Green Microwave-Assisted Synthesis of CoFeRu-Based Electrocatalyst for the Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
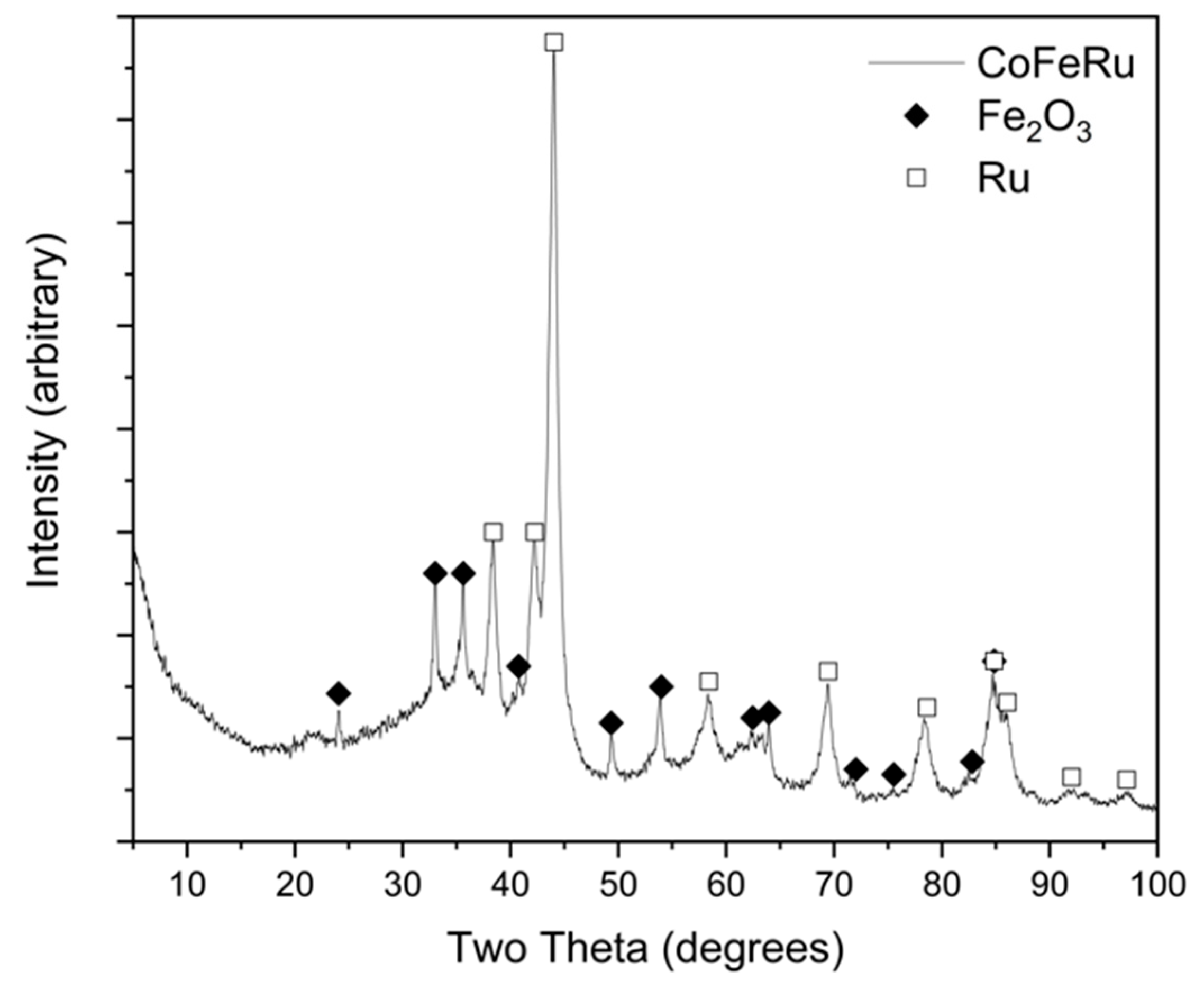
3.1. Physica and Structural Characterization
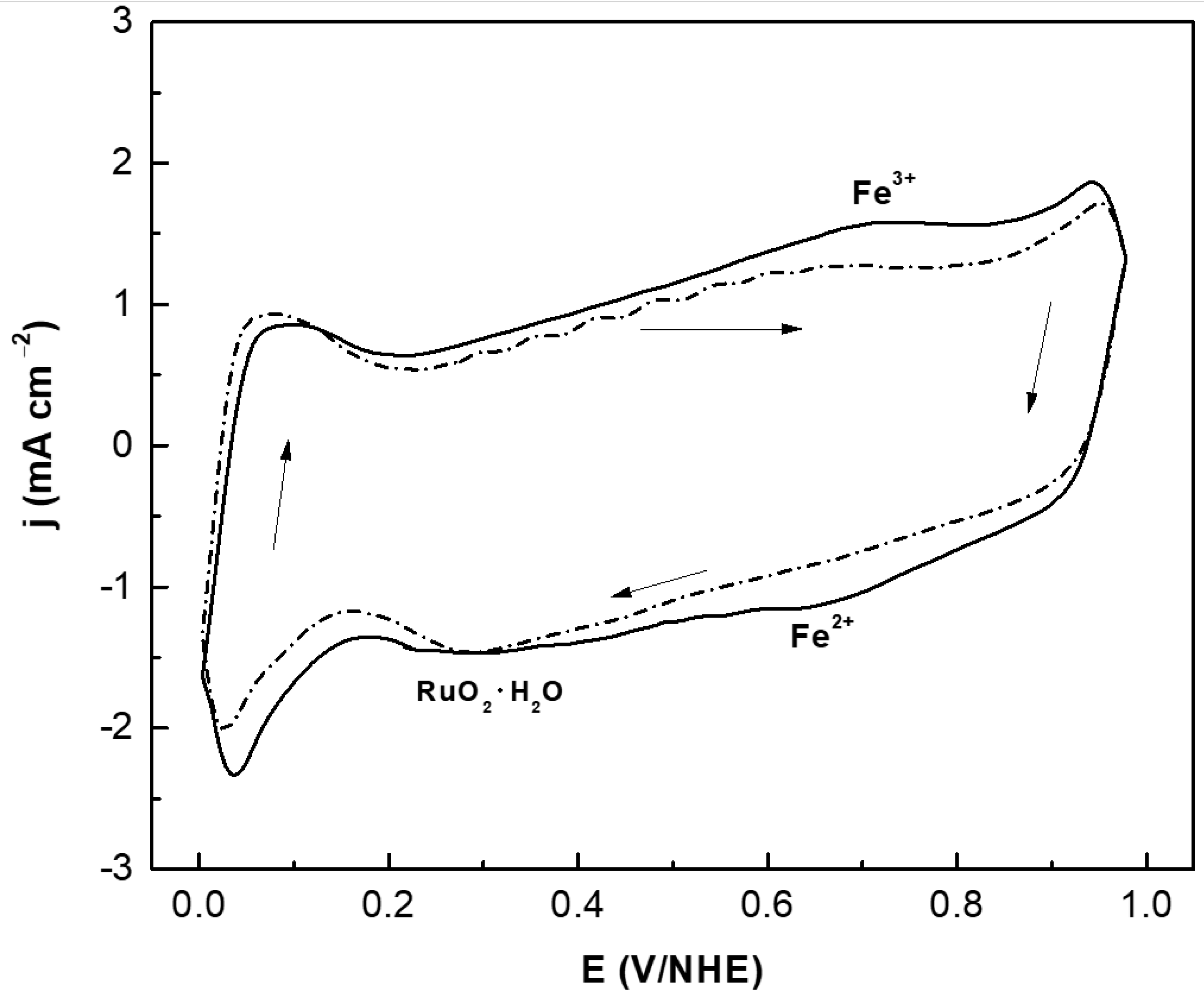
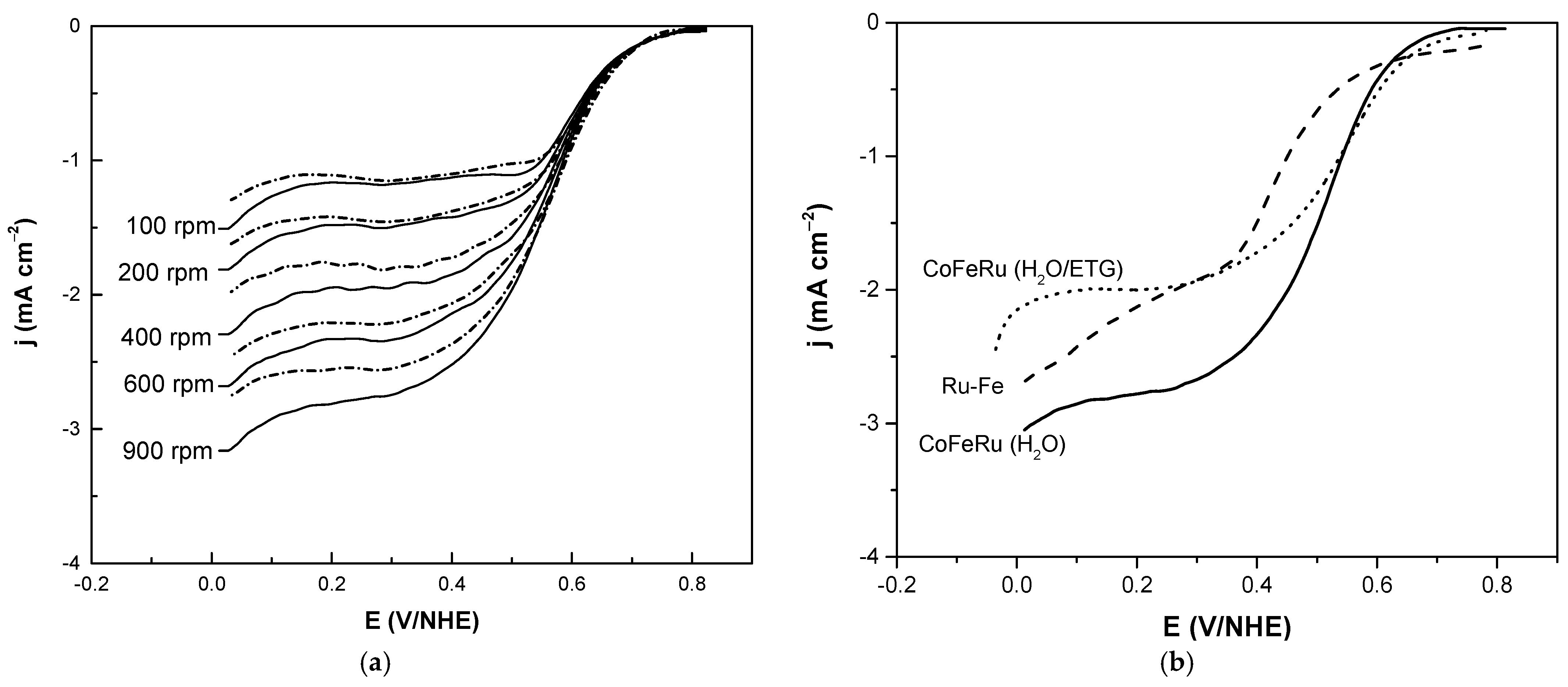
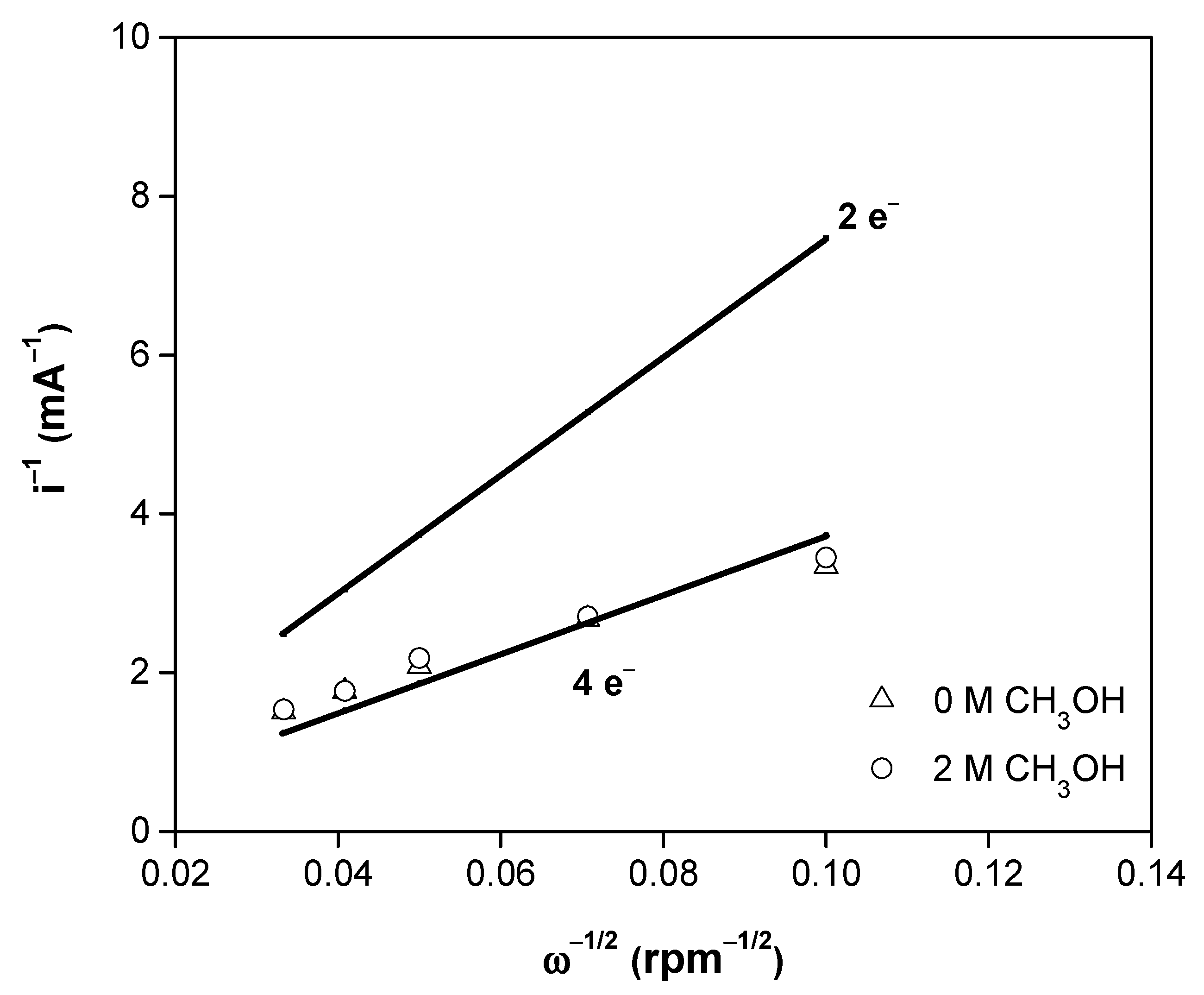
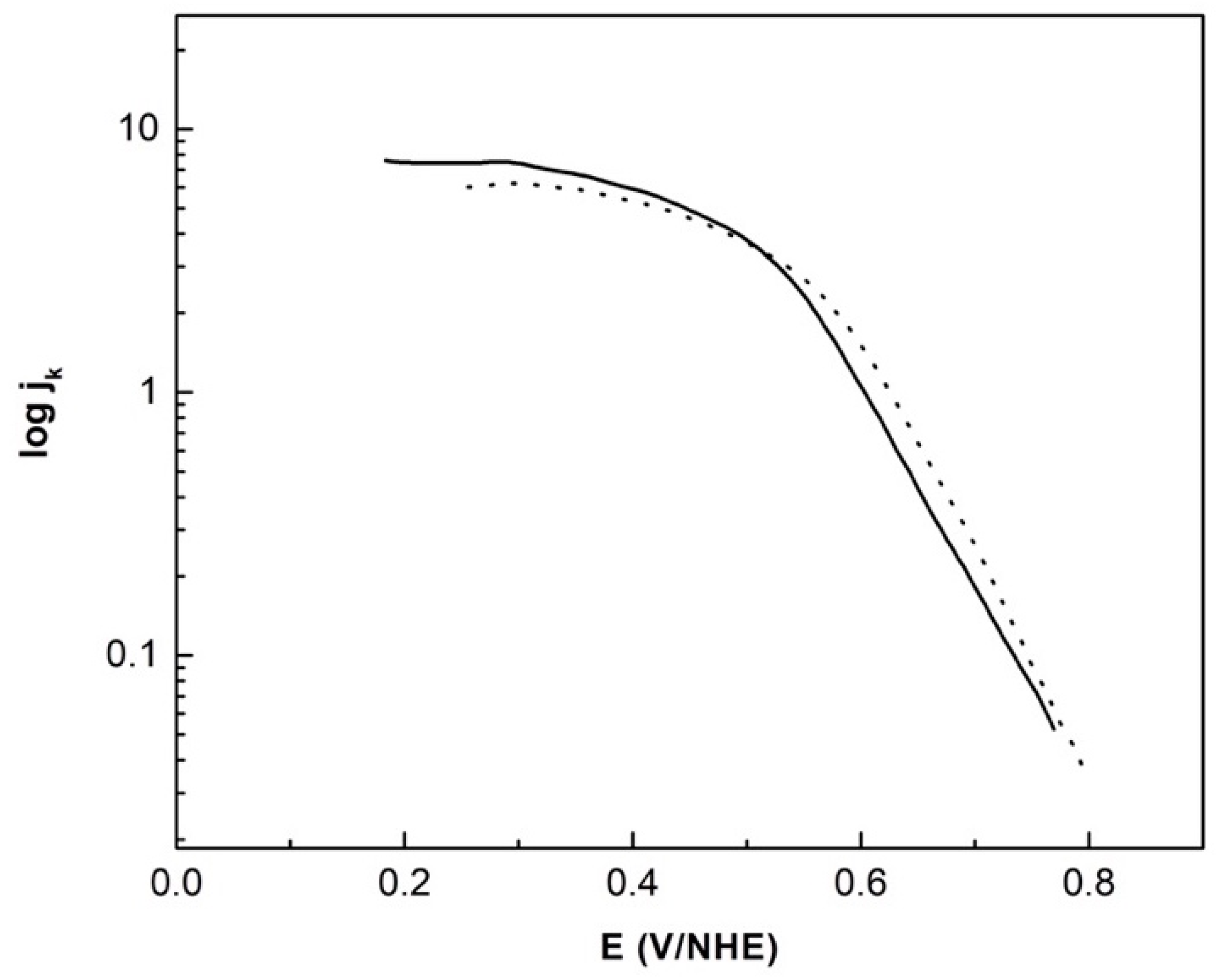
3.2. Electrochemical Studies
- A = geometric area of the glassy carbon disk (0.1963 cm2),
- F = Faraday’s constant (96,485 C mol−1),
- 𝜐 = kinematic viscosity of the electrolyte (0.01 cm2 s−1),
- = diffusion coefficient of O2 in the electrolyte (1.40 × 10−5 cm2 s−1),
- = concentration of O2 in the electrolyte (1.1 × 10−6 mol cm−3),
- ω = electrode rotation velocity (rpm),
- n = number of electrons involved in the oxygen reduction reaction,
- 200 = constant used when ω is expressed in rpm and the current disk (id) in mA.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nekooi, P.; Amini, M. Effect of support type and synthesis conditions on the oxygen reduction activity of RuxSey catalyst prepared by the microwave polyol method. Electrochim. Acta 2010, 55, 3286–3294. [Google Scholar] [CrossRef]
- Stamenković, V.; Schmidt, T.; Ross, P.; Marcović, N. Surface Composition Effects in Electrocatalysis: Kinetics of Oxygen Reduction on Well-Defined Pt3Ni and Pt3Co Alloy Surfaces. J. Phys. Chem. B 2002, 106, 11970–11979. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zhang, F.; Wu, K.; Lu, Z.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Carbon supported Palladium–Iron nanoparticles with uniform alloy structure as methanol-tolerant electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2012, 37, 2993–3000. [Google Scholar] [CrossRef]
