Synthesis of Co–Fe 1D Nanocone Array Electrodes Using Aluminum Oxide Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of AAO Templates
2.2. Electrodeposition of Metal and Alloys
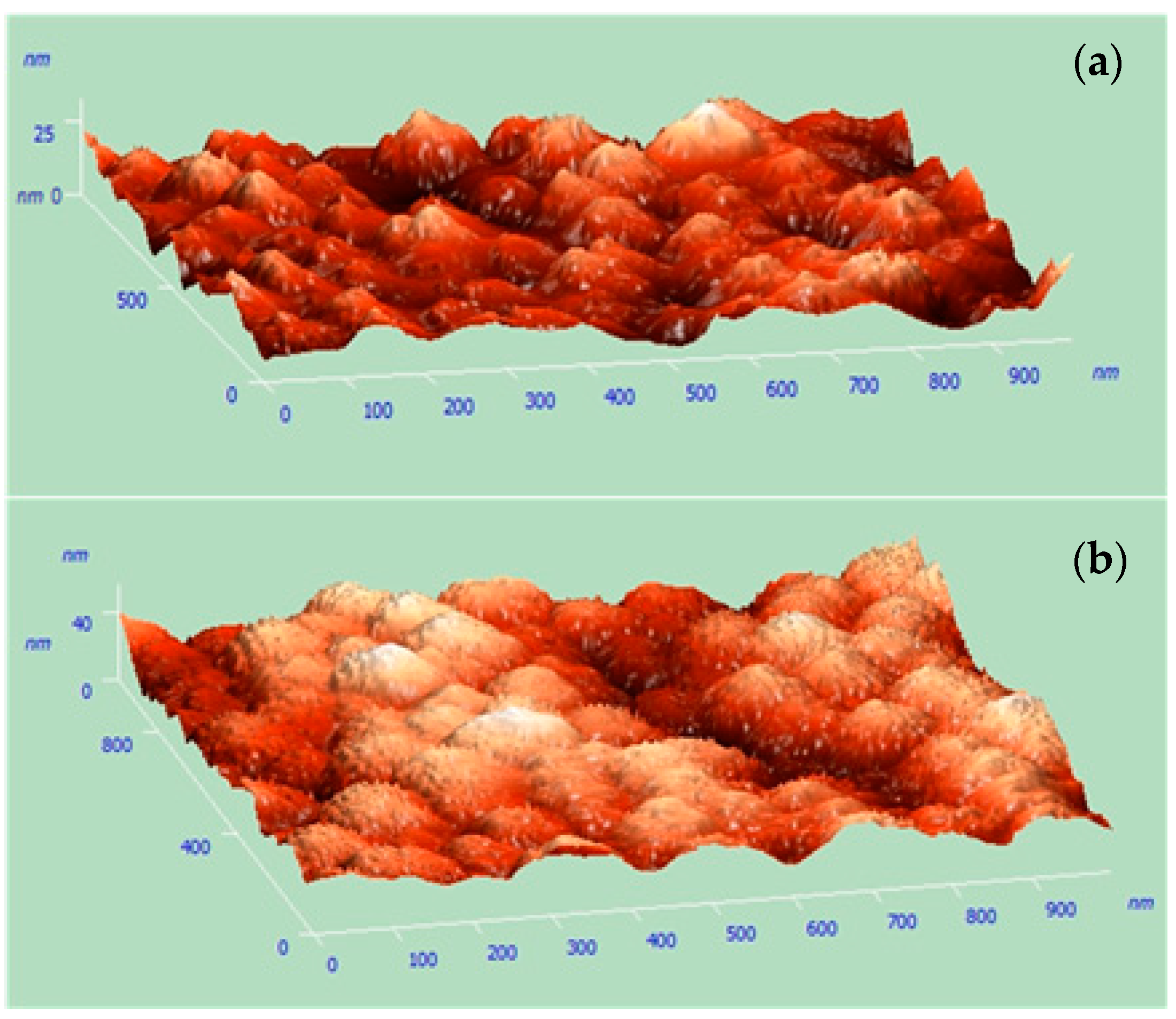
2.3. Microstructal Charaterization
2.4. Electrochemical Characterization
3. Results
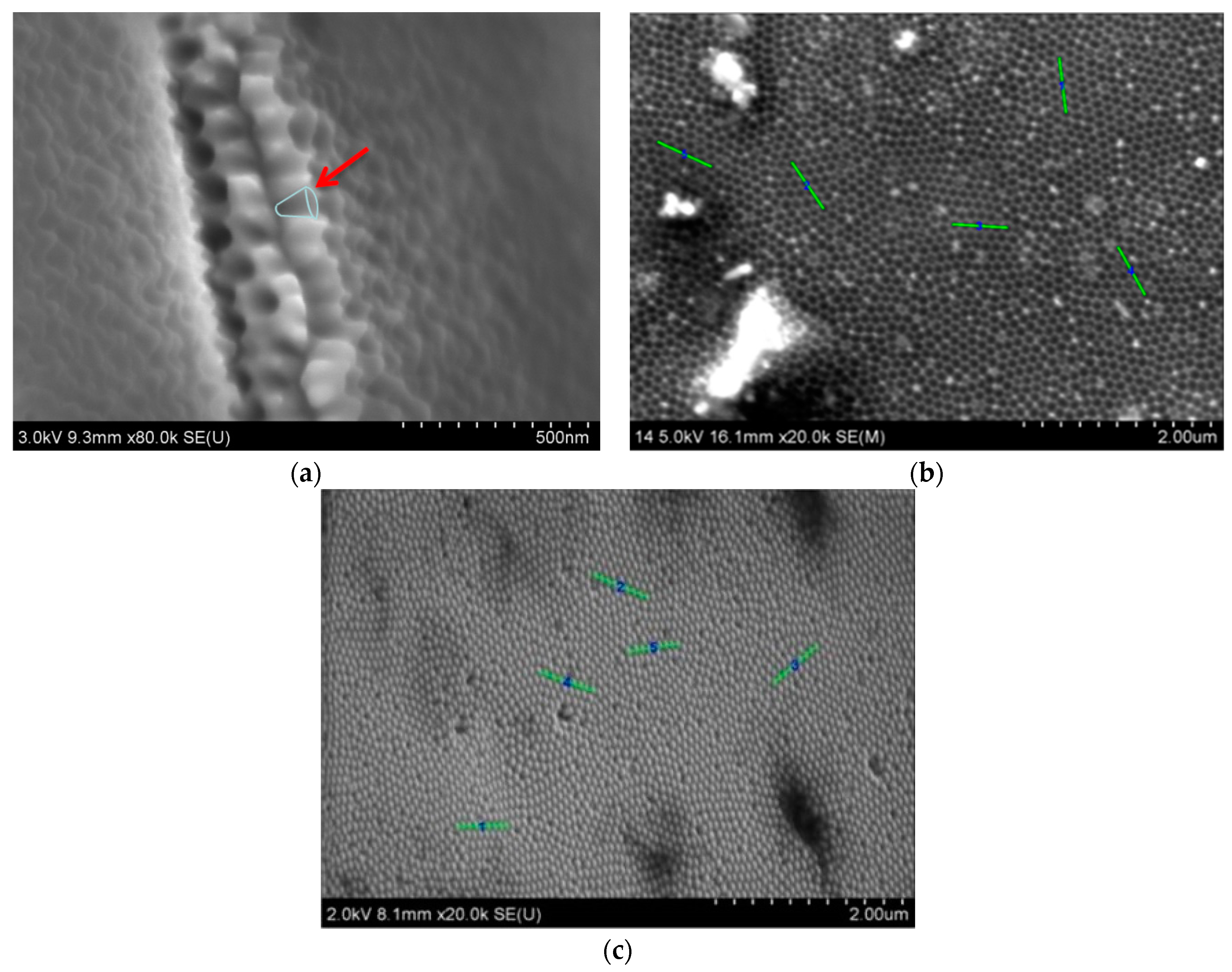
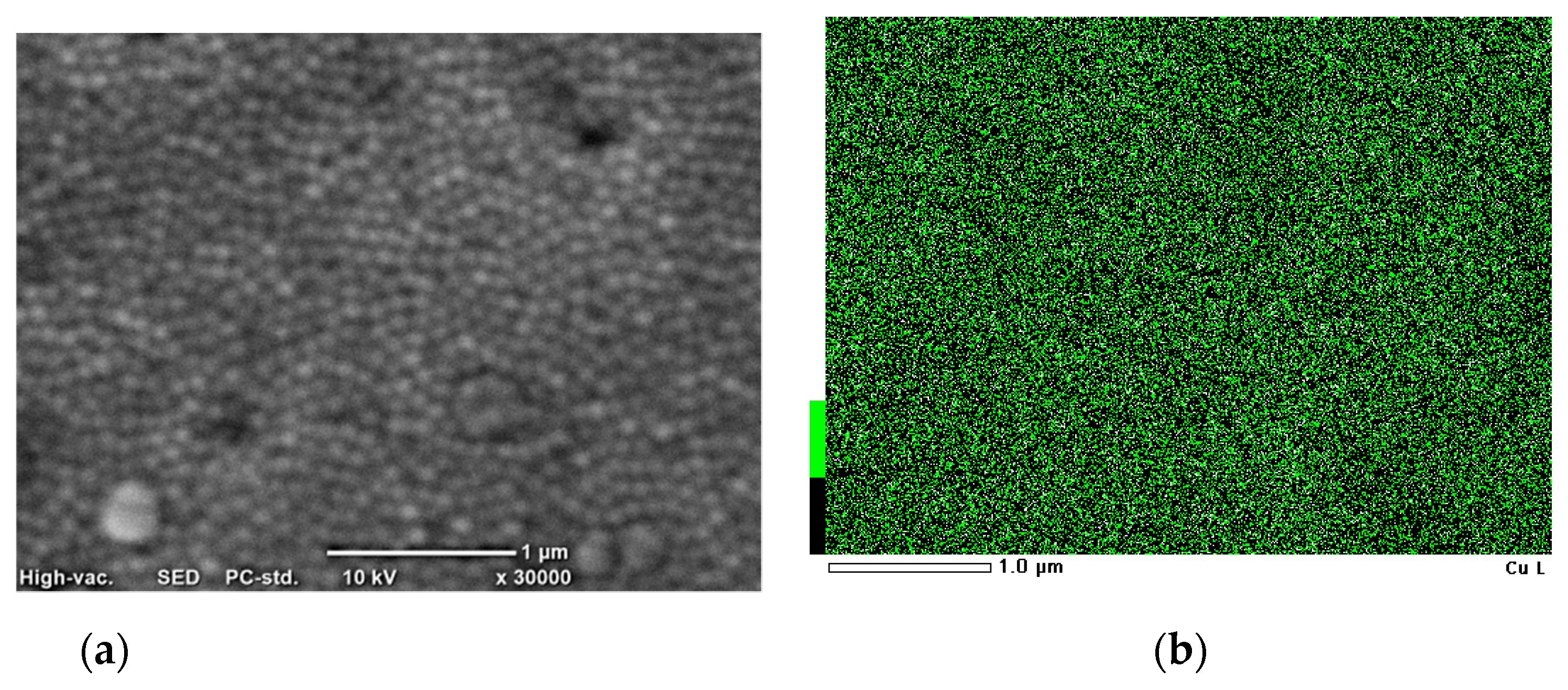
3.1. Characterization of Cu Nanocones
3.2. Electrodeposition of Co–Fe Alloys
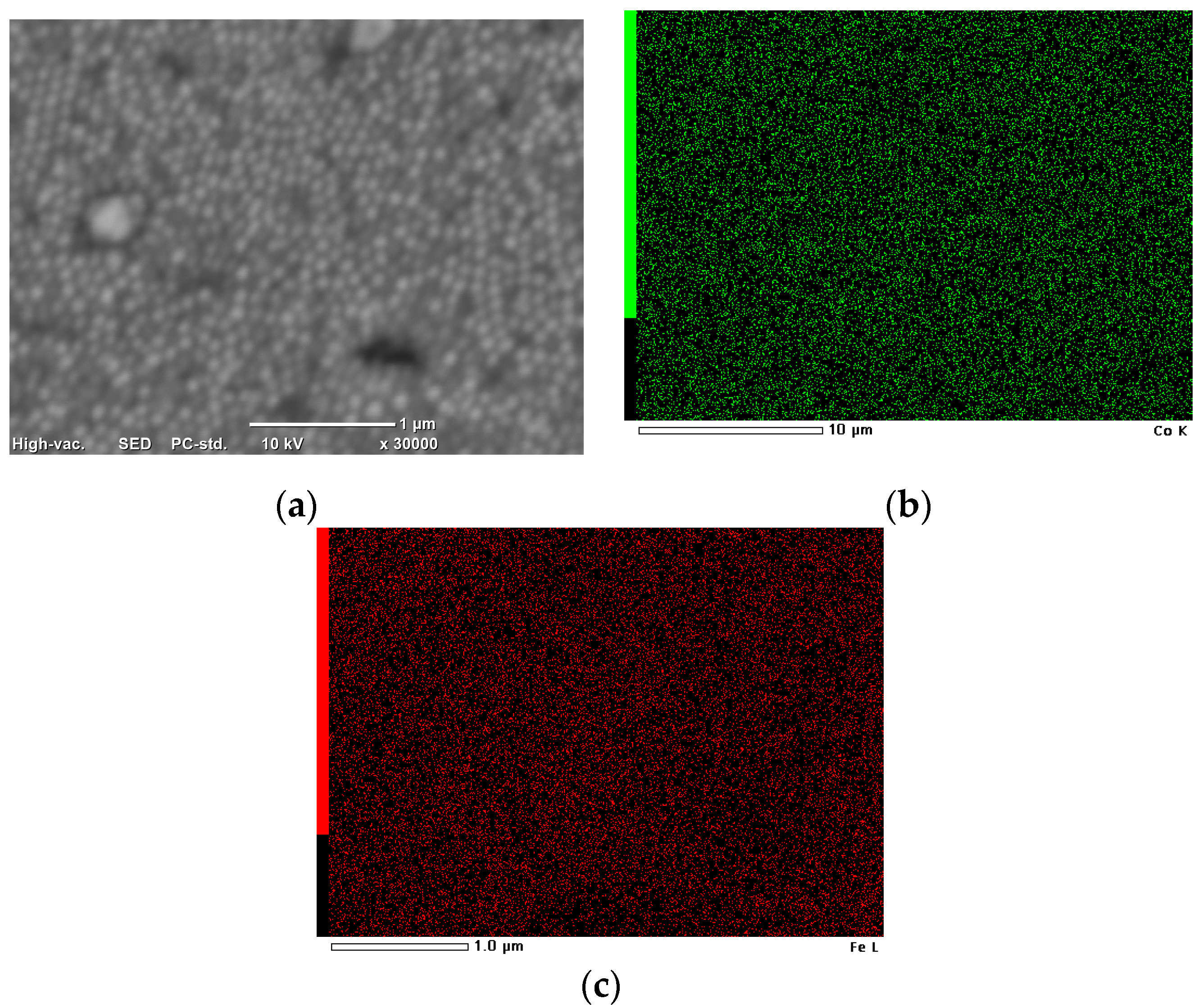
3.3. Synthesis of Co–Fe Nanocones
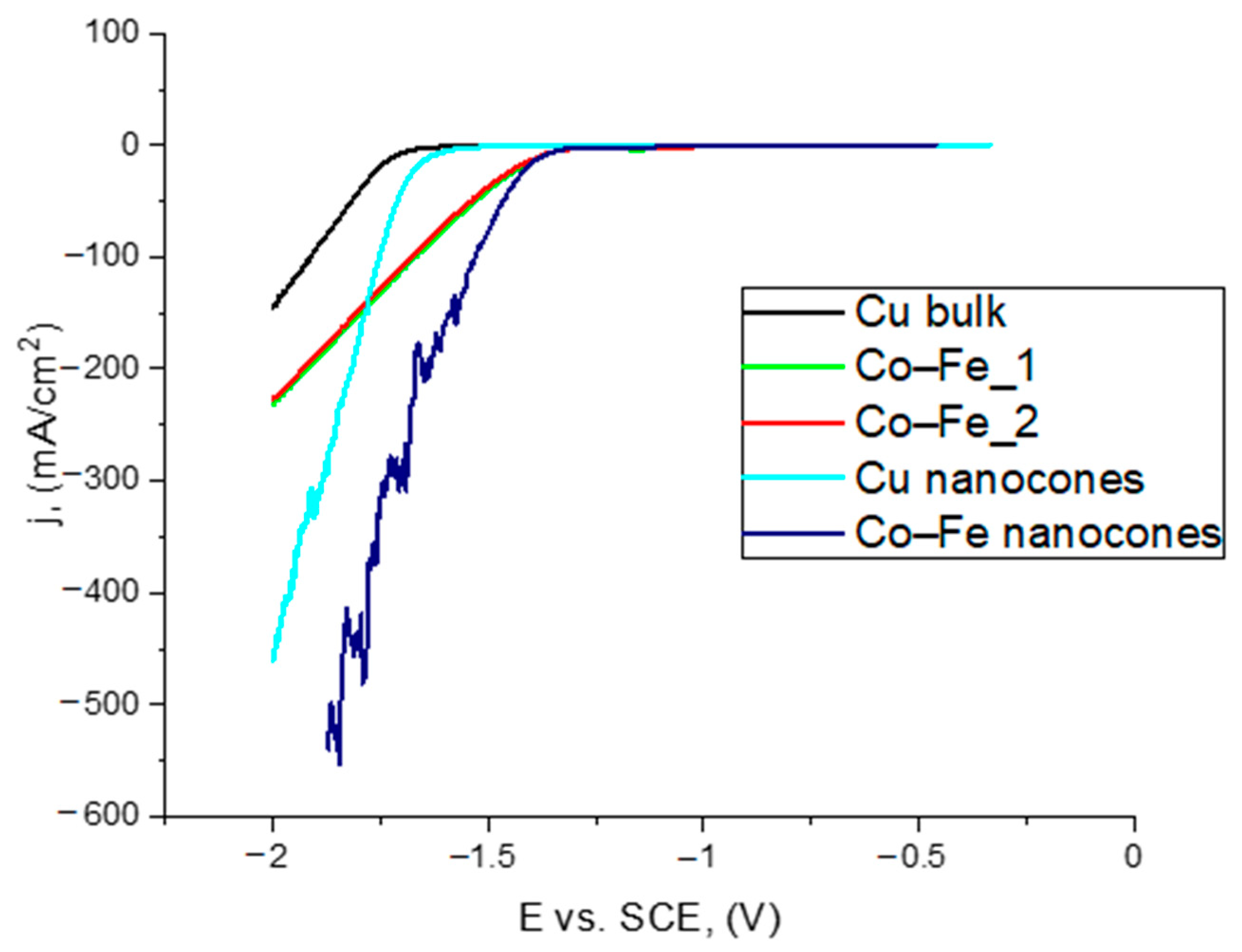
3.4. Catalytic Examination of Nanocones in a Hydrogen Evolution Reaction
4. Conclusions
- It is possible to get nanocones of pure Cu, which was confirmed by mapping analysis. There was no influence from the acid used on the distribution of Cu;
- The copper nanocones showed better electrocatalytic activity than the copper bulk. This was connected with the larger active surface area;
- Co–Fe alloys with different compositions and morphologies were successfully electrodeposited;
- The Co–Fe nanocones were obtained by electrodeposition on templates. The obtained alloy was characterized by a uniform distribution of Co and Fe;
- The nanostructures increased the active surface area of the electrodes;
- The Co–Fe nanocones showed the best electrocatalytic properties. The LSV curve had a sharp character, which may have been caused by the evolution of hydrogen and the active area being blocked by hydrogen bubbles. However, there was no significant increase in the electrocatalytic properties for the Co–Fe nanostructures compared with the bulk materials.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Wei, G.; Yu, Y.; Guo, C.; Jiang, L.; Yu, N. Aluminum alloy AA2024 anodized from the mixed acid system with enhanced mechanical properties. Surf. Interfaces 2018, 13, 46–50. [Google Scholar] [CrossRef]
