Microstructure, Hot Deformation Behavior, and Recrystallization Behavior of Zn-1Fe-1Mg Alloy under Isothermal Compression
Abstract
:1. Introduction
2. Materials and Methods
3. Results
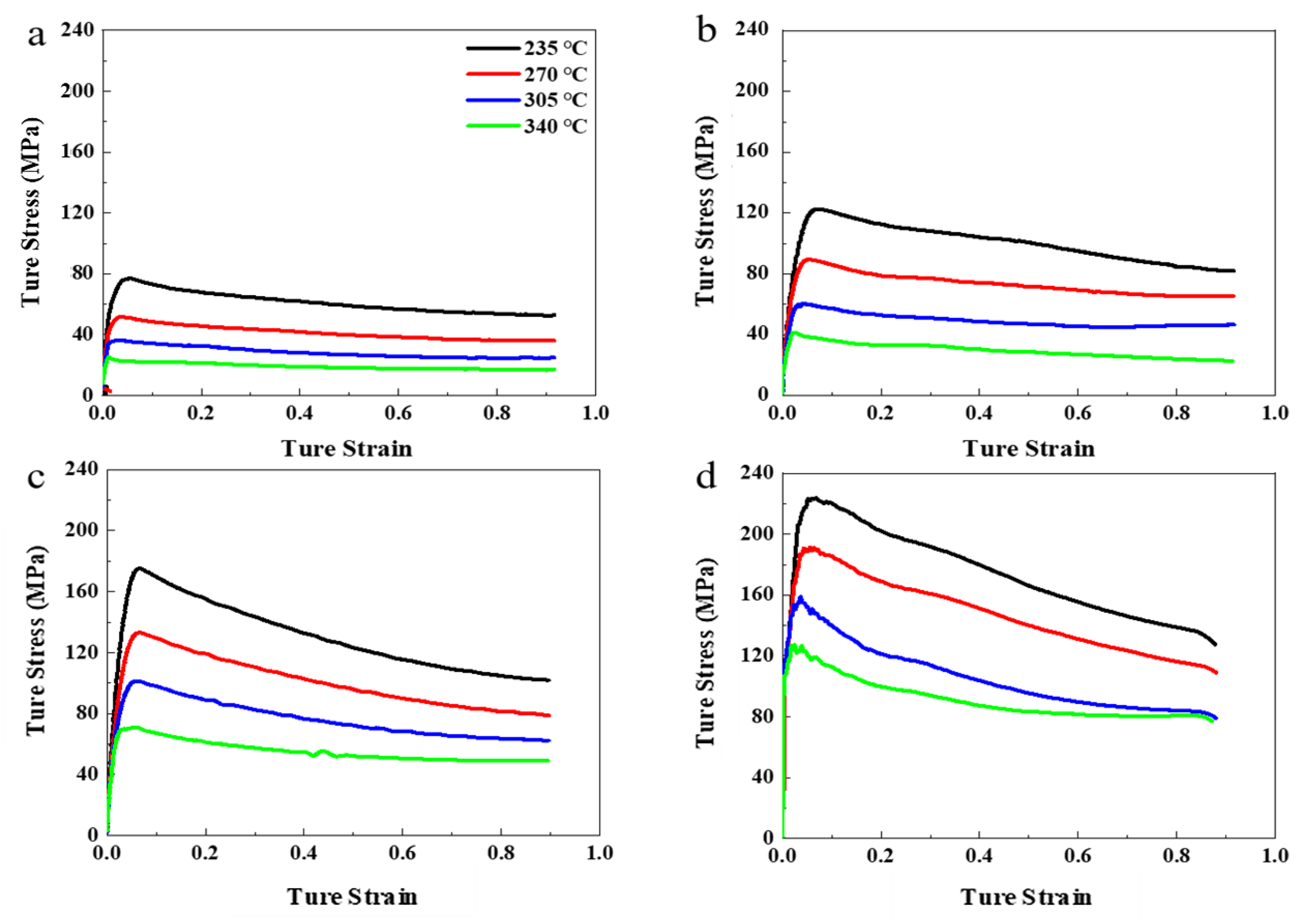
3.1. Flow Stress Behavior
3.2. Constitutive Modeling
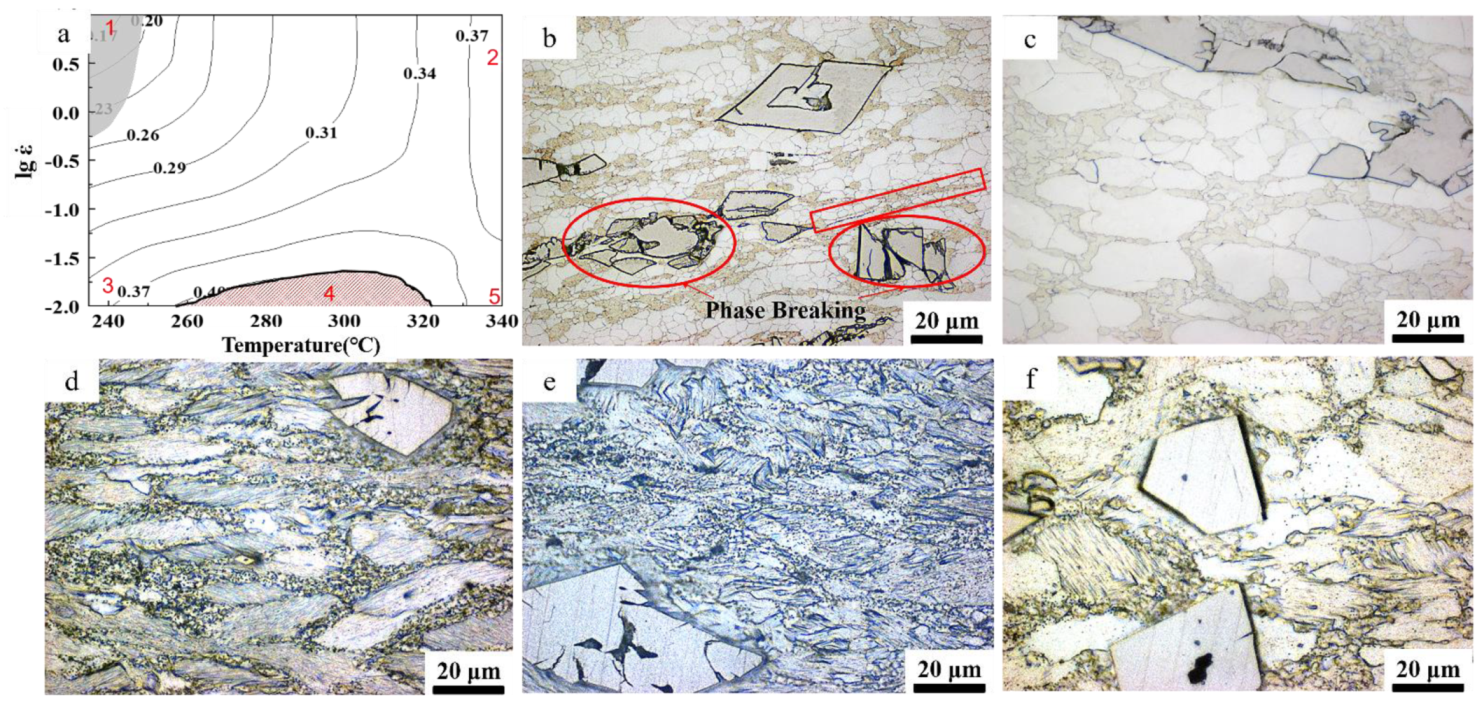
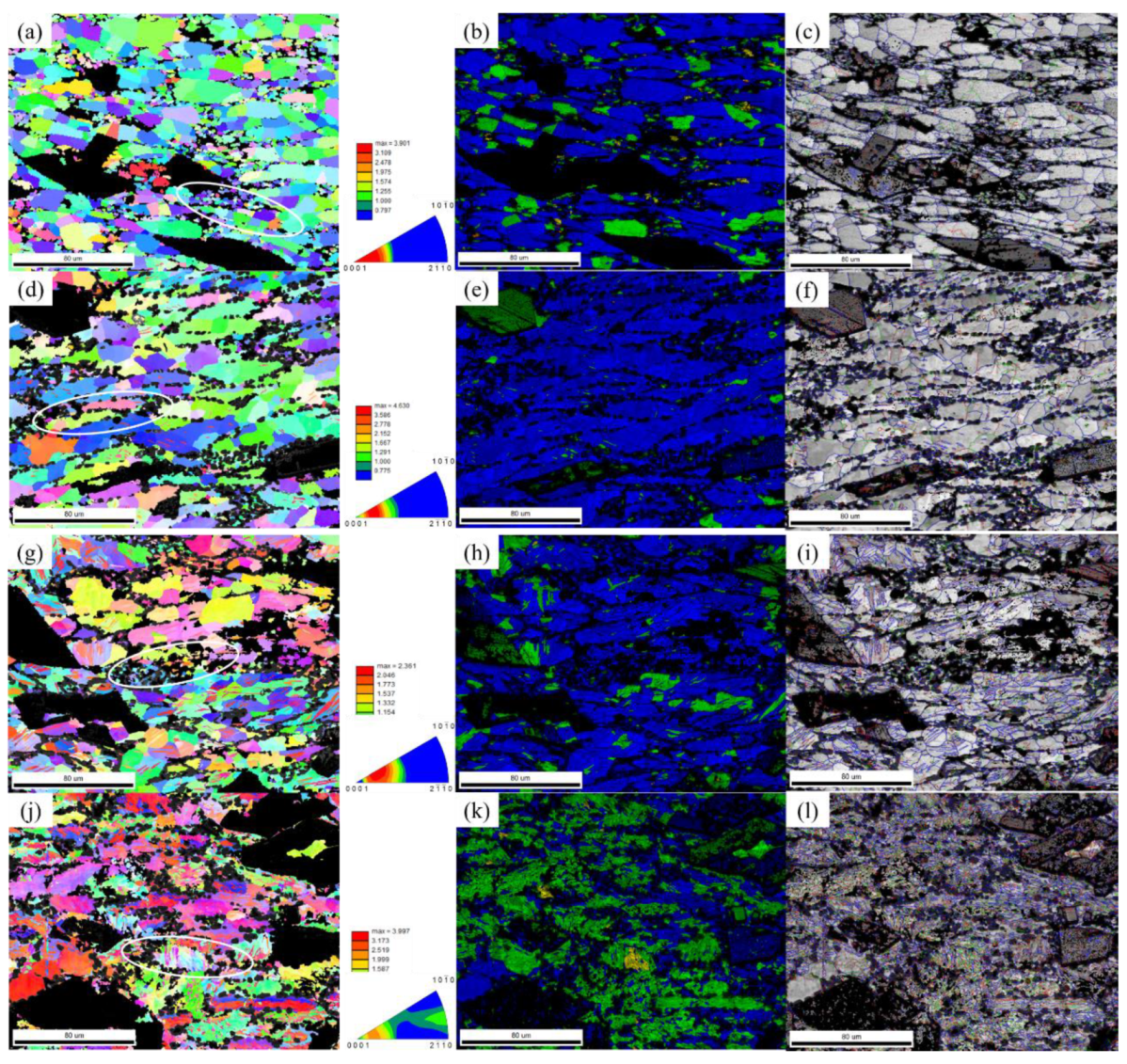
3.3. Microstructural Evolution
3.3.1. TEM Analysis of Deformation Alloys
3.3.2. Influence of Compression Parameters on Grain Structure
Microstructure Evolution with the Changing of Temperature
The Effect of Strain Rate Change on the Grain Structure
4. Conclusions
- In the process of isothermal compression, as the amount of strain accumulates, the flow stress quickly reaches a peak value and then slowly decreases. The peak stress gradually increases as the compression temperature decreases (or as the strain rate increases). The constitutive equation for hot deformation is = 8.33 × 109 × (sinh(0.0104σ))3.803exp(−114326/(RT)). There is a satisfactory consistency between the expected flow stress and the experimental flow stress during the process of hot compression.