- Jeon, M.; Lee, C.; Park, G.; Kang, K. Combinatorial search for oxygen reduction reaction electrocatalysts: A review. J. Power Sources 2012, 216, 400–408. [Google Scholar] [CrossRef]
- Ralph, T.; Hogarth, M. Catalysis for Low Temperature Fuel Cells. PART I: THE CATHODE CHALLENGES. Platinum Met. Rev. 2002, 46, 3–14. [Google Scholar]
- Liu, J.; Jeon, M.; Woo, S. High-throughput screening of binary catalysts for oxygen electroreduction. Appl. Surf. Sci. 2006, 252, 2580–2587. [Google Scholar] [CrossRef]
- Li, W.; Xin, Q.; Yan, Y. Nanostructured Pt–Fe/C cathode catalysts for direct methanol fuel cell: The effect of catalyst composition. Int. J. Hydrogen Energy 2010, 35, 2530–2538. [Google Scholar] [CrossRef]
- Gómez, A.; Rizo, R.; Feliu, J. Some reflections on the understanding of the oxygen reduction reaction at Pt (111). Beilstein J. Nanotech. 2013, 4, 956–967. [Google Scholar] [CrossRef] [Green Version]
- Suárez, K.; Solorza, O. Kinetics and PEMFC performance of RuxMoySez nanoparticles as a cathode catalyst. Electrochim. Acta 2008, 53, 4981–4989. [Google Scholar] [CrossRef]
- Abrego, J.; Wang, Y.; Moreno, A.; Wei, Q.; Cuevas, F.; Arriaga, L.; Sun, S.; Mohamedi, M. Nanostructured Mn2O3/Pt/CNTs selective electrode for oxygen reduction reaction and methanol tolerance in mixed-reactant membraneless micro-DMFC. Electrochim. Acta 2019, 297, 230–239. [Google Scholar] [CrossRef]