- Sadeler, R. Effect of a commercial hard anodizing on the fatigue property of a 2014-T6 aluminium alloy. J. Mater. Sci. 2006, 41, 5803–5809. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.E.; Duszczyk, J.; Katgerman, L. Vickers microhardness of AlSi(Cu) anodic oxide layers formed in H2SO4 at low temperature. Surf. Coat. Technol. 2003, 165, 309–315. [Google Scholar] [CrossRef]
- Choudhary, R.; Mishra, P.; Kain, V.; Singh, K.; Kumar, S.; Chakravartty, J. Scratch behavior of aluminum anodized in oxalic acid: Effect of anodizing potential. Surf. Coat. Technol. 2015, 283, 135–147. [Google Scholar] [CrossRef]
- Ateş, S.; Baran, E.; Yazıcı, B. The nanoporous anodic alumina oxide formed by two-step anodization. Thin Solid Films 2018, 648, 94–102. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, D.-H.; Lee, W.; Cho, S.J.; Hahn, J.-H.; Ahn, H.-S. Tribological properties of nanoporous anodic aluminum oxide film. Surf. Coat. Technol. 2010, 205, 1431–1437. [Google Scholar] [CrossRef]
- Tsyntsaru, N.; Kavas, B.; Sort, J.; Urgen, M.; Celis, J.-P. Mechanical and frictional behaviour of nano-porous anodised aluminium. Mater. Chem. Phys. 2014, 148, 887–895. [Google Scholar] [CrossRef]
- Alcalá, G.; Mato, S.; Skeldon, P.; Thompson, G.; Mann, A.; Habazaki, H.; Shimizu, K. Mechanical properties of barrier-type anodic alumina films using nanoindentation. Surf. Coat. Technol. 2003, 173, 293–298. [Google Scholar] [CrossRef]
- Kikuchi, T.; Hara, Y.; Sakairi, M.; Yonezawa, T.; Yamauchi, A.; Takahashi, H. Corrosion of Al–Sn–Bi alloys in alcohols at high temperatures. Part II: Effect of anodizing on corrosion. Corros. Sci. 2010, 52, 2525–2534. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Nie, X.; Wang, L.; Northwood, D. Corrosion protection properties of anodic oxide coatings on an Al–Si alloy. Surf. Coat. Technol. 