Substituent-Adjusted Electrochromic Behavior of Symmetric Viologens
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Synthesis Procedure and Structure Characterizations
2.3. Synthesis of 1,1′-Dioctyl-4,4′-Bipyridinium Dibromide (OV)
2.4. Synthesis of 1,1′-Didecyl-4,4′-Bipyridinium Dibromide (DeV)
2.5. Synthesis of 1,1′-Didodecyl-4,4′-Bipyridinium Dibromide (DoV)
2.6. Synthesis of 1,1′-Hexadecyl-4,4′-Bipyridinium Dibromide (HV)
2.7. Synthesis of 1,1′-Dibenzyl-4,4′-Bipyridinium Dibromide (BV)
2.8. Performance Measurements
3. Results and Discussion
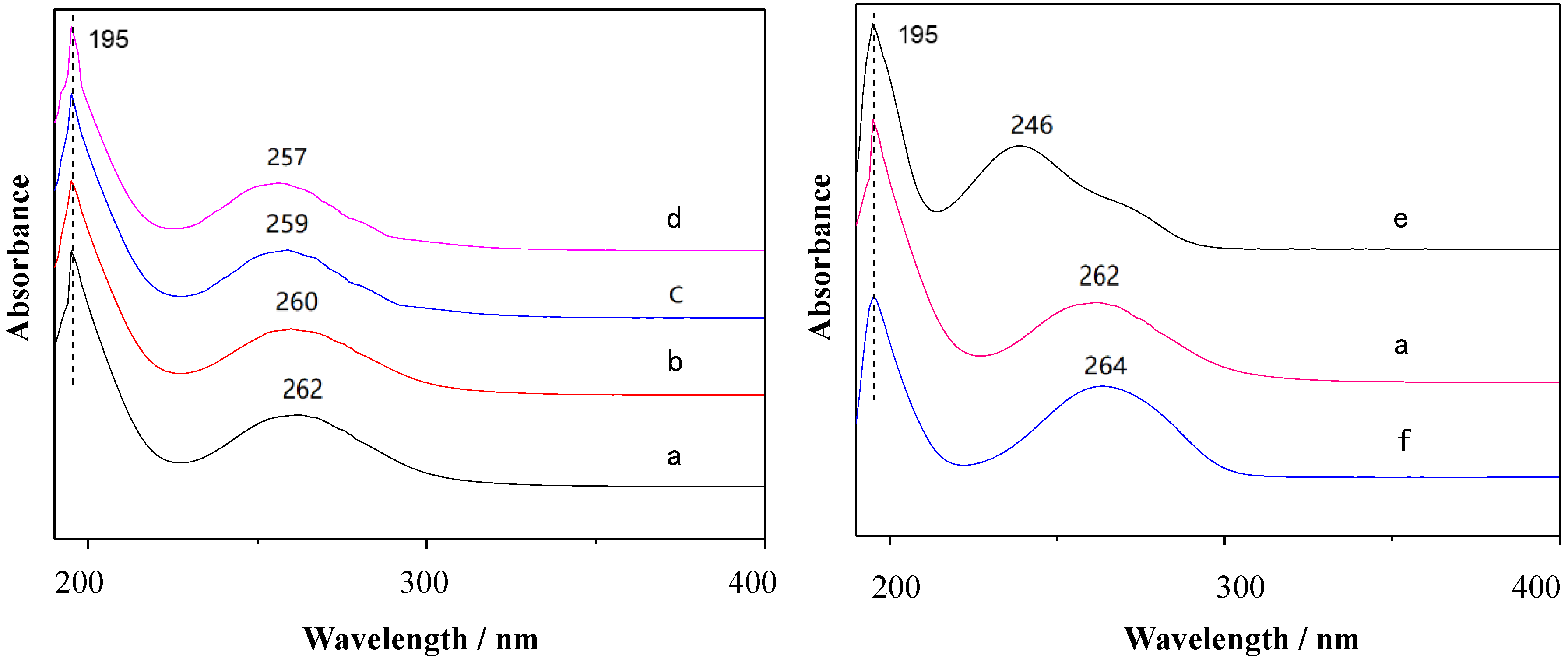
3.1. Photophysical Properties
3.2. Electrochemical Properties
3.3. EC Behaviors
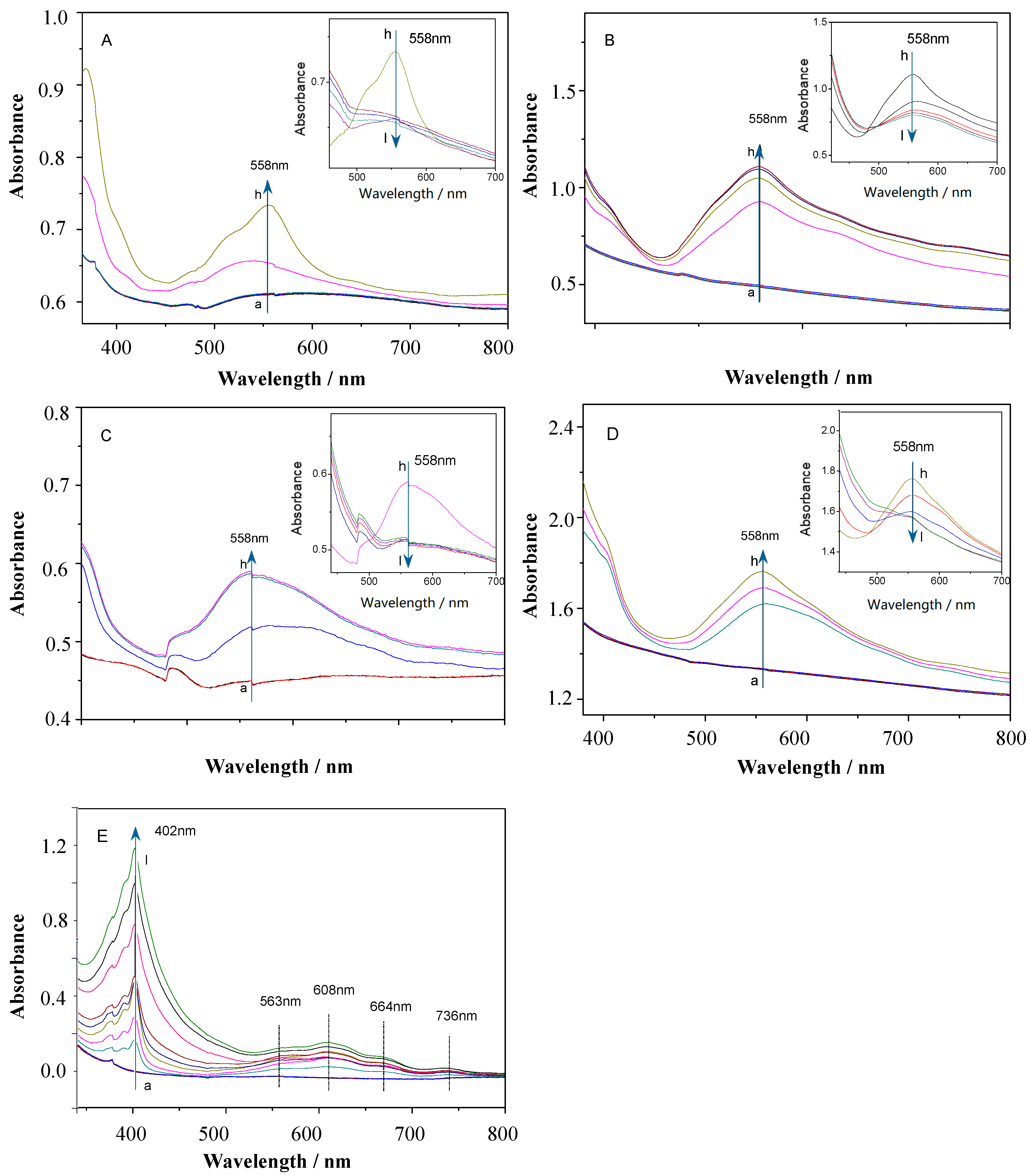
3.3.1. Multipotential UV Absorption Spectroscopy
3.3.2. Color Analysis
3.3.3. Contrast and Response time
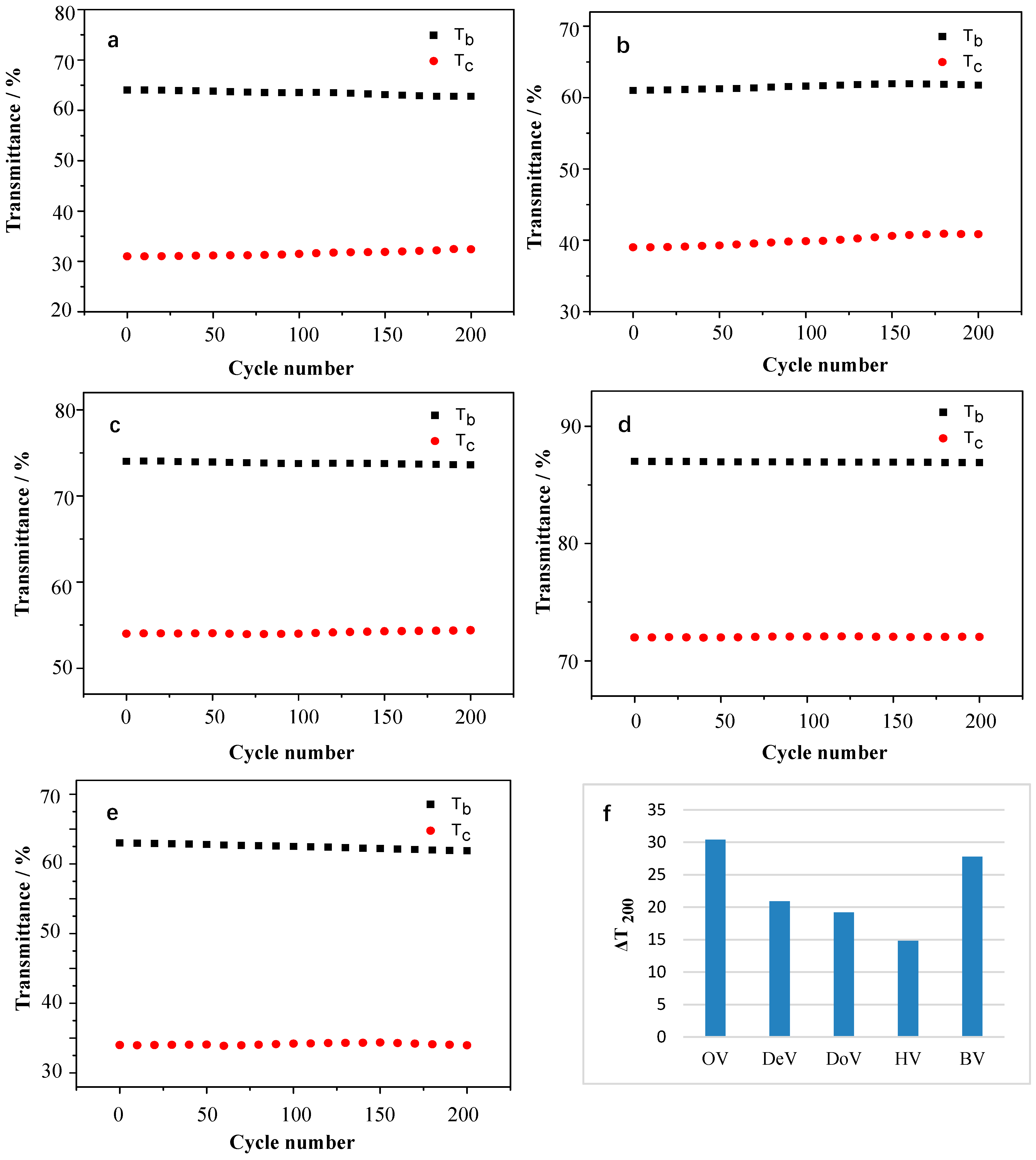
3.3.4. Cyclic Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, H.C.; Lodge, T.P.; Frisbie, C.D. Solution processable, electrochromic ion gels for sub-1 V, flexible displays on plastic. Chem. Mater. 2015, 27, 1420–1425. [Google Scholar] [CrossRef]
- Christiansen, D.T.; Tomlinson, A.L.; Reynolds, J.R. New design paradigm for color control in anodically coloring electrochromic molecules. J. Am. Chem. Soc. 2019, 141, 3859–3862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, S.; Wang, X.J.; Zhang, W.R.; Zheng, W.X.; Zhang, Y.M.; Zhang, S.X.A. A multicolour bistable electronic shelf label based on intramolecular proton-coupled electron transfer. Nat. Mater. 2019, 18, 1335–1342. [Google Scholar] [CrossRef]
- Chang, M.J.; Chen, W.N.; Xue, H.D.; Liang, D.L.; Lu, X.F.; Zhou, G. Conjugation-extended viologens with thiophene derivative bridges: Near-infrared electrochromism, electrofluorochromism, and smart window applications. J. Mater. Chem. C 2020, 8, 16129–16143. [Google Scholar] [CrossRef]