- Li, S.; Tian, S.; Liu, Y.; Jang, Z.; Hasan, S.; Chen, X.; Tsiakaras, P.; Shen, P. Hierarchically skeletal multi-layered Pt-Ni nanocrystals for highly efficient oxygen reduction reaction and methanol oxidation reactions. Chin. J. Catal. 2021, 42, 648–657. [Google Scholar] [CrossRef]
- Bukka, S.; Badam, R.; Vedarajan, R.; Matsumi, N. Photo-generation of ultra-small Pt nanoparticles on carbon–titanium dioxide nanotube composites: A novel strategy for efficient ORR activity with low Pt content. Int. J. Hydrogen Energy 2019, 44, 4745–4753. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, C.; Chang, Y.; Lai, C.; Lim, P.; Tsai, L.; Wang, K. The structure-activity relationship of Pd–Co/C electrocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2010, 35, 1864–1871. [Google Scholar] [CrossRef]
- Mustain, W.; Prakash, J. Kinetics and mechanism for the oxygen reduction reaction on polycrystalline cobalt–palladium electrocatalysts in acid media. J. Power Sources 2007, 170, 28–37. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Zhong, H.; Ma, Y. Cobalt oxyphosphide as oxygen reduction electrocatalyst in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2011, 36, 720–724. [Google Scholar] [CrossRef]
- Ye, S.; Vijh, A. Cobalt-carbonized aerogel nanocomposites electrocatalysts for the oxygen reduction reaction. Int. J. Hydrogen Energy 2005, 30, 1011–1015. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Bai, L.; Han, L.; Dong, S. Nitrogen, cobalt-codoped carbon electrocatalyst for oxygen reduction reaction using soymilk and cobalt salts as precursors. Electrochem. Commun. 2013, 34, 68–72. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, K.; Bezerra, C.; Zhang, J.; Zhang, J. Fe loading of a carbon-supported Fe–N electrocatalyst and its effect on the oxygen reduction reaction. Electrochim. Acta 2009, 54, 6631–6636. [Google Scholar] [CrossRef]
