The Effect of Liquid Slurry-Enhanced Corrosion on the Phase Composition of Selected Portland Cement Pastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples from Ordinary Portland Cement (OPC) Pastes
2.2. Conditions of the Biological Corrosion Process
2.3. Characterization Methods
3. Results and Discussion
3.1. Sample Mass over the Exposure Time of Six Weeks
3.2. Phase Composition of Cement Pastes (Corrosion and Hydration Products)
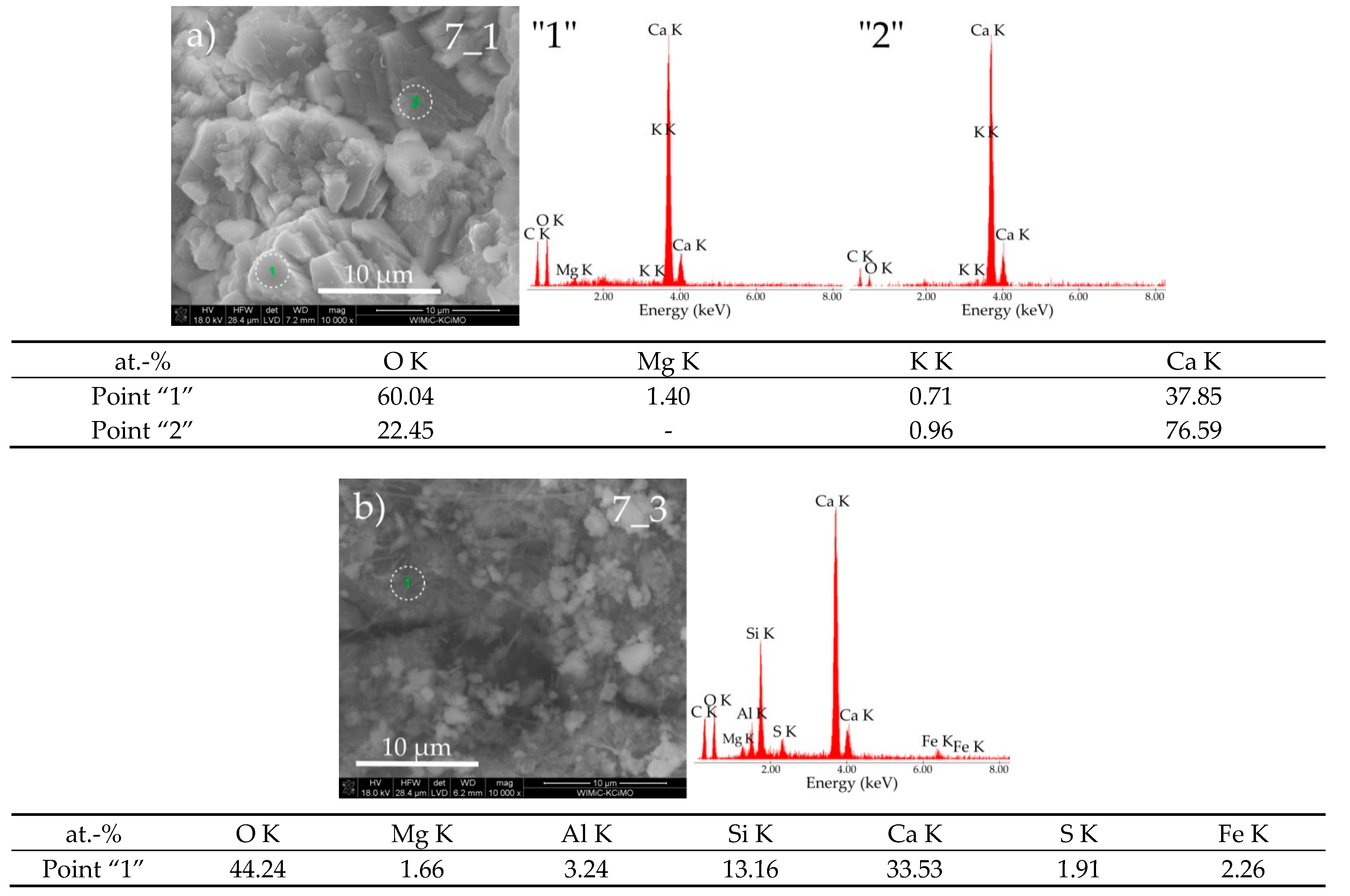
3.3. Sample Morphologies and the Chemical Composition of Corrosion Products
3.4. Biological and Biochemical Aspects of the Corrosion Process-Taumasite Formation Conditions
Ca6[Si(OH)6]2(CO3)2(SO4)2⋅24H2O + CaSO4⋅2H2O + Al2O3⋅xH2O + 3Ca(OH)2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilarski, K.; Pilarska, A.; Witaszek, K.; Dworecki, Z.; Żelaziński, T.; Ekielski, A.; Makowska, A.; Michniewicz, J. The impact of extrusion on the biogas and biomethane yield of plant substrates. J. Ecol. Eng. 2016, 17, 264–272. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Ryniecki, A.; Tomaszyk, K.; Dach, J.; Wolna-Maruwka, A. Utilization of vegetable dumplings waste from industrial production by anaerobic digestion. Int. Agrophys. 2017, 31, 93–102. [Google Scholar] [CrossRef]
