Formation of Nitrogen Doped Titanium Dioxide Surface Layer on NiTi Shape Memory Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Microstructural and Chemical Composition Analysis
2.3. Surface Topography
2.4. Bacterial Colonization
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoeckel, D.; Pelton, A.; Duerig, T. Self-expanding Nitinol stents for the treatment of vascular disease. In Shape Memory Alloys for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2008; ISBN 9781845693442. [Google Scholar]
- Yoneyama, T.; Shuichi, M. Shape Memory Alloys for Biomedical Applications; Woodhead Publishing Limited: Cambridge, UK, 2009; ISBN 978-1-84569-344-2. [Google Scholar]
- Russell, S.M. Design considerations for nitinol bone staples. J. Mater. Eng. Perform. 2009. [Google Scholar] [CrossRef]
- Nili Ahmadabadi, M.; Shahhoseini, T.; Habibi-Parsa, M.; Haj-Fathalian, M.; Hoseinzadeh-Nik, T.; Ghadirian, H. Static and cyclic load-deflection characteristics of NiTi orthodontic archwires using modified bending tests. J. Mater. Eng. Perform. 2009. [Google Scholar] [CrossRef]
- Denkhaus, E.; Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. Crit. Rev. Oncol. Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef]
- Chrzanowski, W.; Szade, J.; Hart, A.D.; Knowles, J.C.; Dalby, M.J. Biocompatible, smooth, plasma-treated nickel-titanium surface—An adequate platform for cell growth. J. Biomater. Appl. 2012. [Google Scholar] [CrossRef] [PubMed]