- Negro, E.; Monteverde, A.; Baglio, V.; Aricò, A.; Specchia, S.; Koper, G. Fe–N supported on graphitic carbon nano-networks grown from cobalt as oxygen reduction catalysts for low-temperature fuel cells. Appl. Catal. B Environ. 2015, 166, 75–83. [Google Scholar] [CrossRef]
- Rivera, R.; Castillo, N.; Soto, A.; Solorza, O. Oxygen reduction on RuxFey cluster electrocatalyst in acid electrolyte. Int. J. Hydrogen Energy 2007, 27, 457–460. [Google Scholar] [CrossRef]
- Alonso, N.; Tributsch, H. Energy conversion catalysis using semiconducting transition metal cluster compounds. Nature 1986, 323, 431–432. [Google Scholar] [CrossRef]
- Alonso, N.; Jaegermann, W.; Tributsch, H.; Hoenle, W.; Yvon, K. Electrocatalysis of oxygen reduction by chalcogenides containing mixed transition metal clusters. J. Am. Chem. Soc. 1987, 109, 3251–3257. [Google Scholar]
- Liu, L.; Lee, J.; Popov, B. Development of ruthenium-based bimetallic electrocatalysts for oxygen reduction reaction. J. Power Sources 2006, 162, 1099–1103. [Google Scholar] [CrossRef]
- Salgado, J.; Fernandes, J.; Botelho, A.; Ferraria, A.; Duarte, R.; Ferreira, M. Pt–Ru nanoparticles supported on functionalized carbon as electrocatalysts for the methanol oxidation. Electrochim. Acta 2011, 56, 8509–8518. [Google Scholar] [CrossRef]
- Khalifeh, M.; Shams, E.; Sharifi, E. Pt–Ru nanoparticles anchored on poly (brilliant cresyl blue) as a new polymeric support: Application as an efficient electrocatalyst in methanol oxidation reaction. Int. J. Hydrogen Energy 2020, 45, 849–860. [Google Scholar] [CrossRef]