- Szymanska, M.; Sosulski, T.; Szara, E.; Pilarski, K. Conversion and properties of anaerobic digestates from biogas production. Przemysl Chem. 2015, 94, 1419–1423. [Google Scholar]
- Dhawan, S.; Bhalla, S.; Bhattacharjee, B. Reinforcement Corrosion in Concrete Structures and Service Life Predictions—A Review. In Proceedings of the 9th International Symposium on Advanced Science and Technology in Experimental Mechanics, New Delhi, India, 1–6 November 2014. [Google Scholar]
- Loreto, G.; Di Benedetti, M.; Iovino, R.; Nanni, A.; Gonzalez, M. Evaluation of corrosion effect in reinforced concrete by chloride exposure. Int. Soc. Opt. Eng. 2011. [Google Scholar] [CrossRef]
- Iverson, W.P. Direct Evidence for the Cathodic Depolarization Theory of Bacterial Corrosion. Science 1966, 151, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.A.; Ray, R.I. Surface analytical techniques for microbiologically influenced corrosion. A review. In Microhiologically Influenced Corrosion Testing; Kearns, J.R., Little, B.J., Eds.; ASTM Publications STP 1232; American Society for Testing and Materials: Philadelphia, PA, USA, 1994; Volume 53. [Google Scholar]
- Kiesse, T.S.; Bonnet, S.; Amiri, O.; Ventura, A. Analysis of corrosion risk due to chloride diffusion for concrete structures in marine environment. Mar. Struct. 2020, 73, 102804. