Characterization of Methacrylate-Based Resins Containing Methacryl-Polyhedral Oligomeric Silsesquioxanes (MA-POSS-8)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Methacrylate-Based Resins
2.2. Degree of Conversion
- Height of the peak of vinyl group
- Height of the peak of benzyl group
2.3. Viscosity
2.4. Martens Hardness and Indentation Modulus
2.5. Water Sorption and Sol Fraction
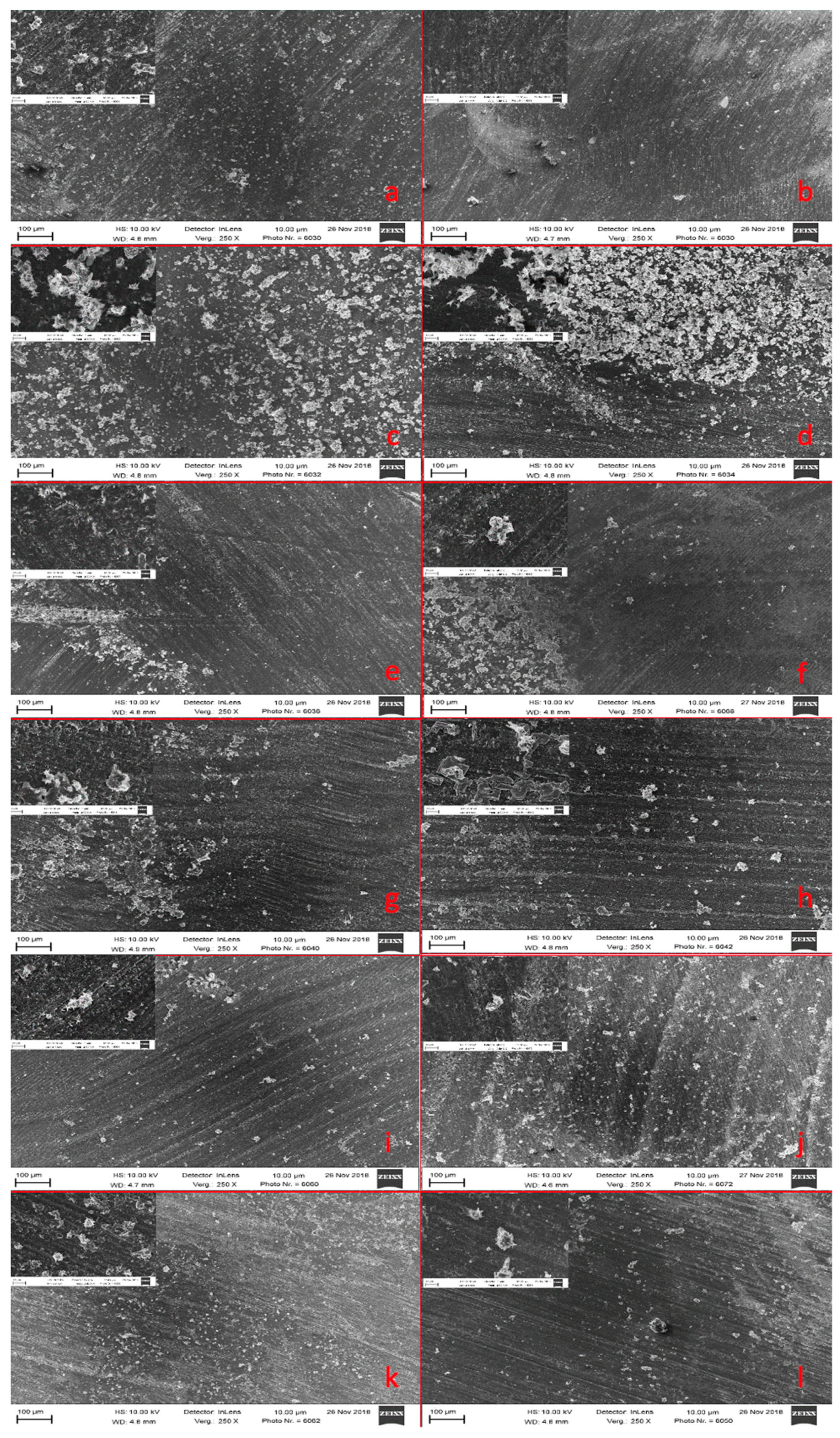
2.6. Mineral Precipitation Capacity
2.7. Statistical Analysis
3. Results
3.1. Degree of Conversion
3.2. Viscosity
3.3. Martens Hardness and Indentation Modulus
3.4. Water Sorption and Sol Fraction
3.5. Mineral Precipitation Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tauböck, T.T.; Zehnder, M.; Schweizer, T.; Stark, W.J.; Attin, T.; Mohn, D. Functionalizing a dentin bonding resin to become bioactive. Dent. Mater. 2014, 30, 868–875. [Google Scholar] [CrossRef]
- Zhang, L.; Weir, M.D.; Hack, G.; Fouad, A.F.; Xu, H.H. Rechargeable dental adhesive with calcium phosphate nanoparticles for long-term ion release. J. Dent. 2015, 43, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Sun, Y.; Xie, W.; Liu, Y.; Song, X. Development of novel dental nanocomposites reinforced with polyhedral oligomeric silsesquioxane (POSS). Dent. Mater. 2010, 26, 456–462. [Google Scholar] [CrossRef]
- Rizk, M.; Hohlfeld, L.; Thanh, L.T.; Biehl, R.; Luhmann, N.; Mohn, D.; Wiegand, A. Bioactivity and properties of a dental adhesive functionalized with polyhedral oligomeric silsesquioxanes (POSS) and bioactive glass. Dent. Mater. 2017, 33, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrand, J.; Lopez, A.; Engqvist, H.; Persson, C. Polyhedral oligomeric silsesquioxane (POSS)-poly(ethylene glycol) (PEG) hybrids as injectable biomaterials. Biomed. Mater. 2012, 7, 35013. [Google Scholar] [CrossRef]
- Rizk, M.; Pohle, A.; Dieckmann, P.; Tauböck, T.T.; Biehl, R.; Wiegand, A. Mineral precipitation, polymerization properties and bonding performance of universal dental adhesives doped with polyhedral oligomeric silsesquioxanes. Int. J. Adhes. Adhes. 2020, 100, 102573. [Google Scholar] [CrossRef]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A nanocage for nanomedicine: Polyhedral oligomeric silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Yu, J.; Sun, Y.; Xie, W. Study of POSS on the Properties of Novel Inorganic Dental Composite Resin. Polymers 2020, 12, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canellas, T.A.T.; de Almeida Neves, A.; Dos Santos, I.K.B.; de Rezende, A.R.P.; Fellows, C.E.; da Silva, E.M. Characterization of low-shrinkage dental composites containing methacrylethyl-polyhedral oligomeric silsesquioxane (ME-POSS). J. Mech. Behav. Biomed. Mater. 2019, 90, 566–574. [Google Scholar] [CrossRef]