- Sandoval, A.; Paraguay, F.; Sebastian, P.; Borja, E. Microwave synthesis of an electrocatalyst based on CoFeRu for the oxygen reduction reaction in the absence and presence of methanol. J. Power Sources 2014, 267, 793–798. [Google Scholar] [CrossRef]
- Wang, J.; Ciucci, F. Boosting Bifunctional Oxygen Electrolysis for N-Doped Carbon via Bimetal Addition. Small 2017, 13, 1604103. [Google Scholar] [CrossRef]
- Gao, D.; Li, H.; Cheng, X. Preparations of Highly Stable RuCo/CNTs Electrocatalysts for Oxygen Reduction Reaction. ECS Trans. 2015, 66, 57–68. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Disalvo, F.; Abruña, H. Multifunctional Electrocatalysts: Ru−M (M = Co, Ni, Fe) for Alkaline Fuel Cells and Electrolyzers. ACS Catal. 2020, 10, 4608–46016. [Google Scholar] [CrossRef]
- Hayes, B. Microwave Synthesis: Chemistry at the Speed of Light; CEM Publishing: Matthews, NC, USA, 2002. [Google Scholar]
- Bernal, M.; Selva, A.; Borja, E.; Magallón, L.; Su, J. Microwave assisted synthesis of metallic Ru for the HOR and ORR. Mater. Res. Express 2020, 7, 025503. [Google Scholar]
- Kappe, C. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem. Int. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Okoli, C.; Kuttiyield, K.; Cole, J.; McCutchen, J.; Tawfik, H.; Adzic, R.; Mahajan, D. Solvent effect in sonochemical synthesis of metal-alloy nanoparticles for use as electrocatalysts. Ultrason. Sonochem. 2018, 41, 427–434. [Google Scholar] [CrossRef] [PubMed]