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Q.; Xu, S.; Wang, N.; Zheng, X. Fabrication of superhydrophobic concrete used in marine environment with anti-corrosion and stable mechanical properties. Constr. Build. Mater. 2020, 251, 118946. [Google Scholar] [CrossRef]
- Grengg, C.; Müller, B.; Staudinger, C.; Mittermayr, F.; Breininger, J.; Ungerböck, B.; Borisov, S.M.; Mayr, T.; Dietzel, M. High-resolution optical pH imaging of concrete exposed to chemically corrosive environments. Cem. Concr. Res. 2019, 116, 231–237. [Google Scholar] [CrossRef]
- Jiang, G.; Zhou, M.; Chiu, T.H.; Sun, X.; Keller, J.; Bond, P.L. Wastewater-Enhanced Microbial Corrosion of Concrete Sewers. Environ. Sci. Technol. 2016, 50, 8084–8092. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Wu, K.; Kan, L. Microbiologically induced corrosion of concrete in sewer structures: A review of the mechanisms and phenomena. Constr. Build. Mater. 2020, 239, 117813. [Google Scholar] [CrossRef]
- Taheri, S.; Ams, M.; Bustamante, H.; Vorreiter, L.; Bevitt, J.J.; Withford, M.; Clark, S.M. Characterizing concrete corrosion below sewer tidal levels at chemically dosed locations. Water Res. 2020, 185, 116245. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bond, P.L.; O’Moore, L.; Wilkie, S.; Hanzic, L.; Johnson, I.; Mueller, K.; Yuan, Z.; Jiang, G. Increased Resistance of Nitrite-Admixed Concrete to Microbially Induced Corrosion in Real Sewers. Environ. Sci. Technol. 