- Korgel, B.A. Materials science: Composite for smarter windows. Nature 2013, 50, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; DiBartolomeo, D.L.; Selkowitz, S.E. Daylighting control performance of a thin-film ceramic electrochromic window: Field study results. Energy Build. 2006, 38, 30–44. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhai, T.T.; Kuang, Z.; Li, J.; Wang, Y.; Gao, Z.; Song, Y.Y.; Xia, X.H. Electrochromic-tuned plasmonics for photothermal sterile window. ACS Nano 2018, 12, 6895–6903. [Google Scholar] [CrossRef]
- Wang, M.; Xing, X.; Perepichka, I.F.; Shi, Y.H.; Zhou, D.Y.; Wu, P.H.; Meng, H. Electrochromic smart windows can achieve an absolute private state through thermochromically engineered electrolyte. Adv. Energy Mater. 2019, 9, 1900433. [Google Scholar] [CrossRef]
- Wu, Q.T. High-tech anti-glare rearview mirror of Jingtai company. Automob. Parts 2007, 11, 47. [Google Scholar]
- Xiao, X.F. Jingtai: The world’s leading supplier of anti-glare rearview mirrors and camera-based active safety systems. Commer. Veh. 2011, 3, 92–95. [Google Scholar]
- Krebs, F.C. The new black. J. Nat. Mater. 2008, 7, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-cleavage-triggered chemosensors and their biological applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. J. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, L.; She, P.; Hou, Y.; Yu, Y.; Zhao, J.; Liu, S.; Zhao, Q. Constructing multi-stimuli-responsive luminescent materials through outer sphere electron transfer in ion pairs. Adv. Opt. Mater. 2019, 7, 1801657. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: Fom new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Mouratis, K.; Tudose, I.V.; Bouranta, A.; Pachiu, C.; Romanitan, C.; Tutunaru, O.; Couris, S.; Koudoumas, E.; Suchea, M. Annealing effect on the properties of electrochromic V2O5 thin films grown by spray deposition technique. Nanomaterials 2020, 10, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, F.J.; Gil-Rostra, J.; Yubero, F.; González-Elipe, A.R. Electrochromism in WOx and WxSiyOz thin films prepared by magnetron sputtering at glancing angles. Nanosci. Nanotechnol. Lett. 2013, 5, 89–93. [Google Scholar] [CrossRef]