- de La Hoz, A.; Díaz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Su, J.; Magallón, L.; Borja, E.; García, J.; Sebastian, P. Oxygen reduction and hydrogen oxidation reactions on Ru-Fe electrocatalyst synthesized by a microwave-assisted synthesis. Mater. Res. Express 2020, 7, 035505. [Google Scholar]
- Max, J.; Chapados, C. Isotope effects in liquid water by infrared spectroscopy. III. H2O and D2O spectra from 6000 to 0 cm−1. J. Chem. Phys. 2009, 131, 184505–184513. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, A.; Lacruz, M. Caracterización de catalizadores de hierro y cobalto soportados sobre sílice sintetizados en ambiente ácido y básico. Rev. Latinam. Metal. Mater. 2006, 26, 20–22. [Google Scholar]
- Ehrhardt, C.; Gjikaj, M.; Brockner, W. Thermal decomposition of cobalt nitrate compounds: Preparation of anhydrous cobalt (II) nitrate and its characterization by Infrared and Raman spectra. Termochim. Acta 2005, 432, 36–40. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, Tables and Charts, 3rd ed.; John Wiley & Sons: Middlesex, London, UK, 2001. [Google Scholar]
- Altamirano, A.; Jiménez, O.; Uribe, J.; Castellanos, R.; Borja, E.; Olivares, J. Methanol resistant ruthenium electrocatalysts for oxygen reduction synthesized by pyrolysis of Ru3(CO)12 in different atmospheres. Int. J. Hydrogen Energy 2009, 34, 7983–7994. [Google Scholar] [CrossRef]
- Ahmed, S.; Ogale, S.; Papaefthymiou, G.; Ramesh, R.; Kofinas, P. Magnetic properties of CoFe2O4 nanoparticles synthesized through a block copolymer nanoreactor route. Appl. Phys. Lett. 2002, 80, 1616–1618. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Nam, Y.; Lee, J. Catalytic nature of iron-nitrogen-graphene heterogeneous catalysts for oxygen evolution reaction and oxygen reduction reaction. Appl. Phys. Lett. 2020, 514, 146073. [Google Scholar] [CrossRef]