2005, 200, 1994–2000. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Durejko, T.; Michalska-Domańska, M.; Łazińska, M.; Aniszewska, J. Characterization of nanoporous anodic aluminum oxide formed on laser pre-treated aluminum. Mater. Charact. 2016, 122, 130–136. [Google Scholar] [CrossRef]
- Meng, X.F.; Wei, G.Y.; Zhao, X.X.; Ge, H.L. Study on Anodic Oxidation of 2024 Aluminum Alloys in Sulfuric-Citric Acid. Mater. Sci. Forum 2014, 788, 236–242. [Google Scholar] [CrossRef]
- Yanagishita, T.; Imaizumi, M.; Kondo, T.; Masuda, H. Formation of porous Al particles by anisotropic anodic etching. Electrochem. Commun. 2017, 78, 26–28. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Meng, X.; Deng, C.; Zhang, L.; Ding, G.; Zeng, H.; Xu, X. Preparation and analysis of anodic aluminum oxide films with continuously tunable interpore distances. Appl. Surf. Sci. 2015, 328, 459–465. [Google Scholar] [CrossRef]
- Jani, A.M.M.; Losic, D.; Voelcker, N.H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 636–704. [Google Scholar] [CrossRef]
- Poges, S.; Jin, J.; Guild, C.; Li, W.-N.; Birnkrant, M.; Suib, S.L. Preparation and characterization of aluminum coatings via electroless plating onto nickel nanowires using ionic liquid plating solution. Mater. Chem. Phys. 2018, 207, 303–308. [Google Scholar] [CrossRef]
- Brzózka, A.; Szeliga, D.; Kurowska-Tabor, E.; Sulka, G. Synthesis of copper nanocone array electrodes and its electrocatalytic properties toward hydrogen peroxide reduction. Mater. Lett. 2016, 174, 66–70. [Google Scholar] [CrossRef]