- van Landuyt, K.L.; Snauwaert, J.; de Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Leitune, V.C.; Collares, F.M.; Trommer, R.M.; Andrioli, D.G.; Bergmann, C.P.; Samuel, S.M. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013, 41, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Um, C.M.; Lee, I.B. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef]
- Provenzi, C.; Collares, F.M.; Cuppini, M.; Samuel, S.M.W.; Alves, A.K.; Bergmann, C.P.; Leitune, V.C.B. Effect of nanostructured zirconium dioxide incorporation in an experimental adhesive resin. Clin. Oral Investig. 2018, 22, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Collares, F.M.; Ogliari, F.A.; Zanchi, C.H.; Petzhold, C.L.; Piva, E.; Samuel, S.M.W. Influence of 2-hydroxyethyl methacrylate concentration on polymer network of adhesive resin. J. Adhes. Dent. 2011, 13, 125–129. [Google Scholar] [CrossRef] [PubMed]
- DIN ISO 14577-1. ISO 14577-1: Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 1: Test Method; ISO: Geneve, Switzerland, 2002. [Google Scholar]
- Klimek, J.; Hellwig, E.; Ahrens, G. Fluoride taken up by plaque, by the underlying enamel and by clean enamel from three fluoride compounds in vitro. Caries Res. 1982, 16, 156–161. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakajima, M.; Hosaka, K.; Ikeda, M.; Foxton, R.M.; Tagami, J. Long-term evaluation of water sorption and ultimate tensile strength of HEMA-containing/-free one-step self-etch adhesives. J. Dent. 2011, 39, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Zackrisson Oskolkova, M.; Bergenholtz, J. Intrinsic viscosity of dispersions of core-shell particles. Colloids Surf. A Physicochem. Eng. Asp. 2003, 225, 119–127. [Google Scholar] [CrossRef]
- Torres, S.A.S.; Silva, G.C.; Maria, D.A.; Campos, W.R.C.; Magalhaes, C.S.; Moreira, A.N. Degree of conversion and hardness of a silorane-based composite resin: Effect of light-curing unit and depth. Oper. Dent. 2014, 39, E137–E146. [Google Scholar] [CrossRef]
- Goncalves, F.; Boaro, L.C.C.; Miyazaki, C.L.; Kawano, Y.; Braga, R.R. Influence of polymeric matrix on the physical and chemical properties of experimental composites. Braz. Oral Res. 2015, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferracane, J.L. Correlation between hardness and degree of conversion during the setting reaction of unfilled dental restorative resins. Dent. Mater. 1985, 1, 11–14. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Huang, L.; Gao, Y.; Ban, J.; Shen, L.; Chen, J. Structure-property relationships in hybrid dental nanocomposite resins containing monofunctional and multifunctional polyhedral oligomeric silsesquioxanes. Int. J. Nanomed. 2014, 9, 841–852. [Google Scholar] [CrossRef] [Green Version]
- Fong, H.; Dickens, S.H.; Flaim, G.M. Evaluation of dental restorative composites containing polyhedral oligomeric silsesquioxane methacrylate. Dent. Mater. 2005, 21, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Sun, Y.; Xie, W. POSS Dental Nanocomposite Resin: Synthesis, Shrinkage, Double Bond Conversion, Hardness, and Resistance Properties. Polymers 2018, 10, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [Green Version]