- Power dissipation is affected by both strain rate and heating temperature. At low temperatures and high strain rates (235~250 °C, 10 s−1~0.5 s−1), the power dissipation factor is very low, and there is a small area of processing instability. As the temperature increases and the strain rate decreases, the power dissipation factor gradually increases, reaching its highest value (0.40) at the temperature of 305 °C and the strain rate of 0.01 s−1.
- The nucleation and growth of DRX are controlled by the mechanism of CDRX. The increase in temperature and the decrease in strain rate reduce the deformed structure and subcrystals inside the grains, but promote the formation of intragranular twins.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rink, L. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies☆. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Ren, Y.; Sun, S.; Zhang, E.; Yan, C.; Liu, Q.; Sun, X.; Shou, F.; Duan, J.; et al. Indirectly extruded biodegradable Zn-0.05wt%Mg alloy with improved strength and ductility: In vitro and in vivo studies. J. Mater. Sci. Technol. 2018, 34, 1618–1627. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.; Griebel, A.; Schaffer, J.; Earley, E.J.; Goldman, J.; Drelich, J.W. Novel high-strength, low-alloys Zn-Mg (<0.1 wt% Mg) and their arterial biodegradation. Mater. Sci. Eng. C 2018, 84, 67–79. [Google Scholar]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5Mg alloy. J. Alloy. Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Yao, C.; Wang, Z.; Tay, S.L.; Zhu, T.; Gao, W. Effects of Mg on microstructure and corrosion properties of Zn–Mg alloy. J. Alloy. Compd. 2014, 602, 101–107. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Li, Z.-L.; Bai, W.-S.; Tuoliken, A.; Yu, J.; Liu, X.-F. (Fe, Mn)Zn13 phase and its core-shell structure in novel biodegradable Zn-Mn-Fe alloys. Mater. Des. 2019, 162, 235–245. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Goldman, J.; Drelich, J.; Aghion, E. The Suitability of Zn–1.3%Fe Alloy as a Biodegradable Implant Material. Metals 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. In vivo performances of pure Zn and Zn–Fe alloy as biodegradable implants. J. Mater. Sci. Mater. Med. 2018, 29, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Micro-alloying with Mn in Zn–Mg alloy for future biodegradable metals application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Xu, X.Q.; Li, D.F.; Guo, S.L.; Wu, X.P.; Du, P. High temperature deformation behavior and processing map of Zn-8Cu-0.3Ti Zn alloy. Chin. J. Nonferrous Met. 2012, 22, 1075–1081. [Google Scholar]
- Yegao, C.; Liqiang, W.; Jiacheng, Y.; YingLi, L.; Huan, Y.; Lina, L.; Shuhong, S.; Yi, Z. Flow Behavior of Zn-10Al-2Cu Alloy During Hot Compression at Elevated Temperature. Mater. Rep. 2016, 30, 483–486, 504. [Google Scholar]
- Liu, Y.; Geng, C.; Zhu, Y.; Peng, J.; Xu, J. Hot Deformation Behavior and Intrinsic Workability of Carbon Nanotube-Aluminum Reinforced ZA27 Composites. J. Mater. Eng. Perform. 2017, 26, 1967–1977. [Google Scholar] [CrossRef]