- Sulka, G.D.; Brzózka, A.; Zaraska, L.; Jaskuła, M. Through-hole membranes of nanoporous alumina formed by anodizing in oxalic acid and their applications in fabrication of nanowire arrays. Electrochim. Acta 2010, 55, 4368–4376. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Salerno, M.; Loria, P.; Matarazzo, G.; Tomè, F.; Diaspro, A.; Eggenhöffner, R. Surface Morphology and Tooth Adhesion of a Novel Nanostructured Dental Restorative Composite. Materials 2016, 9, 203. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Wang, X.; Liu, W.; Shi, Y.; Yang, F. Double-layer anti-reflection coating containing a nanoporous anodic aluminum oxide layer for GaAs solar cells. Opt. Express 2013, 21, 18207–18215. [Google Scholar] [CrossRef] [Green Version]
- Chime, U.K.; Ezema, F.I.; Marques-Hueso, J. Porosity and hole diameter tuning on nanoporous anodic aluminium oxide membranes by one-step anodization. Optik 2018, 174, 558–562. [Google Scholar] [CrossRef]
- Das, G.; Patra, N.; Gopalakrishnan, A.; Zaccaria, R.P.; Toma, A.; Thorat, S.; Di Fabrizio, E.; Diaspro, A.; Salerno, M. Fabrication of large-area ordered and reproducible nanostructures for SERS biosensor application. Analyst 2012, 137, 1785–1792. [Google Scholar] [CrossRef] [Green Version]
- Toccafondi, C.; Zaccaria, R.P.; Dante, S.; Salerno, M. Fabrication of Gold-Coated Ultra-Thin Anodic Porous Alumina Substrates for Augmented SERS. Materials 2016, 9, 403. [Google Scholar] [CrossRef] [Green Version]
- Buijnsters, J.G.; Zhong, R.; Tsyntsaru, N.; Celis, J.-P. Surface Wettability of Macroporous Anodized Aluminum Oxide. ACS Appl. Mater. Interfaces 2013, 5, 3224–3233. [Google Scholar] [CrossRef]
- Cantelli, L.; Santos, J.; Trivinho-Strixino, F. The effect of anodization temperature on optical properties of nanoporous anodic alumina (NAA) films. J. Electroanal. Chem. 2016, 780, 386–390. [Google Scholar] [CrossRef]