- Shabalovskaya, S.A.; Rondelli, G.C.; Undisz, A.L.; Anderegg, J.W.; Burleigh, T.D.; Rettenmayr, M.E. The electrochemical characteristics of native Nitinol surfaces. Biomaterials 2009. [Google Scholar] [CrossRef]
- Arndt, M.; Brück, A.; Scully, T.; Jäger, A.; Bourauel, C. Nickel ion release from orthodontic NiTi wires under simulation of realistic in-situ conditions. J. Mater. Sci. 2005. [Google Scholar] [CrossRef]
- Karagkiozaki, V.; Logothetidis, S.; Laskarakis, A.; Giannoglou, G.; Lousinian, S. AFM study of the thrombogenicity of carbon-based coatings for cardiovascular applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2008. [Google Scholar] [CrossRef]
- Czarnowska, E.; Borowski, T.; Sowińska, A.; Lelątko, J.; Oleksiak, J.; Kamiński, J.; Tarnowski, M.; Wierzchoń, T. Structure and properties of nitrided surface layer produced on NiTi shape memory alloy by low temperature plasma nitriding. Appl. Surf. Sci. 2015. [Google Scholar] [CrossRef]
- Shabalovskaya, S.; Anderegg, J.; Van Humbeeck, J. Critical overview of Nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008, 4, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.; Witkowska, J.; Plocinski, T.; Tarnowski, M.; Wierzchon, T. Structure and corrosion resistance of titanium oxide layers produced on NiTi alloy in low-temperature plasma. Int. J. Mater. Res. 2018. [Google Scholar] [CrossRef]
- Chrzanowski, W.; Abou Neel, E.A.; Armitage, D.A.; Zhao, X.; Knowles, J.C.; Salih, V. In vitro studies on the influence of surface modification of Ni-Ti alloy on human bone cells. J. Biomed. Mater. Res. Part A 2010. [Google Scholar] [CrossRef]