- Marciel, A.; Graça, M.; Bastos, A.; Pereira, L.; Kumar, J.S.; Borges, J.; Vaz, F.; Peres, M.; Magalhães, S.; Lorenz, K.; et al. Molybdenum oxide thin films grown on flexible ITO-coated PET substrates. Materials 2021, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, F.J.; Gil-Rostra, J.; Yubero, F.; Espinós, J.P.; Gonzalez-Elipe, A.R.; Chaboy, J. “In operando” X-ray absorption spectroscopy analysis of structural changes during electrochemical cycling of WO3 and WxSiyOz amorphous electrochromic thin film cathodes. J. Phys. Chem. C 2015, 119, 644–652. [Google Scholar] [CrossRef]
- Wen, R.T.; Granqvist, C.; Niklasson, G. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 2015, 14, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.Y.; Li, X.; Qin, M.M.; Pei, Y.F.; Yang, S.; Lan, Y.W.; Wang, R.; Chen, G.M. Effects of pore size of reverse opal structured PEDOT films on their electrochromic performances. Org. Electron. 2017, 50, 16–24. [Google Scholar] [CrossRef]
- Liang, F.Y.; Li, X.; Zhao, N.; Xu, J. Preparation and elcetrochromic properties of SiO2@PANI photonic crystals. Chin. J. Polym. Sci. 2014, 6, 860–864. [Google Scholar]
- Li, N.; Yu, L.N.; Wei, P.P.; Ji, J.Y.; Zhao, J.P.; Zhang, Q.; Li, Y.; Yin, Y.D. Electrochemical fabrication and sensing application of multicolored silver films. Adv. Mater. Interfaces 2018, 5, 1800277. [Google Scholar] [CrossRef]
- Li, N.; Wei, P.P.; Yu, L.N.; Ji, J.Y.; Zhao, J.P.; Gao, C.B.; Li, Y.; Yin, Y.D. Dynamically switchable multicolor electrochromic films. Small 2019, 15, 1804974. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.W.; Liu, L.W.; Li, X.; Chen, K.; Zheng, Y.P.; Xue, Y.M.; Chen, G.M. Fabrication of metal-supramolecular polymers of FeL/carbon nanomaterials with enhanced electrochromic properties. Compos. Sci. Technol. 2020, 198, 108252. [Google Scholar] [CrossRef]