- Thompson, D.W.; Snyder, P.G.; Castro, L.G.; Yan, L.; Kaipa, P.; Woollam, J.A. Optical characterization of porous alumina from vacuum ultraviolet to midinfrared. J. Appl. Phys. 2005, 97, 113511. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Sarkar, D.K.; Chen, X.-G.; Tong, W. Corrosion performance of superhydrophobic nickel stearate/nickel hydroxide thin films on aluminum alloy by a simple one-step electrodeposition process. Surf. Coat. Technol. 2016, 302, 173–184. [Google Scholar] [CrossRef]
- Tian, F.; Hu, A.; Li, M.; Mao, D. Superhydrophobic nickel films fabricated by electro and electroless deposition. Appl. Surf. Sci. 2012, 258, 3643–3646. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid Fabrication of Large-Area, Corrosion-Resistant Superhydrophobic Mg Alloy Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef]
- Chen, L.J.; Chen, M.; Di Zhou, H.; Chen, J.M. Preparation of super-hydrophobic surface on stainless steel. Appl. Surf. Sci. 2008, 255, 3459–3462. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, K.K.; Lee, S.H.; Ko, J.S. One-step fabrication of nickel nanocones by electrodeposition using CaCl2·2H2O as capping reagent. Appl. Surf. Sci. 2016, 369, 163–169. [Google Scholar] [CrossRef]
- Hang, T.; Hu, A.; Ling, H.; Li, M.; Mao, D. Super-hydrophobic nickel films with micro-nano hierarchical structure prepared by electrodeposition. Appl. Surf. Sci. 2010, 256, 2400–2404. [Google Scholar] [CrossRef]
- Bagheri, H.; Aliofkhazraei, M.; Forooshani, H.M.; Rouhaghdam, A.S. Electrodeposition of the hierarchical dual structured (HDS) nanocrystalline Ni surface with high water repellency and self-cleaning properties. J. Taiwan Inst. Chem. Eng. 2017, 80, 883–893. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly Ordered Anodic Porous Alumina Formation by Self-Organized Anodizing. In Nanostructured Materials in Electrochemistry; Wiley: Hoboken, NJ, USA, 2008; pp. 1–116. [Google Scholar]