- Treimer, S.; Tang, A.; Johnson, D. A Consideration of the Application of Koutecký-Levich Plots in the Diagnoses of Charge-Transfer Mechanisms at Rotated Disk Electrodes. Electroanal. 2002, 14, 165–171. [Google Scholar] [CrossRef]
- Sandoval, A.; Gamboa, S. Analysis of Redox Reactions on Pt-Sn based Nano-catalysts for Direct Methanol Fuel Cell Applications. J. New Mater. Electrochem. Syst. 2018, 21, 021–028. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Trasatti, S. Electrochemical Theory | Electrokinetics. Encyclopedia of Electrochemical Power Sources, 1st ed.; Elsevier Science: Milan, Italy, 2009. [Google Scholar]
- Ramirez, C.; Fernandes, D.; Freire, C.; Villaro, E.; Guerrero, A.; Rodríguez, I. Upgrading the Properties of Reduced Graphene Oxide and Nitrogen-Doped Reduced Graphene Oxide Produced by Thermal Reduction toward Efficient ORR Electrocatalysts. Nanomaterials 2019, 9, 1761. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, C.; Bard, A. Hydrogen Peroxide Production in the Oxygen Reduction Reaction at Different Electrocatalysts as Quantified by Scanning Electrochemical Microscopy. J. Anal. Chem. 2009, 81, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, A.; Nabae, Y.; Hayakawa, T.; Okajima, T.; Ohsaka, T. Fe-containing polyimide-based high-performance ORR catalysts in acidic medium: A kinetic approach to study the durability of catalysts. Catal. Sci. Technol. 2015, 5, 475–483. [Google Scholar] [CrossRef]