- Hu, T.; Chu, C.L.; Yin, L.H.; Pu, Y.P.; Dong, Y.S.; Guo, C.; Sheng, X.B.; Chung, J.C.; Chu, P.K. In vitro biocompatibility of titanium-nickel alloy with titanium oxide film by H2O2 oxidation. Trans. Nonferrous Met. Soc. China 2007. [Google Scholar] [CrossRef]
- Levintant, N. Analysis of the mechanical and shape memory behaviour of nitrogen ion-implanted NiTi alloy. Vacuum 2007. [Google Scholar] [CrossRef]
- Larsson, C.; Emanuelsson, L.; Thomsen, P.; Ericson, L.E.; Aronsson, B.O.; Kasemo, B.; Lausmaa, J. Bone response to surface modified titanium implants—Studies on the tissue response after 1 year to machined and electropolished implants with different oxide thicknesses. J. Mater. Sci. Mater. Med. 1997, 8, 721–729. [Google Scholar] [CrossRef]
- Ohtsu, N.; Yamasaki, K.; Taniho, H.; Konaka, Y.; Tate, K. Pulsed anodization of NiTi alloy to form a biofunctional Ni-free oxide layer for corrosion protection and hydrophycility. Surf. Coat. Tech. 2021, 412. [Google Scholar] [CrossRef]
- Mahmud, A.; Wu, Z.; Zhang, J.; Liu, Y.; Yang, H. Surface oxidation of NiTi and its effects on thermal and mechanical properties. Intermetallics 2018, 103, 52–62. [Google Scholar] [CrossRef]
- Chlanda, A.; Witkowska, J.; Morgiel, J.; Nowińska, K.; Choińska, E.; Swieszkowski, W.; Wierzchoń, T. Multi-scale characterization and biological evaluation of composite surface layers produced under glow discharge conditions on NiTi shape memory alloy for potential cardiological application. Micron 2018. [Google Scholar] [CrossRef] [PubMed]