- Li, J.; Hu, L.; Li, C.; Gao, X. Tailoring Hexagonally Packed Metal Hollow-Nanocones and Taper-Nanotubes by Template-Induced Preferential Electrodeposition. ACS Appl. Mater. Interfaces 2013, 5, 10376–10380. [Google Scholar] [CrossRef]
- Stepniowski, W.J.; Moneta, M.; Karczewski, K.; Michalska-Domanska, M.; Czujko, T.; Mol, J.M.; Buijnsters, J.G. Fabrication of copper nanowires via electrodeposition in anodic aluminum oxide templates formed by combined hard anodizing and electrochemical barrier layer thinning. J. Electroanal. Chem. 2018, 809, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Kurowska, E.; Brzózka, A.; Jarosz, M.; Sulka, G.; Jaskuła, M. Silver nanowire array sensor for sensitive and rapid detection of H2O2. Electrochim. Acta 2013, 104, 439–447. [Google Scholar] [CrossRef]
- Ngamlerdpokin, K.; Tantavichet, N. Electrodeposition of nickel–copper alloys to use as a cathode for hydrogen evolution in an alkaline media. Int. J. Hydrogen Energy 2014, 39, 2505–2515. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nat. Cell Biol. 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Ahn, S.H.; Hwang, S.J.; Yoo, S.J.; Choi, I.; Kim, H.-J.; Jang, J.H.; Nam, S.W.; Lim, T.-H.; Lim, T.; Kim, S.-K.; et al. Electrodeposited Ni dendrites with high activity and durability for hydrogen evolution reaction in alkaline water electrolysis. J. Mater. Chem. 2012, 22, 15153–15159. [Google Scholar] [CrossRef]
- Nagaura, T.; Takeuchi, F.; Inoue, S. Fabrication and structural control of anodic alumina films with inverted cone porous structure using multi-step anodizing. Electrochim. Acta 2008, 53, 2109–2114. [Google Scholar] [CrossRef]
- Sourmail, T. Near equiatomic FeCo alloys: Constitution, mechanical and magnetic properties. Prog. Mater. Sci. 2005, 50, 816–880. [Google Scholar] [CrossRef]