2020, 54, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Standard PN-EN 197-1:2012. Cement—Part 1: Composition, Requirements and Compliance Criteria for Common Cements. Available online: https://sklep.pkn.pl/pn-en-197-1-2012p.html (accessed on 14 October 2013).
- Diamond, S. Cement Paste Micostructure—An Overview at Several Levels. In Proceedings of the Conference on Hydraulic Cement Paste: Their Structure and Properties, Sheffield, UK, 8–9 April 1976. [Google Scholar]
- Taumasite Expert Group: The Taumasite from of Sulfate Attack: Risks, Diagnosis, Remedial Works, and Guidance on New Construction; Raport of the Taumasite Expert Group; DETR: London, UK, 1999.
- Van Aardt, J.H.P.; Visser, S. Taumasite formation: A cause of deterioration of Portland cement and related substances in the presence of sulphates. Cem. Concr. Res. 1975, 5, 225–232. [Google Scholar] [CrossRef]
- Irassar, E.; Bonavetti, V.; González, M. Microstructural study of sulfate attack on ordinary and limestone Portland cements at ambient temperature. Cem. Concr. Res. 2003, 33, 31–41. [Google Scholar] [CrossRef]
- Skalny, J.; Marchand, J.M.; Odler, I. Sulfate Attack on Concrete; Spon Press: London, UK; New York, NY, USA, 2002. [Google Scholar]