- Helebrant, A.; Jonášová, L.; Šanda, L. The influence of simulated body fluid composition on carbonated hydroxyapatite formation. Ceram. Silik. 2002, 46, 9–14. [Google Scholar]
| Methacrylate-Based Resin | BisGMA | TEGDMA | HEMA |
|---|---|---|---|
| 1 | 40 | 35 | 25 |
| 2 | 50 | 25 | 25 |
| 3 | 60 | 15 | 25 |
| 4 | 40 | 25 | 35 |
| 5 | 50 | 15 | 35 |
| 6 | 60 | 5 | 35 |
| Methacrylate-Based Resin | MA-POSS-8 (wt%) | DC (%) | η (mPa*s) | HM (N/mm2) | EIT (kN/mm2) | WS (µg/mm3) |
|---|---|---|---|---|---|---|
| 1 | 0 | 58.2 ± 0.4 a | 66.2 ± 2.6 d | 149.1 ± 9.5 ab | 4.0 ± 0.1 ab | 105.4 ± 7.5 bc |
| 5 | 55.6 ± 2.1 A | 65.9 ± 0.9 C | 144.0 ± 6.9 A | 3.9 ± 0.1 A | 101.6 ± 7.9 BC | |
| 2 | 0 | 57.6 ± 4.3 a | 146.0 ± 3.3 c | 161.3 ± 2.3 a* | 4.2 ± 0.1 a* | 100.5 ± 5.9 bc |
| 5 | 57.2 ± 2.2 A | 136.9 ± 1.4 B | 138.1 ± 2.5 AB* | 3.9 ± 0.0 A* | 92.7 ± 6.9 C | |
| 3 | 0 | 57.4 ± 0.9 a | 422.3 ± 16.8 a* | 137.7 ± 7.9 b | 4.0 ± 0.1 ab | 93.9 ± 10.6 c |
| 5 | 55.0 ± 0.7 A | 333.9 ± 24.7 A* | 151.4 ± 4.7 A | 4.1 ± 0.1 A | 86.0 ± 5.5 C | |
| 4 | 0 | 56.4 ± 1.8 a | 60.0 ± 0.8 d | 110.4 ± 14.6 c | 3.4 ± 0.3 c | 135.7 ± 13.2 a |
| 5 | 56.4 ± 1.5 A | 62.1 ± 0.2 C | 101.4 ± 6.9 C | 3.3 ± 0.2 B | 122.9 ± 8.0 A | |
| 5 | 0 | 57.8 ± 1.3 a | 137.3 ± 3.5 c | 156.8 ± 9.0 a | 4.2 ± 0.2 a | 115.2 ± 7.9 b |
| 5 | 55.8 ± 2.2 A | 133.9 ± 1.5 B | 147.4 ± 5.3 A | 3.9 ± 0.1 A | 109.5 ± 22.1 AB | |
| 6 | 0 | 59.8 ± 1.1 a | 338.7 ± 25.1 b | 119.1 ± 7.0 c | 3.7 ± 0.2 bc | 112.4 ± 9.8 b |
| 5 | 57.2 ± 0.4 A | 312.3 ± 2.1 A | 126.6 ± 4.7 B | 3.8 ± 0.2 A | 112.6 ± 13.1 AB |
| Methacrylate-Based Resin | MA-POSS-8 (wt.%) | Ca/P Ratio |
|---|---|---|
| 1 | 0 | 0.40 ± 0.07 |
| 5 | 0.73 ± 0.22 | |
| 2 | 0 | 0.80 ± 0.24 |
| 5 | 1.12 ± 0.06 | |
| 3 | 0 | 1.30 ± 0.05 |
| 5 | 0.54 ± 0.25 | |
| 4 | 0 | 0.47 ± 0.08 |
| 5 | 1.12 ± 0.24 | |
| 5 | 0 | 0.88 ± 0.19 |
| 5 | 1.29 ± 0.06 | |
| 6 | 0 | 1.12 ± 0.03 |
| 5 | 0.55 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreutz, M.; Wiegand, A.; Stawarczyk, B.; Lümkemann, N.; Rizk, M. Characterization of Methacrylate-Based Resins Containing Methacryl-Polyhedral Oligomeric Silsesquioxanes (MA-POSS-8). Materials 2021, 14, 1680. https://doi.org/10.3390/ma14071680
Kreutz M, Wiegand A, Stawarczyk B, Lümkemann N, Rizk M. Characterization of Methacrylate-Based Resins Containing Methacryl-Polyhedral Oligomeric Silsesquioxanes (MA-POSS-8). Materials. 2021; 14(7):1680. https://doi.org/10.3390/ma14071680
Chicago/Turabian StyleKreutz, Marietta, Annette Wiegand, Bogna Stawarczyk, Nina Lümkemann, and Marta Rizk. 2021. "Characterization of Methacrylate-Based Resins Containing Methacryl-Polyhedral Oligomeric Silsesquioxanes (MA-POSS-8)" Materials 14, no. 7: 1680. https://doi.org/10.3390/ma14071680