- Sowińska, A.; Czarnowska, E.; Tarnowski, M.; Witkowska, J.; Wierzchoń, T. Structure and hemocompatibility of nanocrystalline titanium nitride produced under glow-discharge conditions. Appl. Surf. Sci. 2018, 436. [Google Scholar] [CrossRef]
- Witkowska, J.; Rudnicki, J.; Piekoszewski, W.; Raugh, G.; Morgiel, J.; Wierzchoń, T. Influence of low temperature plasma oxynitriding on the mechanical behavior of NiTi shape memory alloys. Vacuum 2018. [Google Scholar] [CrossRef]
- Witkowska, J.; Kamiński, J.; Płociński, T.; Tarnowski, M.; Wierzchoń, T. Corrosion resistance of NiTi shape memory alloy after hybrid surface treatment using low-temperature plasma. Vacuum 2017. [Google Scholar] [CrossRef]
- Wierzchoń, T.; Czarnowska, E.; Grzonka, J.; Sowińska, A.; Tarnowski, M.; Kamiński, J.; Kulikowski, K.; Borowski, T.; Kurzydłowski, K.J. Glow discharge assisted oxynitriding process of titanium for medical application. Appl. Surf. Sci. 2015, 334. [Google Scholar] [CrossRef]
- Kamiński, J.; Witkowska, J.; Wierzchoń, T. Corrosion resistance of NiTi shape memory alloy after nitriding and oxynitriding processes under glow discharge conditions for medical applications. In Proceedings of the Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2016. [Google Scholar]