- Bensted, J. Taumasite sulphate attack—Its scientific background and ramifications in constructions. In Proceedings of the Conference Materials of the Symposium Kurdowski—Science of Cement and Concrete, Kraków, Poland, 20–21 June 2001; pp. 189–198. [Google Scholar]
- Kohler, S.; Heinz, D.; Urbonas, L. Effect of ettringite on taumasite formation. Cem. Concr. Res. 2006, 36, 697–706. [Google Scholar] [CrossRef]
- Blanco-Varela, M.T.; Aguilera, J.; Martınez-Ramırez, S. Effect of cement C3A content, temperature, and storage medium on taumasite formation in carbonated mortars. Cem. Concr. Res. 2006, 36, 707–715. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look. Part 1: Summary of experimental results. Cem. Concr. Res. 2002, 32, 915–921. [Google Scholar] [CrossRef]

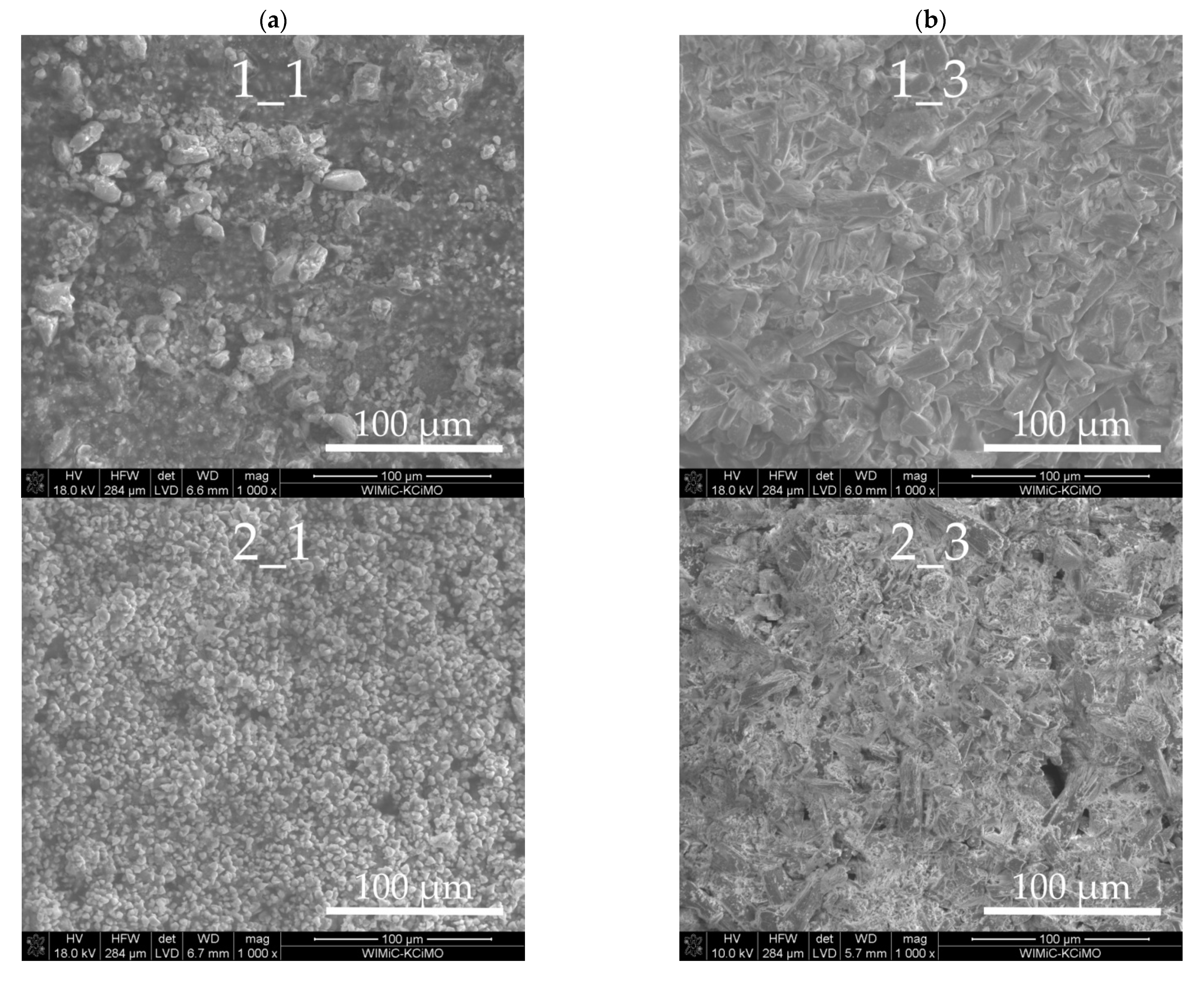
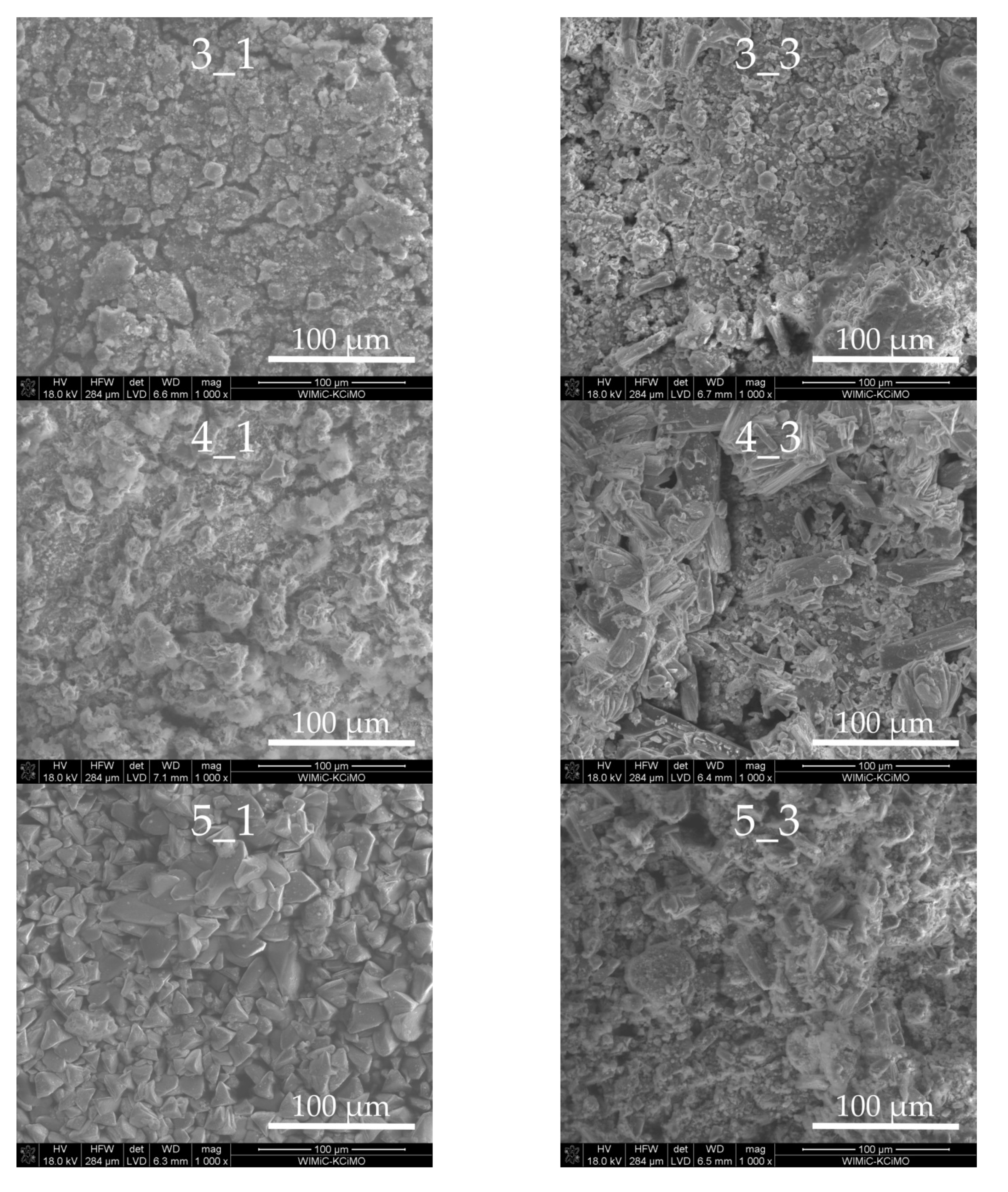
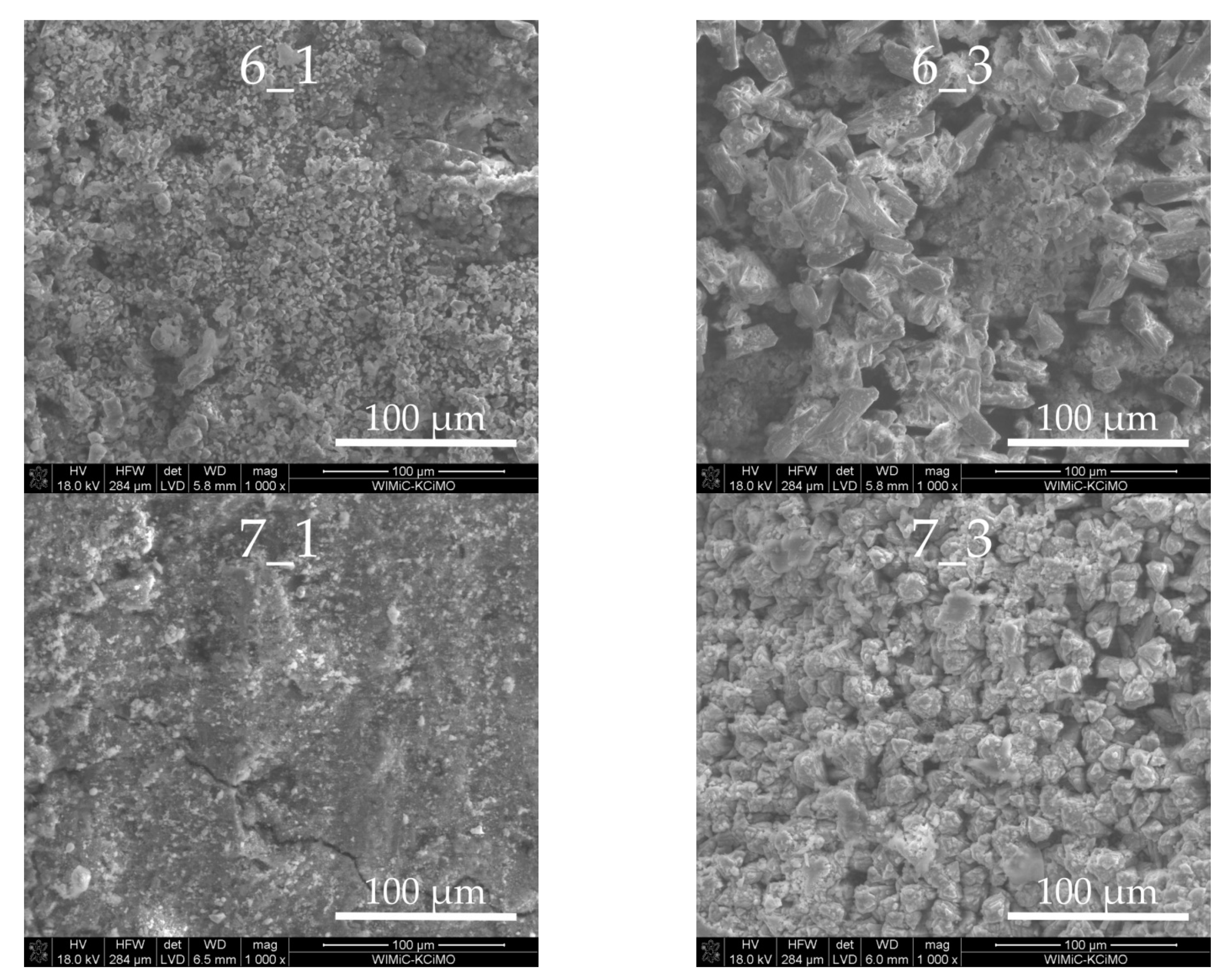

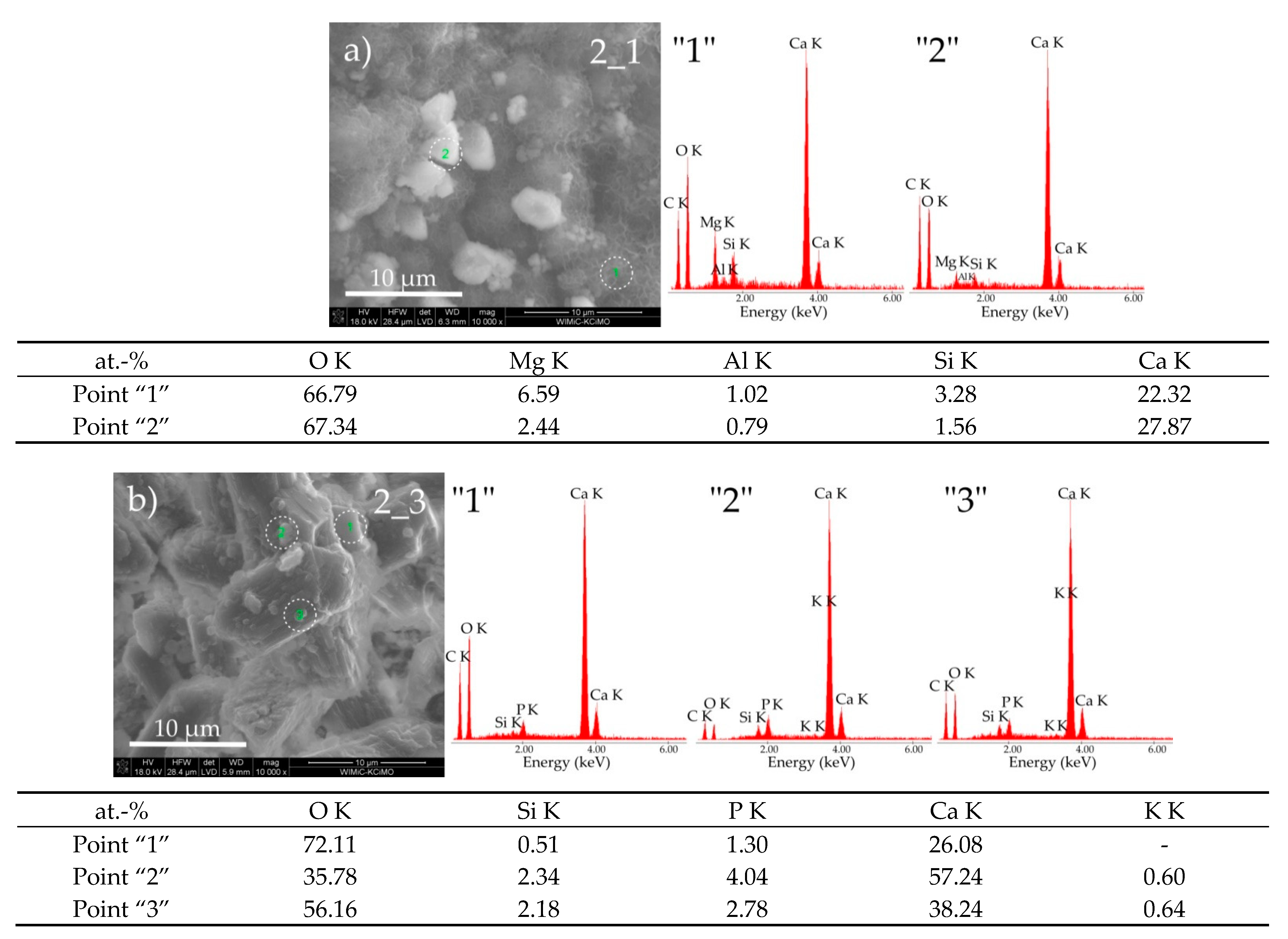
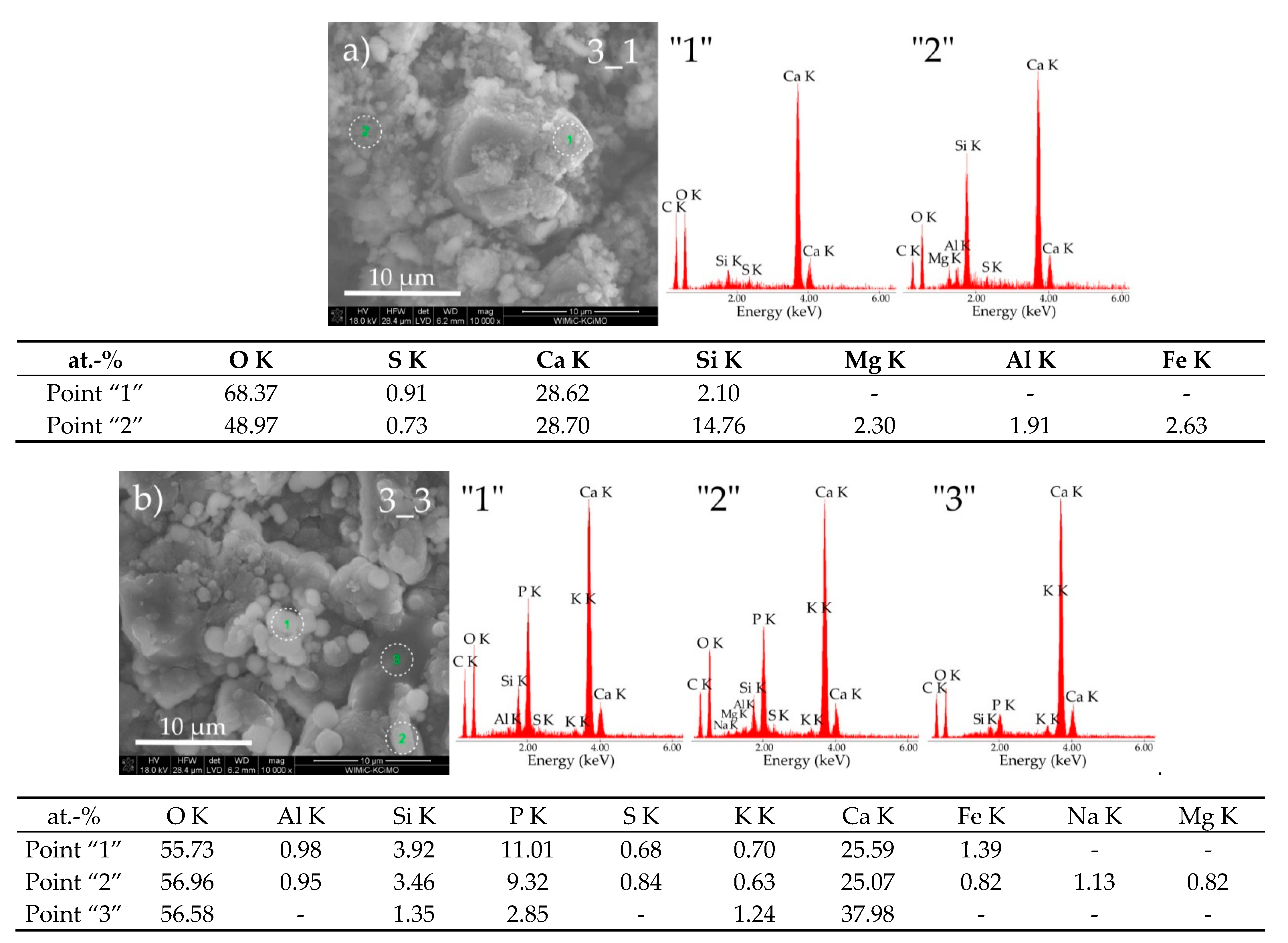
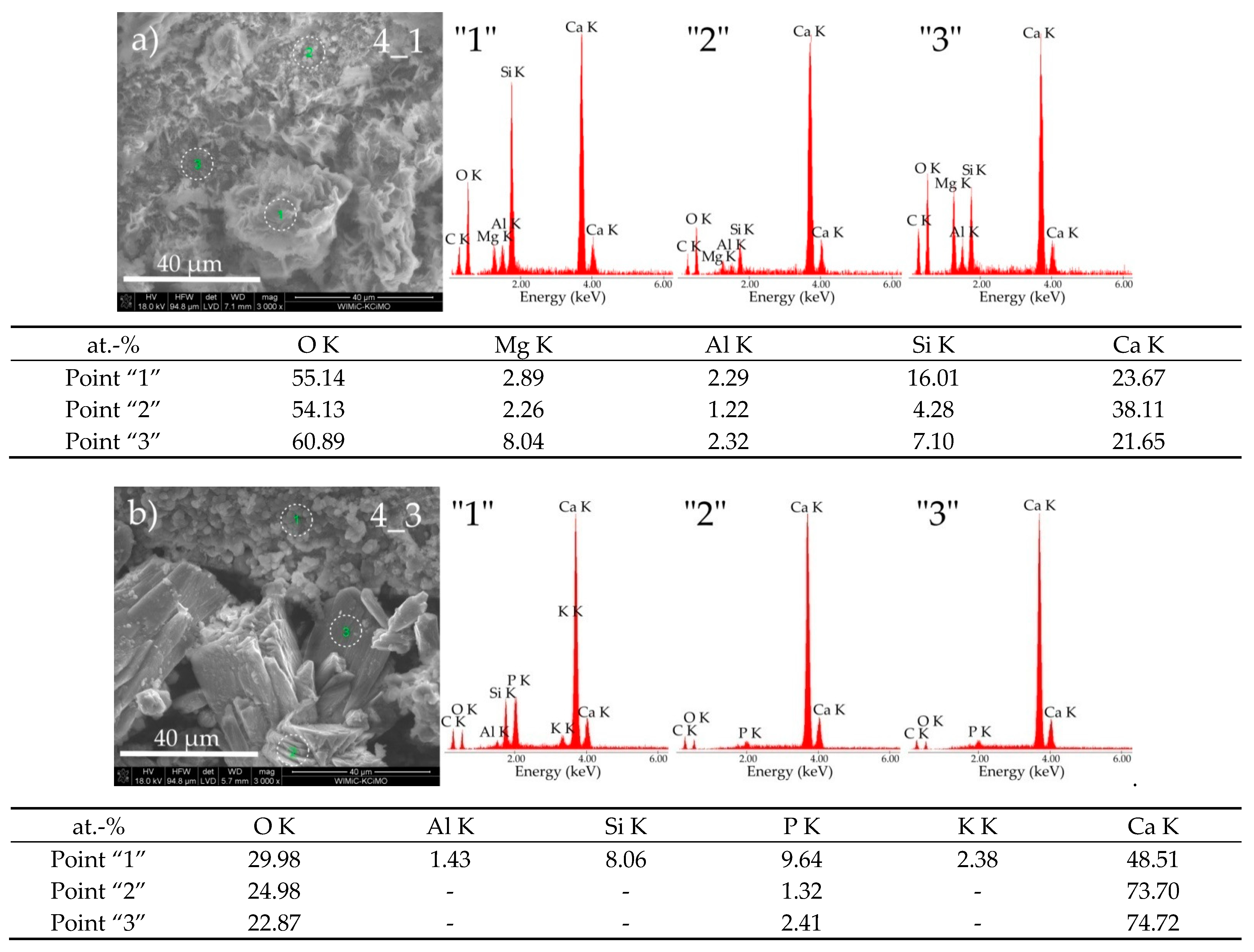
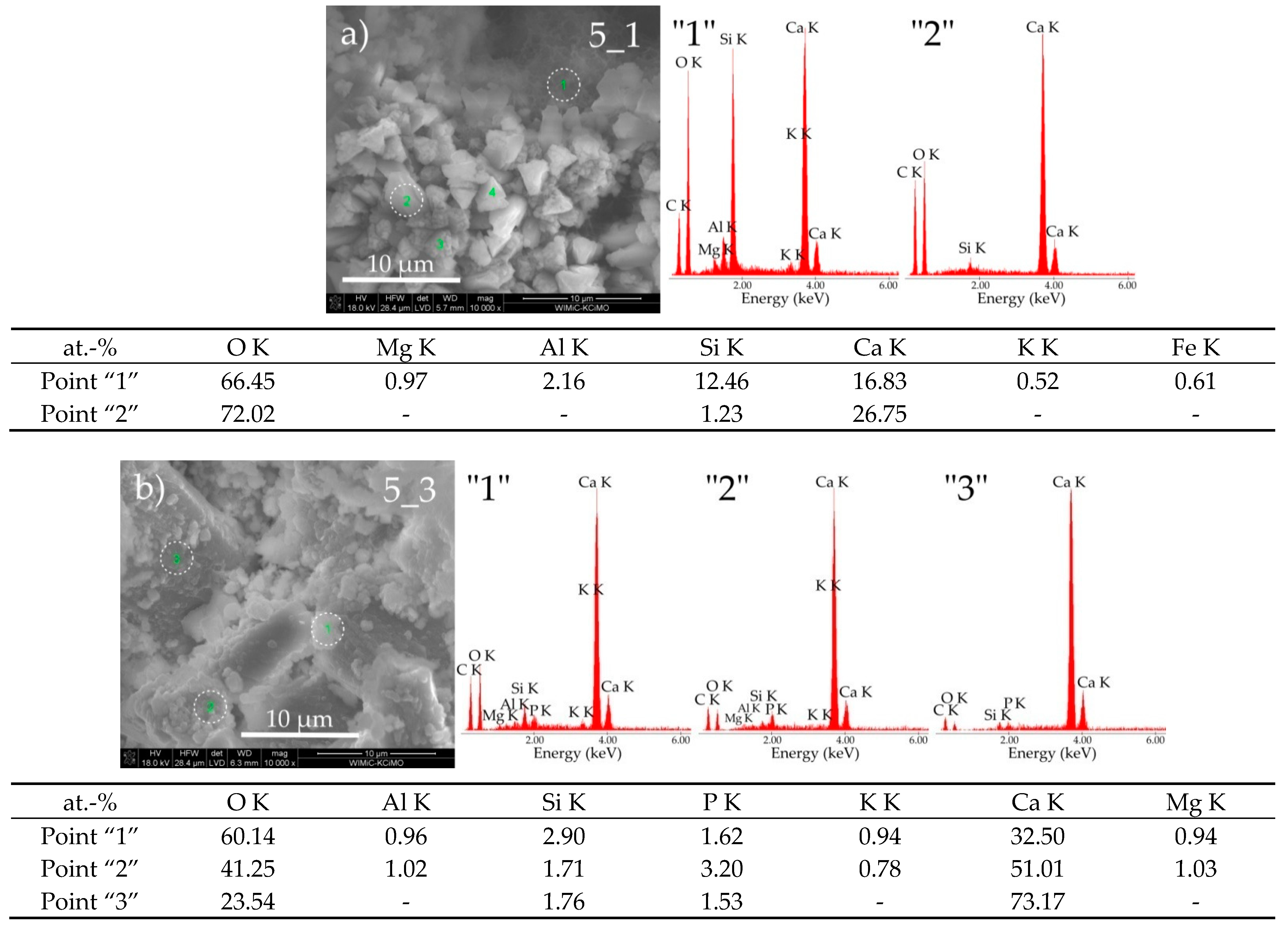
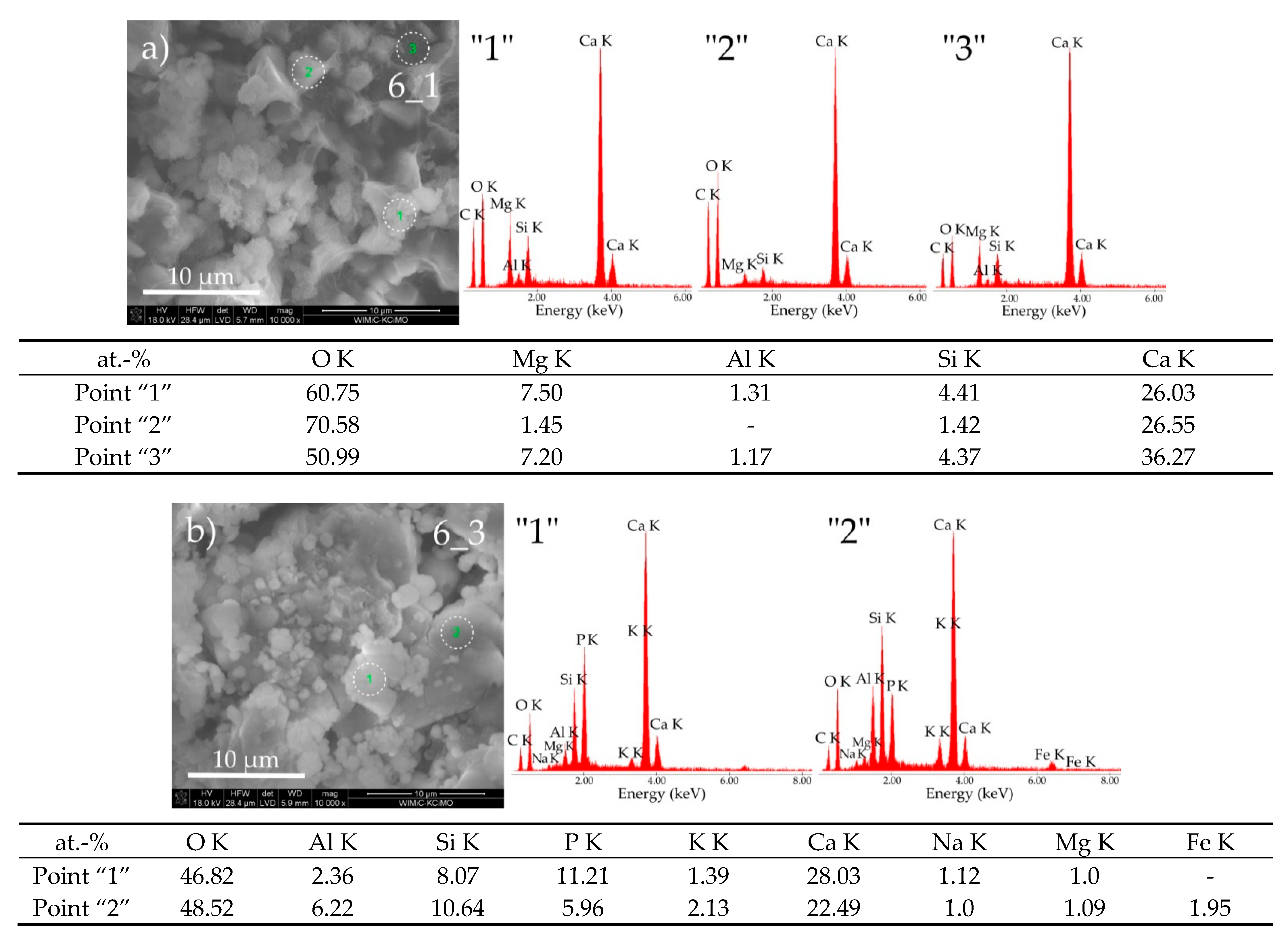
| Oxides/ Ions [%] | Chełm CEM I 42.5N | Rudniki CEM I 42.5R | Górażdze CEM I 42.5R | Ożarów CEM I 42.5N | Odra CEM I 42.5R | Warta CEM I 42.5R | Małogoszcz CEM I 42.5R |
|---|---|---|---|---|---|---|---|