- Anastasijević, N.; Dimitrijević, Z.; Adžić, R. Oxygen Reduction on a Ruthenium Electrode in Acid Electrolytes. Electrochim. Acta 1986, 31, 1125–1130. [Google Scholar] [CrossRef]


| Electrocatalyst | at.% | ||||
|---|---|---|---|---|---|
| Co | Fe | Ru | O | C | |
| CoFeRu (H2O) | 0.47 | 17.52 | 13.30 | 47.47 | 21.24 |
| CoFeRu (H2O/ETG) [26] | 1.70 | 1.70 | 46.60 | 33.00 | 17.00 |
| Electrocatalyst | Methanol (mol L−1) | Open Circuit Potential (V/NHE) | Tafel Slope (V dec−1) | Charge Transfer Coefficient | Exchange Current Density (mA cm−2) |
|---|---|---|---|---|---|
| CoFeRu (H2O) | 0 | 0.811 (0.002) | 0.129 (0.001) | 0.420 (0.004) | 1.36 (0.09) × 10−5 |
| 2 | 0.823 (0.0006) | 0.126 (0.002) | 0.430 (0.007) | 1.55 (0.19) × 10−5 | |
| CoFeRu (ETG/H2O) [26] | 0 | 0.782 | 0.193 | 0.306 | 2.49 × 10−4 |
| 2 | 0.780 | 0.203 | 0.306 | 3.20 × 10−4 | |
| Ru-Fe [35] | 0 | 0.772 | 0.267 | 0.22 | 1.7 × 10−3 |
| 2 | 0.774 | 0.270 | 0.22 | 2.0 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval, A.; Borja, E.; Magallón, L.; Su, J. Green Microwave-Assisted Synthesis of CoFeRu-Based Electrocatalyst for the Oxygen Reduction Reaction. Materials 2021, 14, 1662. https://doi.org/10.3390/ma14071662
Sandoval A, Borja E, Magallón L, Su J. Green Microwave-Assisted Synthesis of CoFeRu-Based Electrocatalyst for the Oxygen Reduction Reaction. Materials. 2021; 14(7):1662. https://doi.org/10.3390/ma14071662
Chicago/Turabian StyleSandoval, Antonia, Edgar Borja, Lorena Magallón, and Javier Su. 2021. "Green Microwave-Assisted Synthesis of CoFeRu-Based Electrocatalyst for the Oxygen Reduction Reaction" Materials 14, no. 7: 1662. https://doi.org/10.3390/ma14071662








