Rev. B 1991, 44, 13298–13307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Gong, H.; Zhou, B.; Xiao, H.; Zhang, H.; Liu, Z.; Zu, X. An AIMD+U simulation of low-energy displacement events in UO2. J. Nucl. Mater. 2020, 540, 152379. [Google Scholar] [CrossRef]
- Jiang, M.; Xiao, H.; Peng, S.; Yang, G.; Gong, H.; Liu, Z.; Qiao, L.; Zu, X. Ab initio molecular dynamics simulation of the radiation damage effects of GaAs/AlGaAs superlattice. J. Nucl. Mater. 2019, 516, 228–237. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Li, P.C.; Zhao, F.G.; Xiao, H.Y.; Zhang, H.B.; Gong, H.F.; Zhang, S.; Liu, Z.J.; Zu, X.T. First-Principles Study of Thermo-Physical Properties of Pu-Containing Gd2Zr2O7. Nanomaterials 2019, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciftja, O.; Escamilla, L.; Mills, R. Shape-Dependent Energy of an Elliptical Jellium Background. Adv. Condens. Matter Phys. 2015, 2015, 851356. [Google Scholar] [CrossRef] [Green Version]
- Li, X.B.; Liu, X.Q.; Liu, X.; Han, D.; Zhang, Z.; Han, X.D.; Sun, H.B.; Zhang, S.B. Role of Electronic Excitation in the Amorphization of Ge-Sb-Te Alloys. Phys. Rev. Lett. 2011, 107, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolowskitinten, K.; Bialkowski, J.; Vonderlinde, D. Ultrafast laser-induced order-disorder transitions in semiconductors. Phys. Rev. B 1995, 51, 14186–14198. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.T.; Hughes, R.W.; Rooke, J.; Knee, C.S.; Reading, J. The pyrochlore family—A potential panacea for the frustrated perovskite chemist. Dalton Trans. 2004, 3032–3041. [Google Scholar] [CrossRef]
- Malkin, B.Z.; Zakirov, A.R.; Popova, M.N.; Klimin, S.A.; Chukalina, E.P.; Antic-Fidancev, E.; Goldner, P.; Aschehoug, P.; Dhalenne, G. Optical spectroscopy of Yb2Ti2O7 and Y2Ti2O7: Yb3+ and crystal-field parameters in rare-earth titanate pyrochlores. Phys. Rev. B 2004, 70, 9. [Google Scholar] [CrossRef]
- Raj, A.K.V.; Rao, P.P.; Sreena, T.S.; Sameera, S.; James, V.; Renju, U.A. Remarkable changes in the photoluminescent properties of Y2Ce2O7: Eu3+ red phosphors through modification of the cerium oxidation states and oxygen vacancy ordering. Phys. Chem. Chem. Phys. 2014, 16, 23699–23710. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, R.S.; Marza, V.B.; Carda, J.B. Electronic absorption spectroscopy and colour of chromium-doped solids. J. Mater. Chem. 2002, 12, 2825–2832. [Google Scholar] [CrossRef]
- Yamazaki, S.; Yamashita, T.; Matsui, T.; Nagasaki, T. Thermal expansion and solubility limits of plutonium-doped lanthanum zirconates. J. Nucl. Mater. 2001, 294, 183–187. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Chartier, A.; Meis, C.; Weber, W.J.; Corrales, L.R. Theoretical study of disorder in Ti-substituted La2Zr2O7. Phys. Rev. B 2002, 65, 11. [Google Scholar] [CrossRef]
- Lian, J.; Wang, L.; Chen, J.; Sun, K.; Ewing, R.C.; Matt Farmer, J.; Boatner, L.A. The order–disorder transition in ion-irradiated pyrochlore. Acta Mater. 2003, 51, 1493–1502. [Google Scholar] [CrossRef]
- Flores-Ruiz, H.M.; Naumis, G.G. Mean-square-displacement distribution in crystals and glasses: An analysis of the intrabasin dynamics. Phys. Rev. E 2012, 85, 041503. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Li, M.; Li, P.; Xiao, H.; Zhang, H.; Zu, X. Defect formation and its effect on the thermodynamic properties of Pu2Zr2O7 pyrochlore: A first-principles study. J. Am. Ceram. Soc. 2020. [Google Scholar] [CrossRef]


| Compounds | a0 | xO48f | Eg (eV) | d<La-O48f > | d<La-O8b > | d<Pu-O48f> | d<Pu-O8b> | d<Zr-O48f> |
|---|---|---|---|---|---|---|---|---|
| La2Zr2O7 | 10.879 | 0.333 | 3.58 | 2.635 | 2.339 | – | – | 2.106 |
| Cal. [2] | 10.696 | 0.3346 | 3.52 | 2.589 | 2.316 | – | – | 2.096 |
| Exp. [45] | 10.805 | 0.332 | – | 2.635 | 2.339 | – | – | 2.105 |
| Pu2Zr2O7 | 10.802 | 0.335 | 2.12 | – | – | 2.587 | 2.317 | 2.099 |
| Cal. [36] | 10.802 | 0.335 | 2.37 | – | – | 2.615 | 2.339 | 2.117 |
| Exp. [44] | 10.70 | – | – | – | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Li, M.; Xiao, H.; Liu, Z.; Zu, X. A Comparative Study of Electron Radiation Responses of Pu2Zr2O7 and La2Zr2O7: An abinitio Molecular Dynamics Study. Materials 2021, 14, 1516. https://doi.org/10.3390/ma14061516
Zhang S, Li M, Xiao H, Liu Z, Zu X. A Comparative Study of Electron Radiation Responses of Pu2Zr2O7 and La2Zr2O7: An abinitio Molecular Dynamics Study. Materials. 2021; 14(6):1516. https://doi.org/10.3390/ma14061516
Chicago/Turabian StyleZhang, Shounuo, Menglu Li, Haiyan Xiao, Zijiang Liu, and Xiaotao Zu. 2021. "A Comparative Study of Electron Radiation Responses of Pu2Zr2O7 and La2Zr2O7: An abinitio Molecular Dynamics Study" Materials 14, no. 6: 1516. https://doi.org/10.3390/ma14061516