| 1_3 | 2_3 | 3_3 | 4_3 | 5_3 | 6_3 | 7_3 | |
| SiO2 | 21.70 | 20.81 | 21.66 | 21.56 | 19.36 | 22.47 | 24.27 |
| Al2O3 | 3.30 | 4.69 | 5.14 | 4.76 | 5.75 | 6.05 | 4.29 |
| Fe2O3 | 4.56 | 3.77 | 2.77 | 3.14 | 2.83 | 2.72 | 2.80 |
| CaO | 65.52 | 65.79 | 65.36 | 64.94 | 66.99 | 63.67 | 64.01 |
| MgO | 1.12 | 1.33 | 1.46 | 1.92 | 1.68 | 1.91 | 1.10 |
| SO3 | 3.14 | 2.65 | 2.52 | 2.63 | 2.20 | 2.12 | 2.30 |
| K2O | 0.41 | 0.77 | 0.86 | 0.71 | 1.05 | 0.80 | 1.10 |
| Na2O | 0.20 | 0.14 | 0.15 | 0.30 | 0.11 | 0.20 | 0.10 |
| Cl− | 0.05 | 0.05 | 0.08 | 0.04 | 0.03 | 0.06 | 0.03 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Parameter | Unit | Value |
|---|---|---|
| Arsen | mg/L | <0.001 |
| Nitrates | mg/L | 1.60 ± 0.03 |
| Cyanides | mg/L | <0.005 |
| Fluorides | mg/L | 0.41 ± 0.01 |
| Magnesium | mg/L | 14.00 ± 0.11 |
| Copper | mg/L | <0.003 |
| Lead | mg/L | <0.001 |
| Mercury | mg/L | <0.0001 |
| Sulfate | mg/L | 175.0 ± 12.1 |
| Total hardness CaCO3 | mg/L | 377.0 ± 18.6 |
| Calcium | mg/L | 87.0 ± 1.9 |
| Iron | mg/L | 0.20 ± 0.01 |
| Total trihalomethanes (THM) | µg/L | 3.0 ± 0.5 |
| Total chlorates and chlorites | mg/L | 0.100 ± 0.006 |
| Parameter | Unit | Value |
|---|---|---|
| Kjeldahl total nitrogen | mg/L | 1240.0 ± 60.4 |
| Ammonium nitrogen | mg/L | 1050.0 ± 51.1 |