- Liang, F.Y.; Li, X.; Zhang, D.Q. Electrochromic properties of acid dye doped poly(3,4-ethyl-enedioxy-thiophene) by electropolymerization. J. Nanosci. Nanotechnol 2015, 15, 3130–3134. [Google Scholar] [CrossRef]
- Sonmez, G.; Meng, H.; Zhang, Q.; Wudl, F. A highly stable, new electrochromic polymer: Poly(1,4-bis(2-(3′,4′-ethylenedioxy) thienyl)-2-methoxy-5-2″-ethylhexyloxybenzene). Adv. Funct. Mater. 2003, 13, 726–731. [Google Scholar] [CrossRef]
- Electrochromic Materials and Devices; Mortimer, R.J., Rosseinsky, D.R., Monk, P.M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; ISBN 9783527679850. [Google Scholar]
- Shah, K.W.; Wang, S.X.; Soo, D.X.Y.; Xu, J. Viologen-based electrochromic materials: From small molecules, polymers and composites to their applications. Polymers 2019, 11, 1839. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.J.; Zheng, C.N.; Wang, L.X.; Lu, C.B.; Zhang, B.; Chen, Y.; Li, M.Q.; Zhai, G.Q.; Zhuang, X.D. Viologen-inspired functional materials: Synthetic strategies and applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Hwang, E.; Seo, S.; Bak, S.; Lee, H.; Min, M.; Lee, H. An electrolyte-free flexible electrochromic device using electrostatically strong graphene quantum dot-viologen nanocomposites. Adv. Mater. 2014, 26, 5129–5136. [Google Scholar] [CrossRef] [PubMed]
- Giuseppina, A.C.; Sante, C.; Agostina, L.C.; Amerigo, B. Changes in fatigue recovery and muscle damage enzymes after deep-sea water thalassotherapy. Appl. Sci. 2020, 10, 8372–8383. [Google Scholar]
- Yu, H.F.; Chen, K.I.; Yeh, M.H.; Ho, K.C. Effect of trifluoromethyl substituents in benzyl-based viologen on the electrochromic performance: Optical contrast and stability. Sol. Energy Mater Sol. Cells 2019, 200, 110020–110022. [Google Scholar] [CrossRef]
- Dmitrieva, E.; Rosenkranz, M.; Alesanco, Y.; Viñuales, A. UiO66-NH2 as self-sacrificing template for Fe/N-doped hierarchically porous carbon with high electrochemical performance for oxygen reduction in microbial fuel cells. Electrochim. Acta. 2019, 323, 134792–134799. [Google Scholar] [CrossRef]