- Kim, D.; Park, D.-Y.; Yoo, B.; Sumodjo, P.; Myung, N. Magnetic properties of nanocrystalline iron group thin film alloys electrodeposited from sulfate and chloride baths. Electrochim. Acta 2003, 48, 819–830. [Google Scholar] [CrossRef]
- Koza, J.A.; Karnbach, F.; Uhlemann, M.; Mccord, J.; Mickel, C.; Gebert, A.; Baunack, S.; Schultz, L. Electrocrystallisation of CoFe alloys under the influence of external homogeneous magnetic fields—Properties of deposited thin films. Electrochim. Acta 2010, 55, 819–831. [Google Scholar] [CrossRef]
- Díaz-Ortiz, A.; Drautz, R.; Fähnle, M.; Dosch, H.; Sanchez, J.M. Structure and magnetism in bcc-based iron-cobalt alloys. Phys. Rev. B 2006, 73, 13–18. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Lv, X.; Bai, J.; Yu, W.; Liu, J. Electrocatalytic hydrogen evolution on iron-cobalt nanoparticles encapsulated in nitrogenated carbon nanotube. Int. J. Hydrogen Energy 2019, 44, 16478–16486. [Google Scholar] [CrossRef]
- Skibinska, K.; Smola, G.; Bialo, L.; Kutyla, D.; Kolczyk-Siedlecka, K.; Kwiecinska, A.; Wojnicki, M.; Zabinski, P. Influence of Annealing Time of Aluminum AA1050 on the Quality of Cu and Co Nanocones. J. Mater. Eng. Perform. 2020, 29, 8025–8035. [Google Scholar] [CrossRef]
- Kutyła, D.; Palarczyk, M.; Kołczyk, K.; Kowalik, R.; Żabiński, P. Electrodeposition of Electroactive Co–B and Co–B–C Alloys for Water Splitting Process in 8 M NaOH Solutions. Electrocatalysis 2017, 9, 189–203. [Google Scholar] [CrossRef] [Green Version]
- Zabinski, P.R.; Meguro, S.; Asami, K.; Hashimoto, K. Electrodeposited Co–Fe and Co–Fe–C Alloys for Hydrogen Evolution in a Hot 8 kmol m-3 NaOH Solution. Mater. Trans. 2003, 44, 2350–2355. [Google Scholar] [CrossRef] [Green Version]
- Zabinski, P.R.; Meguro, S.; Asami, K.; Hashimoto, K. Electrodeposited Co-Ni-Fe-C Alloys for Hydrogen Evolution in a Hot 8 kmol·m−3 NaOH. Mater. Trans. 2006, 47, 2860–2866. [Google Scholar] [CrossRef] [Green Version]