| Total nitrogen | mg/L | 1350.0 ± 63.5 |
| Nitrite nitrogen | mg/L | 0.032 ± 0.003 |
| Nitrate nitrogen | mg/L | 0.31 ± 0.05 |
| Chrome | mg/L | 0.40 (-) * |
| Cadmium | mg/L | 0.05 ± 0.01 |
| Nickel | mg/L | 0.09 ± 0.01 |
| Lead | mg/L | <0.5 |
| Mercury | mg/L | <0.003 |
| Calcium | mg/L | 68 (-) * |
| Magnesium | mg/L | 4.37 ± 0.87 |
| Total phosphorous | mg/L | 352 (-) |
| Potassium | mg/L | 684.0 ± 15.4 |
| Dry mass | mg/L | 1.31 ± 0.06 |
| Sample | I–Initial Mass | Uncertainty of the Result [±] | II–Exposure Time Two Weeks | Uncertainty of the Result [±] | III–Exposure Time Four Weeks | Uncertainty of the Result [±] | IV–Exposure Time Six Weeks | Uncertainty of the Result [±] |
|---|---|---|---|---|---|---|---|---|
| Chełm (1) | 136 | 0.26 | 142 | 0.27 | 138 | 0.26 | 137 | 0.26 |
| Rudniki (2) | 139 | 0.26 | 139 | 0.26 | 138 | 0.26 | 140 | 0.26 |
| Górażdże (3) | 139 | 0.26 | 133 | 0.25 | 136 | 0.26 | 138 | 0.26 |
| Ożarów (4) | 138 | 0.26 | 136 | 0.26 | 135 | 0.25 | 138 | 0.26 |
| Odra (5) | 143 | 0.27 | 143 | 0.27 | 141 | 0.27 | 141 | 0.27 |
| Warta(6) | 136 | 0.26 | 139 | 0.26 | 135 | 0.25 | 134 | 0.25 |
| Małogoszcz (7) | 137 | 0.26 | 136 | 0.26 | 135 | 0.25 | 135 | 0.25 |
| Sample | Phase Composition/Abbreviation/ICDD * | ||||
|---|---|---|---|---|---|