- Marek, L.; Waclaw, G.; Carl, W.S. The electrochemical properties of some alkylviologens. J. Electroanal. 1998, 10, 486–491. [Google Scholar]
- Cook, S.K.; Horrocks, B.R. Heterogeneous electron-transfer rates for the reduction of viologen derivatives at platinum and bismuth electrodes in acetonitrile. Chem. Electro. Chem. 2017, 4, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, B.; Wang, J.; Zhao, H.; Ma, W.; Xu, L.; Zhang, W.; Zhou, K.; Du, Y.; He, G. Electrochromic poly(chalcogenoviologen)s as anode materials for high-performance organic radical lithium-ion batteries. Angew. Chem. Int. Ed. 2019, 58, 8468–8473. [Google Scholar] [CrossRef]
- Gorkem, E.G.; Levent, T. A unique processable green polymer with a transmissive oxidized state for realization of potential rgb-based electrochromic device applications. J. Adv. Funct. Mater. 2008, 18, 2026–2030. [Google Scholar]
- Oh, H.; Seo, D.G.; Yun, T.Y.; Kim, C.Y.; Moon, H.C. Voltage-Tunable Multicolor, Sub-1.5 V, Flexible electrochromic devices based on ion gels. Appl. Mater. Interfaces 2017, 9, 7658–7665. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Vasantha, V.S.; Ho, K.-C. A study of ion exchange at the poly(butyl viologen)-electrolyte interface by SECM. Electrochim. Acta 2008, 53, 6244–6251. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; Li, C.J.J. Synthesis, characterization and electrochromic property research of symmetric viologen. J. Beijing Inst. Fashion. Tech. 2013, 33, 5–11. [Google Scholar]
- Jiang, Q.L. The synthesis of viologen derivates and solid electrochromic devices. Master’s Thesis, Southwest University, Chong Qing, China, 2007. [Google Scholar]
- Liang, F.Y.; Dong, Z.Y.; Li, X. Preparation and characterization of poly(3,4-dibromothiophene) and its polymer electrochromic device. J. Beijing Inst. Fashion Tech. 2012, 32, 8–13. [Google Scholar]