- Bociaga, D.; Komorowski, P.; Batory, D.; Szymanski, W.; Olejnik, A.; Jastrzebski, K.; Jakubowski, W. Silver-doped nanocomposite carbon coatings (Ag-DLC) for biomedical applications—Physiochemical and biological evaluation. Appl. Surf. Sci. 2015, 355, 388–397. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Feng, X.; Li, H. Thermal-Sprayed Photocatalytic Coatings for Biocidal Applications: A Review. J. Therm. Spray Technol. 2020. [Google Scholar] [CrossRef]
- Lela̧tko, J.; Goryczka, T.; Wierzchoń, T.; Ossowski, M.; Łosiewicz, B.; Rówiński, E.; Morawiec, H. Structure of low temperature nitrided/oxidized layer formed on NiTi shape memory alloy. In Proceedings of the Solid State Phenomena; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2010. [Google Scholar]
- Bakir, M. Haemocompatibility of titanium and its alloys. J. Biomater. Appl. 2012, 6, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sun, T.; Wang, Y. Bioactive Titanium Oxide Coatings Fabricated on NiTi SMA via Thermal Treatment for Medical Applications. Procedia Eng. 2016, 141, 115–120. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Kustosz, R.; Altyntsev, I.; Darlak, M.; Wierzchoń, T.; Tarnowski, M.; Gawlikowski, M.; Gonsior, M.; Kościelniak-Ziemniak, M. The tin coatings utilisation as blood contact surface modification in implantable rotary left ventricle assist device religaheart rot. Arch. Metall. Mater. 2015, 60. [Google Scholar] [CrossRef] [Green Version]

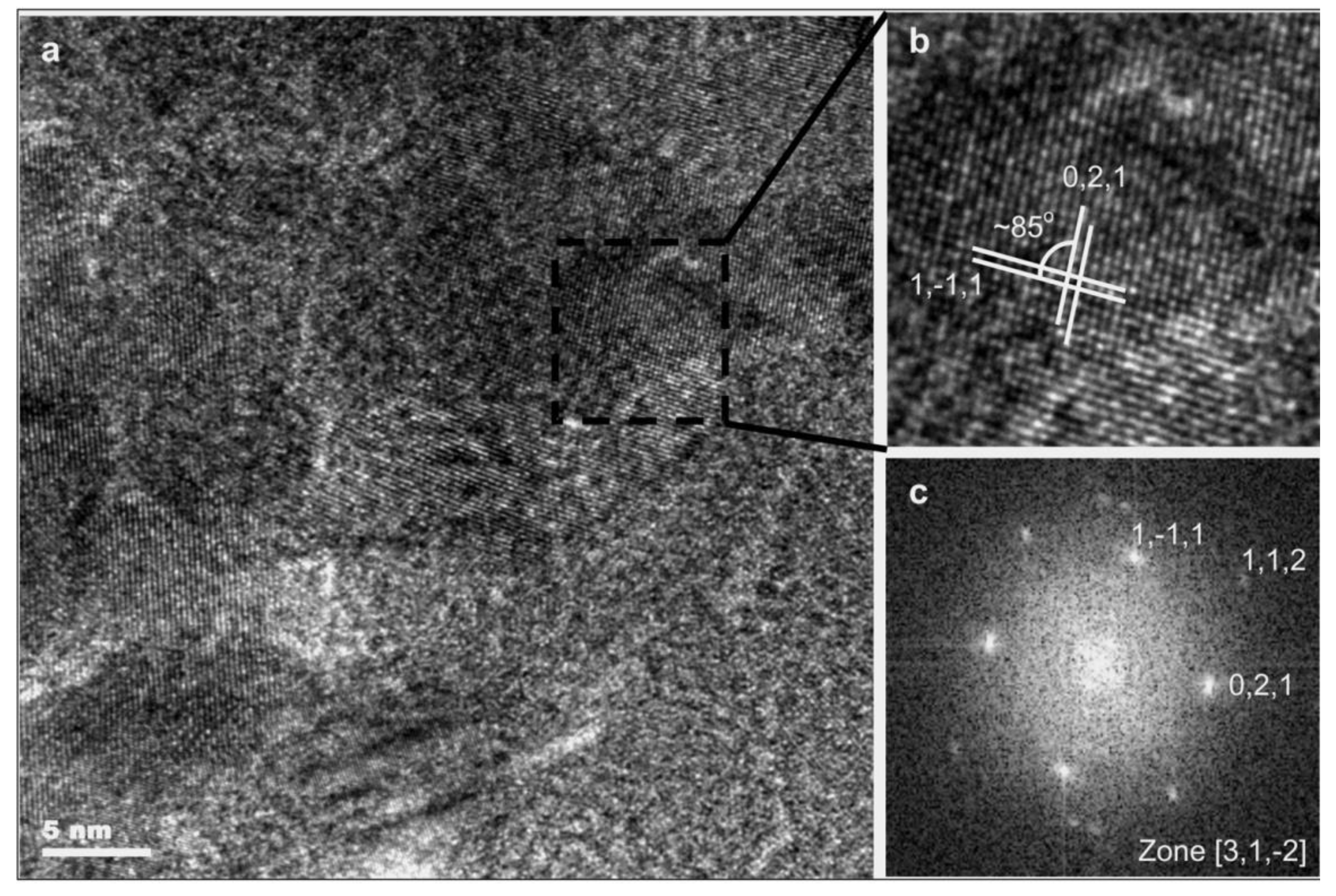
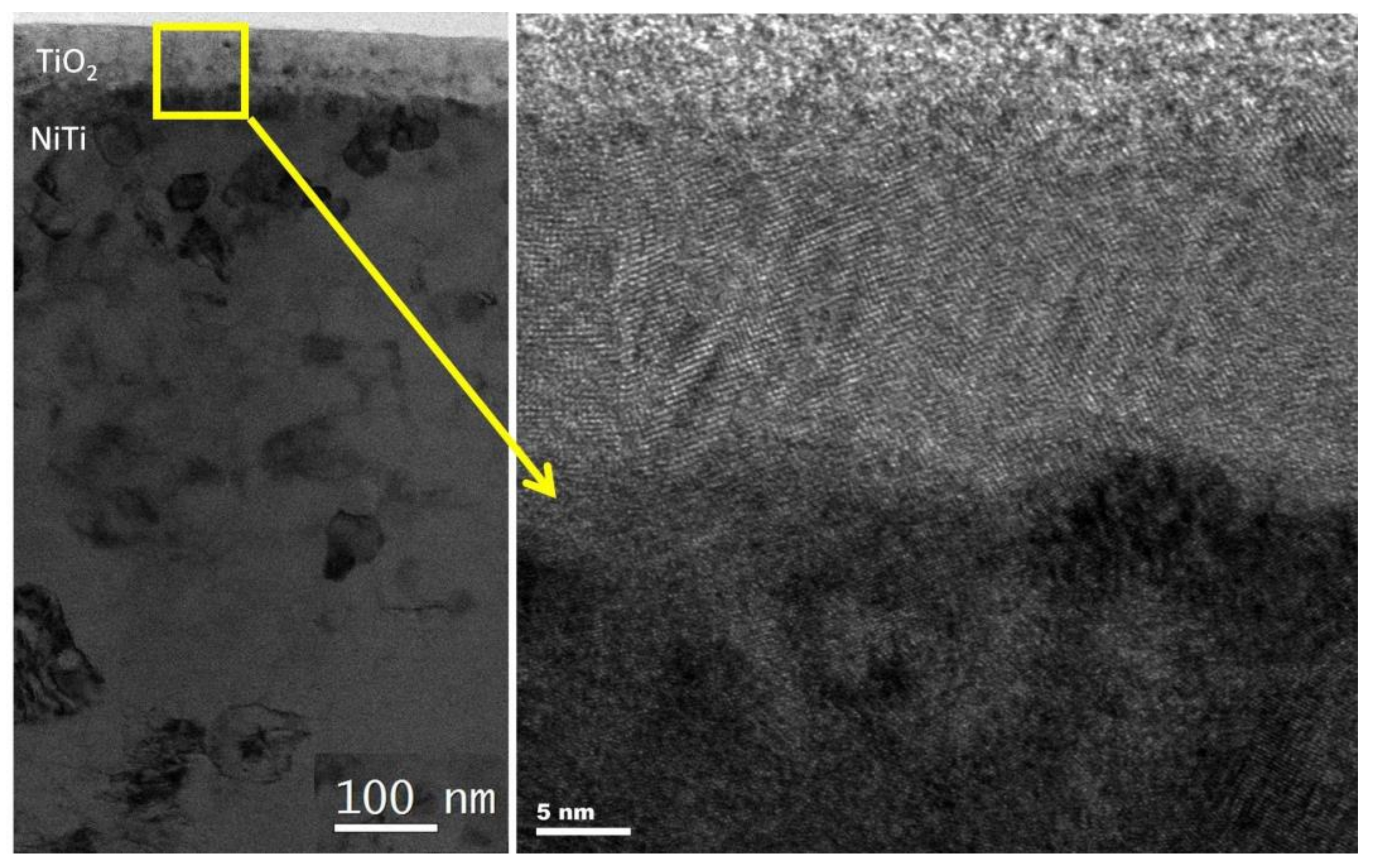
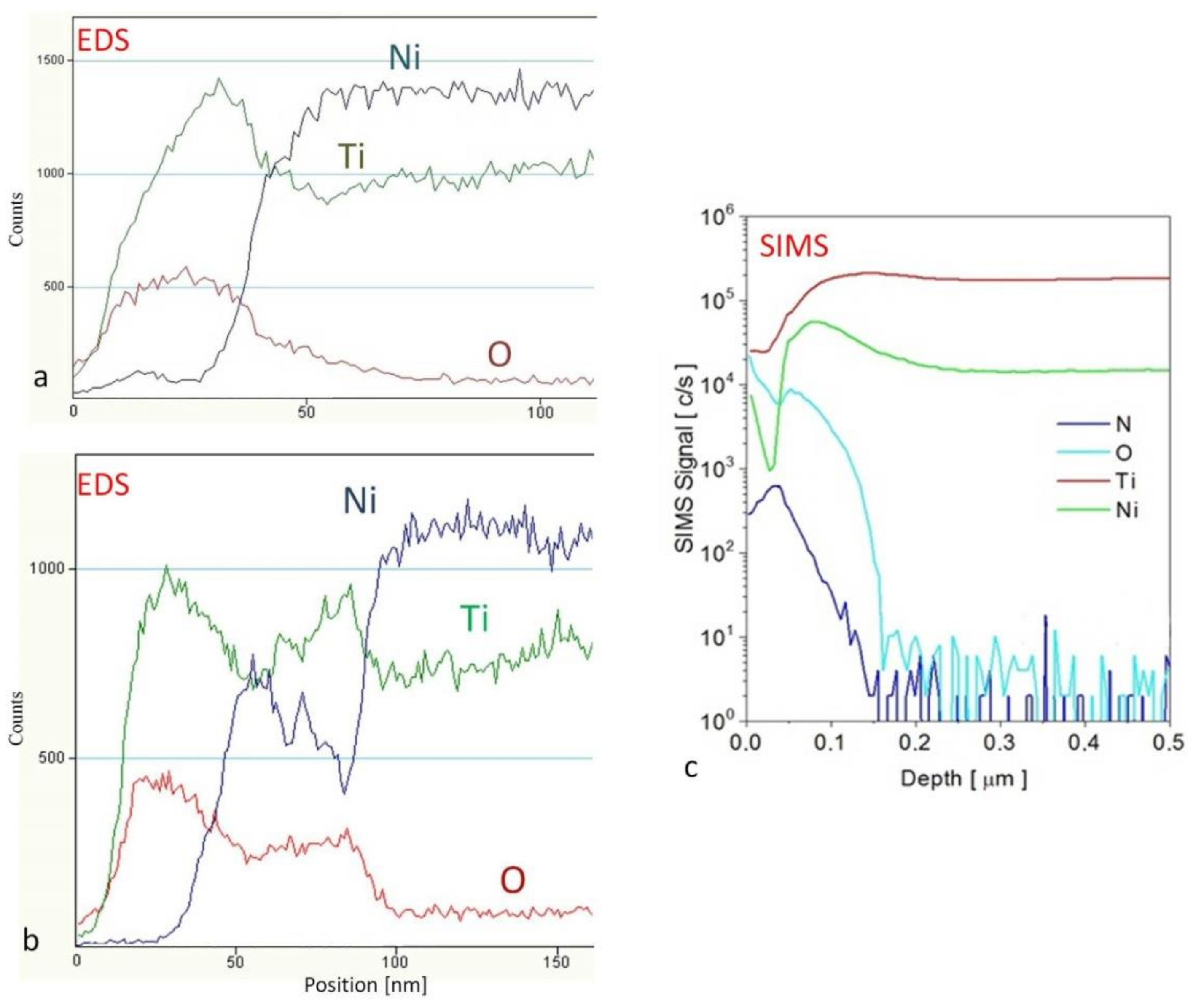
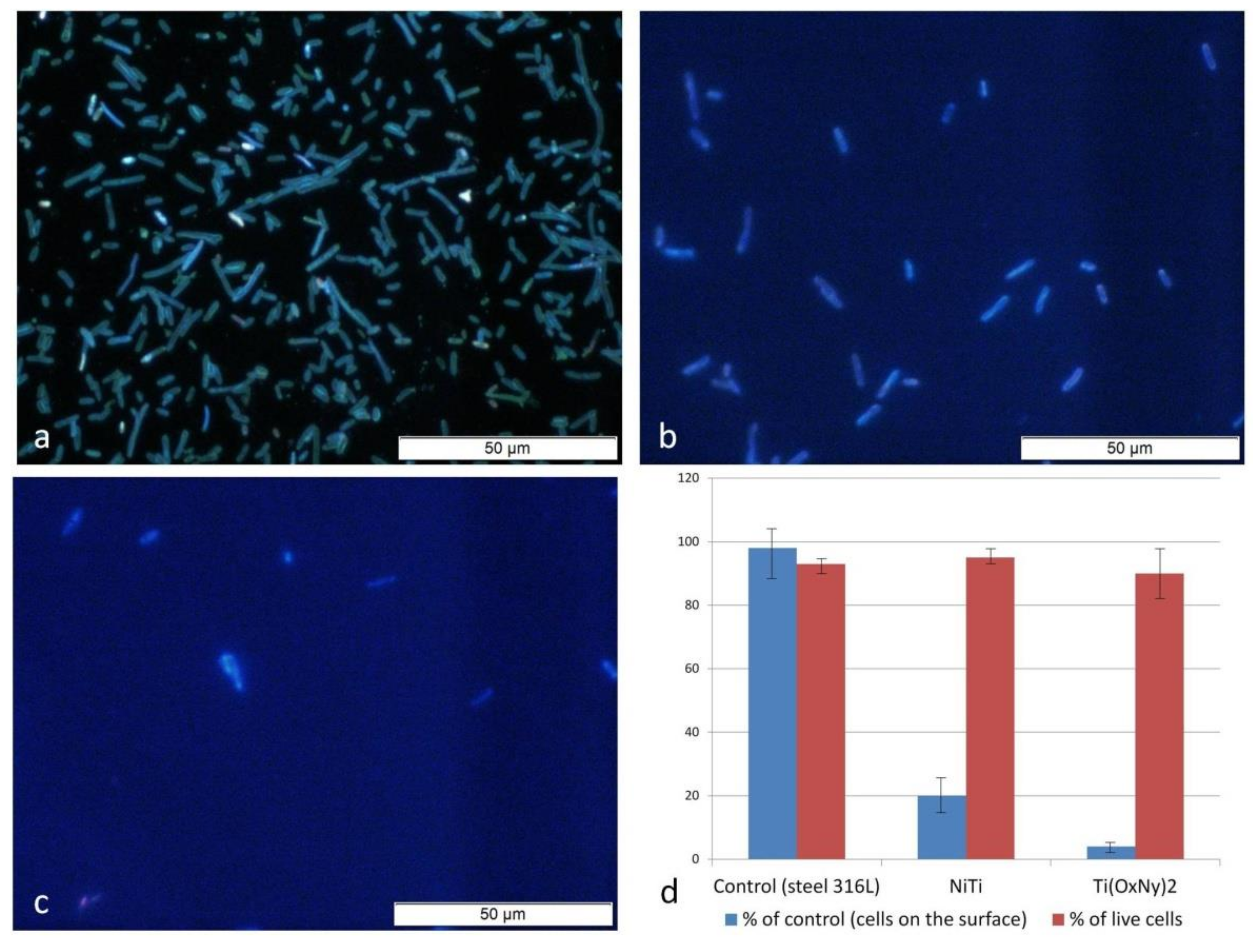
| Method | Material | Ra (nm) | Rq (nm) | Rz (nm) |