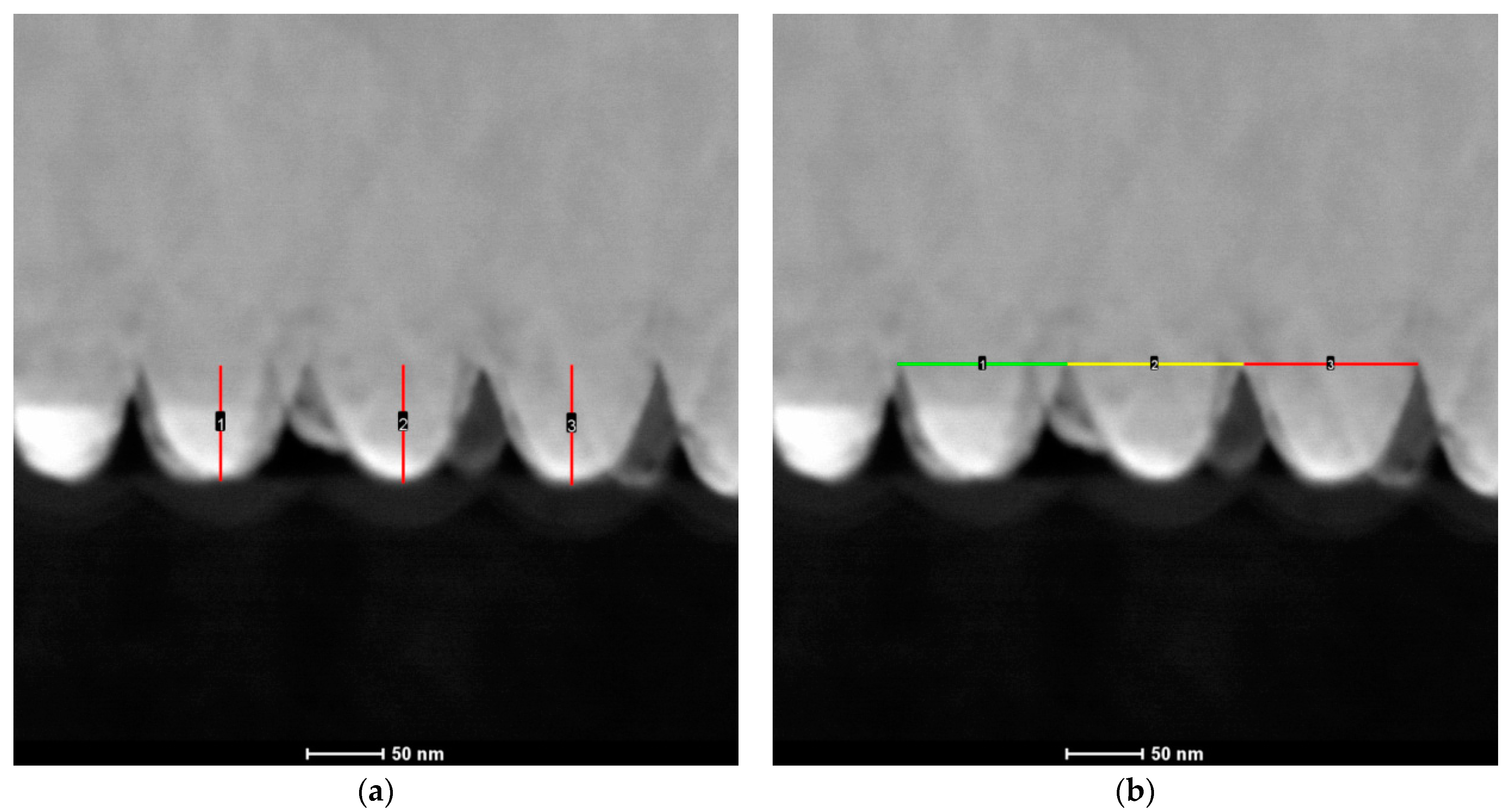

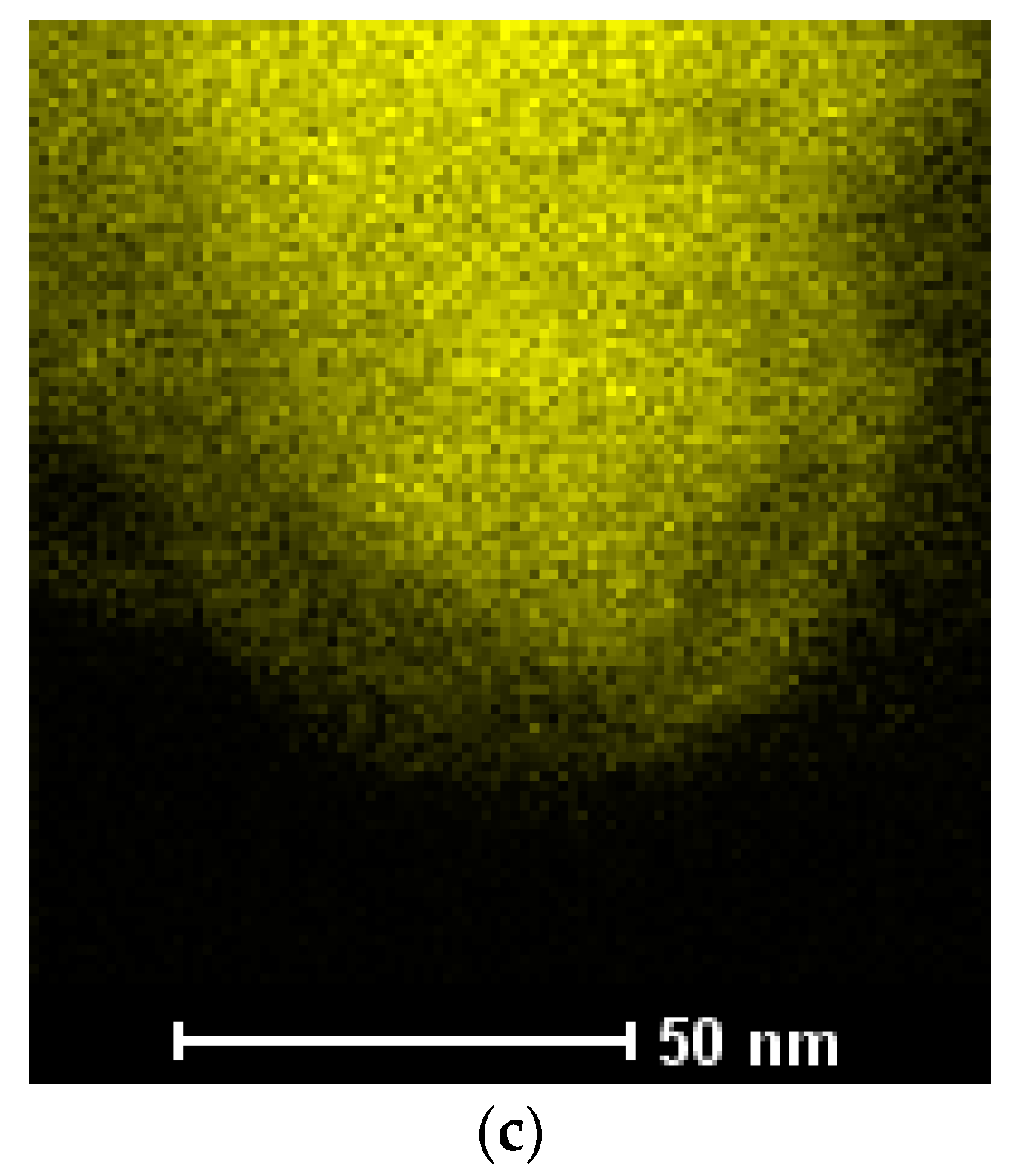

| Sample Name | Electrolyte Composition (mM) | Mass Co in Deposit (%) | Mass Fe in Deposit (%) |
|---|---|---|---|
| Co–Fe_1 | 6.5 CoSO4, 1.6 FeSO4 and 98.4 Na2SO4 | 81.90 | 18.10 |
| Co–Fe_2 | 6.5 CoSO4, 3.3 FeSO4 and 96.8 Na2SO4 | 67.11 | 32.89 |
| Material | Overpotential (mV/dec) | Solution (M) | Temperature (°C) | References |
|---|---|---|---|---|
| Co | 145 | 8 NaOH | 90 | [49] |
| Co–Fe–C | 35 | 8 NaOH | 90 | [50] |
| Co–Ni–Fe–C | 36 | 8 NaOH | 90 | [51] |
| Material | EONSET (V) | Calculated Active Area (cm2) |
|---|---|---|
| Cu bulk | −1.72 | 1.20 |
| Cu nanocones obtained in 0.3 M H2C2O4 | −1.69 | 1.64 |
| Co–Fe_1 | −1.39 | 1.20 |
| Co–Fe_2 | −1.42 | 1.20 |
| Co–Fe nanocones | −1.41 | 1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skibińska, K.; Kołczyk-Siedlecka, K.; Kutyła, D.; Gajewska, M.; Żabiński, P. Synthesis of Co–Fe 1D Nanocone Array Electrodes Using Aluminum Oxide Template. Materials 2021, 14, 1717. https://doi.org/10.3390/ma14071717
Skibińska K, Kołczyk-Siedlecka K, Kutyła D, Gajewska M, Żabiński P. Synthesis of Co–Fe 1D Nanocone Array Electrodes Using Aluminum Oxide Template. Materials. 2021; 14(7):1717. https://doi.org/10.3390/ma14071717
Chicago/Turabian StyleSkibińska, Katarzyna, Karolina Kołczyk-Siedlecka, Dawid Kutyła, Marta Gajewska, and Piotr Żabiński. 2021. "Synthesis of Co–Fe 1D Nanocone Array Electrodes Using Aluminum Oxide Template" Materials 14, no. 7: 1717. https://doi.org/10.3390/ma14071717
APA StyleSkibińska, K., Kołczyk-Siedlecka, K., Kutyła, D., Gajewska, M., & Żabiński, P. (2021). Synthesis of Co–Fe 1D Nanocone Array Electrodes Using Aluminum Oxide Template. Materials, 14(7), 1717. https://doi.org/10.3390/ma14071717