| Substituent Viologens | First Oxidation Potential Epa1(V) | Second Oxidation Potential Epa2(V) | First Reduction Potential Epc1(V) | Second Reduction Potential Epc2(V) |
|---|---|---|---|---|
| OV | −0.34 | −0.60 | −0.62 | −0.98 |
| DeV | −0.27 | −0.62 | −0.55 | −0.86 |
| DoV | −0.24 | −0.62 | −0.50 | −0.94 |
| HV | −0.24 | −0.70 | −0.47 | −0.95 |
| BV | −0.21 | −0.87 | −0.39 | −0.90 |
| Viologen Films | Color Variations | Potential/V | L * | a * | b * | ΔEab * |
|---|---|---|---|---|---|---|
| OV |  | −0.9 −0.6 | 62.17 69.38 | −6.43 35.22 | 33.12 −11.70 | 61.61 |
| DeV |  | −0.9 −0.6 | 53.37 56.27 | 31.52 3.69 | −49.34 −12.67 | 45.88 |
| DoV | 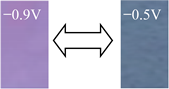 | −0.9 −0.5 | 56.38 61.25 | 20.45 −2.86 | −4.36 −32.14 | 36.59 |
| HV |  | −0.9 −0.5 | 59.34 63.70 | 2.58 23.15 | −41.21 −43.47 | 21.25 |
| BV | 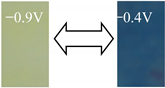 | −0.9 −0.4 | 48.86 54.37 | −12.53 9.35 | 10.68 −37.76 | 53.44 |
| Viologen Films | RTb (s) | RTc (s) | ΔTmax (%) |
|---|---|---|---|
| OV | 0.95 | 8.16 | 33 |
| DeV | 6.72 | 5.42 | 22 |
| DoV | 5.38 | 4.35 | 20 |
| HV | 4.25 | 3.23 | 15 |
| BV | 5.94 | 4.56 | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Yuan, L.; Guan, F.; Li, X.; Wang, R.; Xu, J.; Qin, Y.; Chen, G. Substituent-Adjusted Electrochromic Behavior of Symmetric Viologens. Materials 2021, 14, 1702. https://doi.org/10.3390/ma14071702
Zhang Q, Yuan L, Guan F, Li X, Wang R, Xu J, Qin Y, Chen G. Substituent-Adjusted Electrochromic Behavior of Symmetric Viologens. Materials. 2021; 14(7):1702. https://doi.org/10.3390/ma14071702
Chicago/Turabian StyleZhang, Qun, Li Yuan, Fanglan Guan, Xin Li, Rui Wang, Jian Xu, Yanyan Qin, and Guangming Chen. 2021. "Substituent-Adjusted Electrochromic Behavior of Symmetric Viologens" Materials 14, no. 7: 1702. https://doi.org/10.3390/ma14071702
APA StyleZhang, Q., Yuan, L., Guan, F., Li, X., Wang, R., Xu, J., Qin, Y., & Chen, G. (2021). Substituent-Adjusted Electrochromic Behavior of Symmetric Viologens. Materials, 14(7), 1702. https://doi.org/10.3390/ma14071702