- Shen, C.; Liu, X.; Fan, B.; Lan, P.; Zhou, F.; Li, X.; Wang, H.; Xiao, X.; Li, L.; Zhao, S.; et al. Mechanical properties, in vitro degradation behavior, hemocompatibility and cytotoxicity evaluation of Zn–1.2Mg alloy for biodegradable implants. RSC Adv. 2016, 6, 86410–86419. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Loffredo, S.; Demir, A.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, Q.; Yan, X.; Ran, X.; Wang, L.; Zhou, J.G.; Hu, T.; Wang, G. Mechanical properties, degradation behaviors and biocompatibility evaluation of a biodegradable Zn-Mg-Cu alloy for cardiovascular implants. Mater. Lett. 2019, 234, 294–297. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.; Zhang, H.; Pei, J.; Xue, G.; Yuan, G. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys. Mater. Charact. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Xue, P.; Ma, M.; Li, Y.; Li, X.; Yuan, J.; Shi, G.; Wang, K.; Zhang, K. Microstructure, Mechanical Properties, and in Vitro Corrosion Behavior of Biodegradable Zn-1Fe-xMg Alloy. Materials 2020, 13, 4835. [Google Scholar] [CrossRef]
- Ningqi, P.; Guangbo, T.; Zhengdong, L. Correcting method of flow stress curve for hot compression. Hot Work. Technol. 2012, 41, 12–15. [Google Scholar]
- Li, H.Y.; Liu, Y.; Lu, X.C.; Su, X.J. Constitutive modeling for hot deformation behavior of ZA27 alloy. J. Mater. Sci. 2012, 47, 5411–5418. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Tang, J.; Zhang, J. Influence of strain rate on hot deformation behavior and recrystallization behavior under isothermal compression of Al-Zn-Mg-Cu alloy. J. Alloy. Compd. 2019, 809, 151788. [Google Scholar] [CrossRef]
- McQueen, H.; Ryan, N. Constitutive analysis in hot working. Mater. Sci. Eng. A 2002, 322, 43–63. [Google Scholar] [CrossRef]
- Rout, M.; Pal, S.K.; Singh, S.B. Finite element modeling of hot rolling: Steady- and unsteady-state analyses. In Computational Methods and Production Engineering; Paulo Davim, J., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 83–124. [Google Scholar]
- Lin, Y.C.; Zhao, C.-Y.; Chen, M.-S.; Chen, D.-D. A novel constitutive model for hot deformation behaviors of Ti–6Al–4V alloy based on probabilistic method. Appl. Phys. A 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Prasad, Y.V.R.K.; Gegel, H.L.; Doraivelu, S.M.; Malas, J.C.; Morgan, J.T.; Lark, K.A.; Barker, D.R. Modeling of dynamic material behavior in hot deformation: Forging of Ti-6242. Met. Mater. Trans. A 1984, 15, 1883–1892. [Google Scholar] [CrossRef]
- Xu, X.Q.; Li, D.F.; Guo, S.L.; Wu, X.P. Microstructure evolution of Zn–8Cu–0.3 Ti alloy during hot deformation. Trans. Nonferrous Met. Soc. China 2012, 22, 1606–1612. [Google Scholar] [CrossRef]






| Zn | Mg | Fe | Cu | Si | Ni |
|---|---|---|---|---|---|
| Balance | 1.19 | 0.97 | 0.02 | 0.02 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, P.; Ma, M.; Li, Y.; Li, X.; Yuan, J.; Shi, G.; Wang, K.; Zhang, K. Microstructure, Hot Deformation Behavior, and Recrystallization Behavior of Zn-1Fe-1Mg Alloy under Isothermal Compression. Materials 2021, 14, 1735. https://doi.org/10.3390/ma14071735
Xue P, Ma M, Li Y, Li X, Yuan J, Shi G, Wang K, Zhang K. Microstructure, Hot Deformation Behavior, and Recrystallization Behavior of Zn-1Fe-1Mg Alloy under Isothermal Compression. Materials. 2021; 14(7):1735. https://doi.org/10.3390/ma14071735
Chicago/Turabian StyleXue, Penghao, Minglong Ma, Yongjun Li, Xinggang Li, Jiawei Yuan, Guoliang Shi, Kaikun Wang, and Kui Zhang. 2021. "Microstructure, Hot Deformation Behavior, and Recrystallization Behavior of Zn-1Fe-1Mg Alloy under Isothermal Compression" Materials 14, no. 7: 1735. https://doi.org/10.3390/ma14071735





