Electrodeposition of Copper and Brass Coatings with Olive-Like Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrodeposition of Zn, Cu, and Cu-Zn Coatings on Steel
2.2. Characterization
3. Results and Discussion
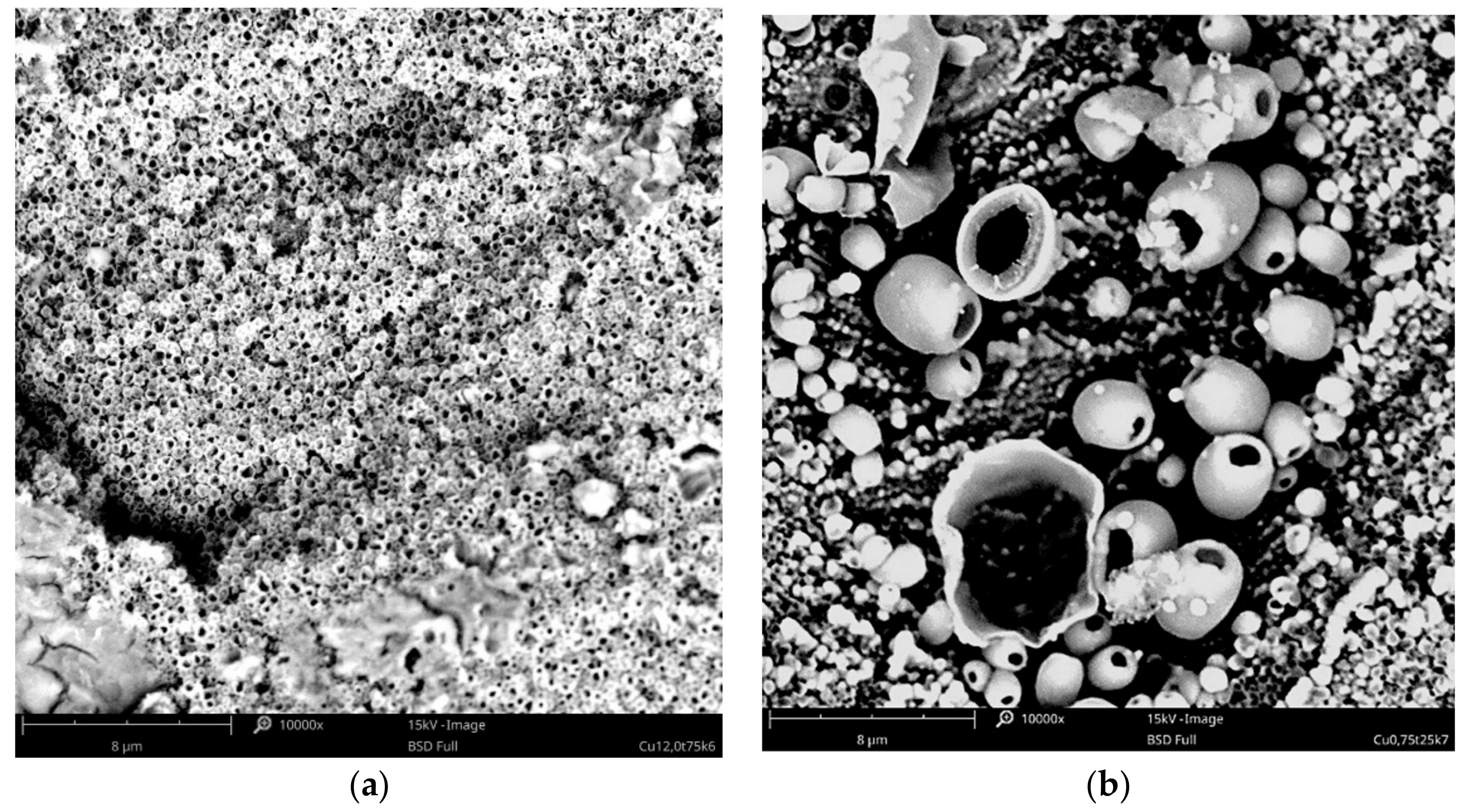