|---|---|---|---|---|
| Optical Profilometer | NiTi | 59 ± 8 | 81 ± 12 | 1247 ± 187 |
| TiO2 | 173 ± 15 | 227 ± 27 | 2760 ± 331 | |
| Ti(OxNy)2 | 144 ± 10 | 192 ± 12 | 2430 ± 315 | |
| Atomic Force Microscope | NiTi | 5.6 ± 1.9 | 7.5 ± 2.5 | 88.6 ± 11.3 |
| TiO2 | 26.1 ± 4.8 | 32.9 ± 5.1 | 264.7 ± 31.2 | |
| Ti(OxNy)2 | 18.8 ± 3.5 | 23.4 ± 4.2 | 184.5 ± 15.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarnowski, M.; Witkowska, J.; Morgiel, J.; Jakubowski, W.; Walkowiak, B.; Borowski, T.; Wierzchoń, T. Formation of Nitrogen Doped Titanium Dioxide Surface Layer on NiTi Shape Memory Alloy. Materials 2021, 14, 1575. https://doi.org/10.3390/ma14061575
Tarnowski M, Witkowska J, Morgiel J, Jakubowski W, Walkowiak B, Borowski T, Wierzchoń T. Formation of Nitrogen Doped Titanium Dioxide Surface Layer on NiTi Shape Memory Alloy. Materials. 2021; 14(6):1575. https://doi.org/10.3390/ma14061575
Chicago/Turabian StyleTarnowski, Michał, Justyna Witkowska, Jerzy Morgiel, Witold Jakubowski, Bogdan Walkowiak, Tomasz Borowski, and Tadeusz Wierzchoń. 2021. "Formation of Nitrogen Doped Titanium Dioxide Surface Layer on NiTi Shape Memory Alloy" Materials 14, no. 6: 1575. https://doi.org/10.3390/ma14061575
APA StyleTarnowski, M., Witkowska, J., Morgiel, J., Jakubowski, W., Walkowiak, B., Borowski, T., & Wierzchoń, T. (2021). Formation of Nitrogen Doped Titanium Dioxide Surface Layer on NiTi Shape Memory Alloy. Materials, 14(6), 1575. https://doi.org/10.3390/ma14061575