| Ca(OH)2 /P/ [4–773] * | CaCO3/K/ [24–27] * | C2S/L/ [33–302] * | C3A/G/ [38–1429] * | C3S·CO2·SO3·15H2O/T/[46–1360] * | |
| 1_3 | + | + | + | − | + |
| 2_3 | + | + | + | − | + |
| 3_3 | + | + | + | − | + |
| 4_3 | + | + | + | + | − |
| 5_3 | + | + | + | + | − |
| 6_3 | + | + | + | + | − |
| 7_3 | + | + | + | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durczak, K.; Pyzalski, M.; Pilarski, K.; Brylewski, T.; Sujak, A. The Effect of Liquid Slurry-Enhanced Corrosion on the Phase Composition of Selected Portland Cement Pastes. Materials 2021, 14, 1707. https://doi.org/10.3390/ma14071707
Durczak K, Pyzalski M, Pilarski K, Brylewski T, Sujak A. The Effect of Liquid Slurry-Enhanced Corrosion on the Phase Composition of Selected Portland Cement Pastes. Materials. 2021; 14(7):1707. https://doi.org/10.3390/ma14071707
Chicago/Turabian StyleDurczak, Karol, Michał Pyzalski, Krzysztof Pilarski, Tomasz Brylewski, and Agnieszka Sujak. 2021. "The Effect of Liquid Slurry-Enhanced Corrosion on the Phase Composition of Selected Portland Cement Pastes" Materials 14, no. 7: 1707. https://doi.org/10.3390/ma14071707
APA StyleDurczak, K., Pyzalski, M., Pilarski, K., Brylewski, T., & Sujak, A. (2021). The Effect of Liquid Slurry-Enhanced Corrosion on the Phase Composition of Selected Portland Cement Pastes. Materials, 14(7), 1707. https://doi.org/10.3390/ma14071707