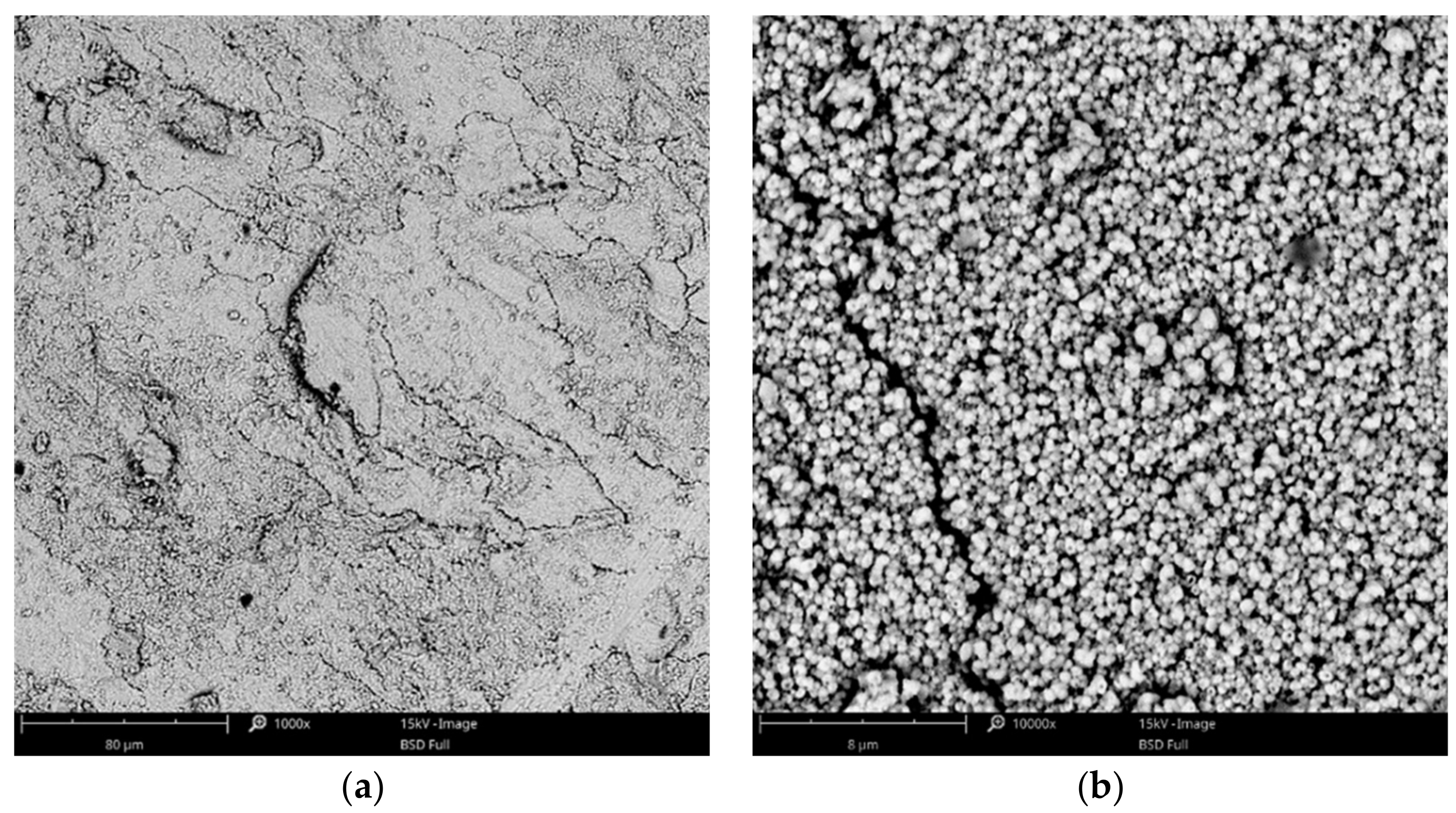
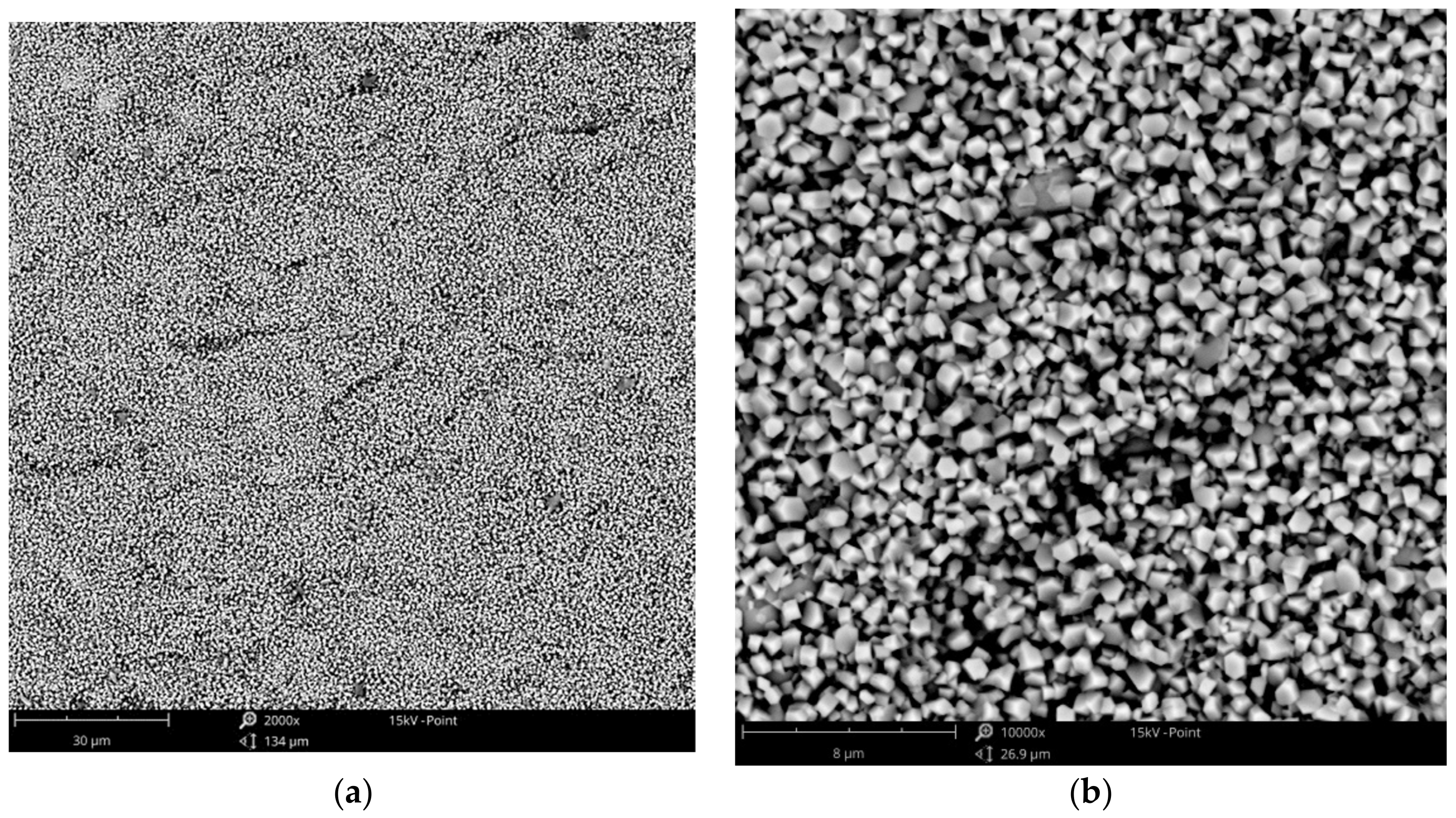
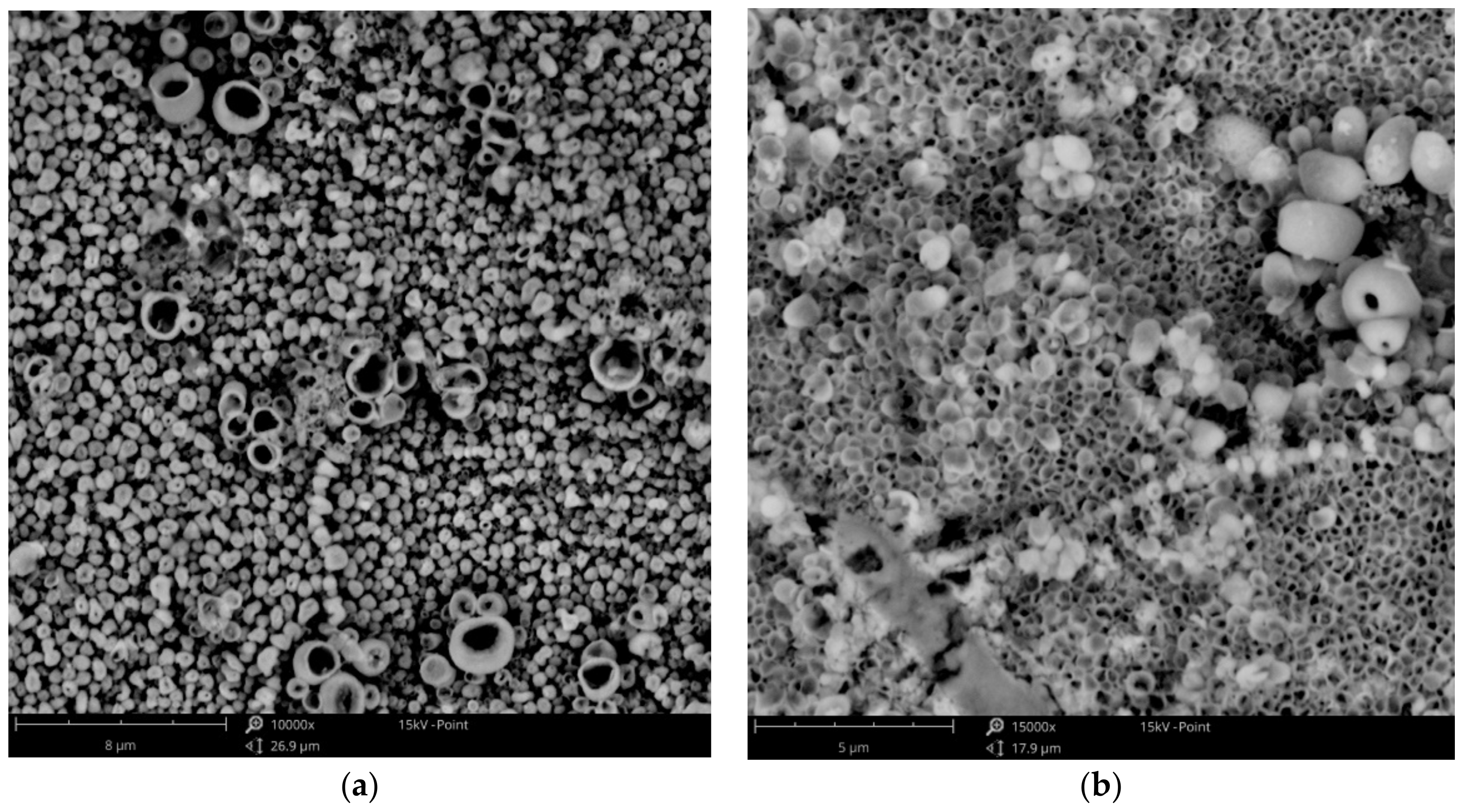
3.1. Surface Morphology—SEM Analysis
3.1.1. Copper Coatings
3.1.2. Zinc Coatings
3.1.3. Cu-Zn Coatings
3.2. Chemical Composition of the Cu-Zn Coatings
3.3. Current Efficiency and Electrodeposition Rate
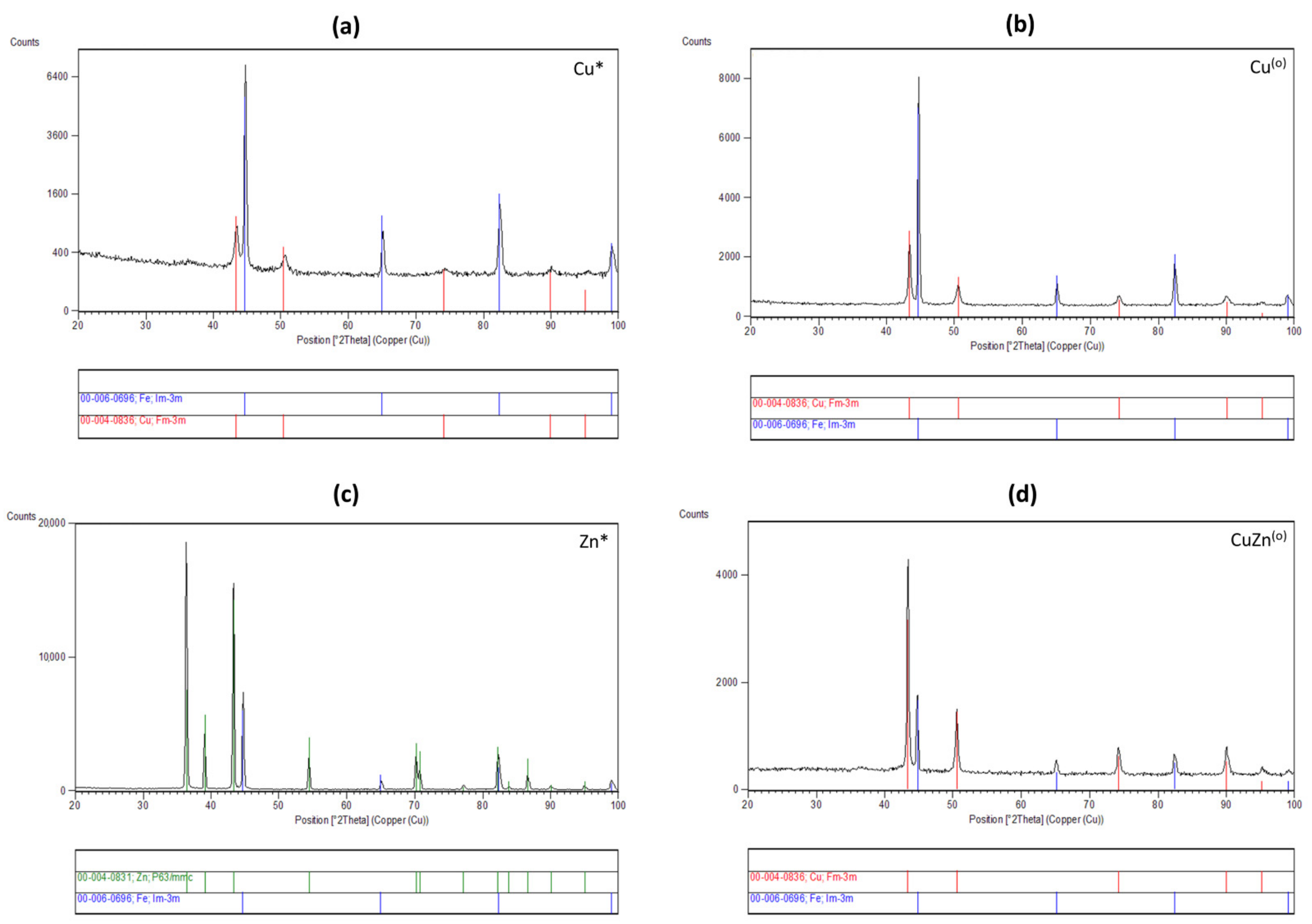
3.4. Phase Composition of the Coatings
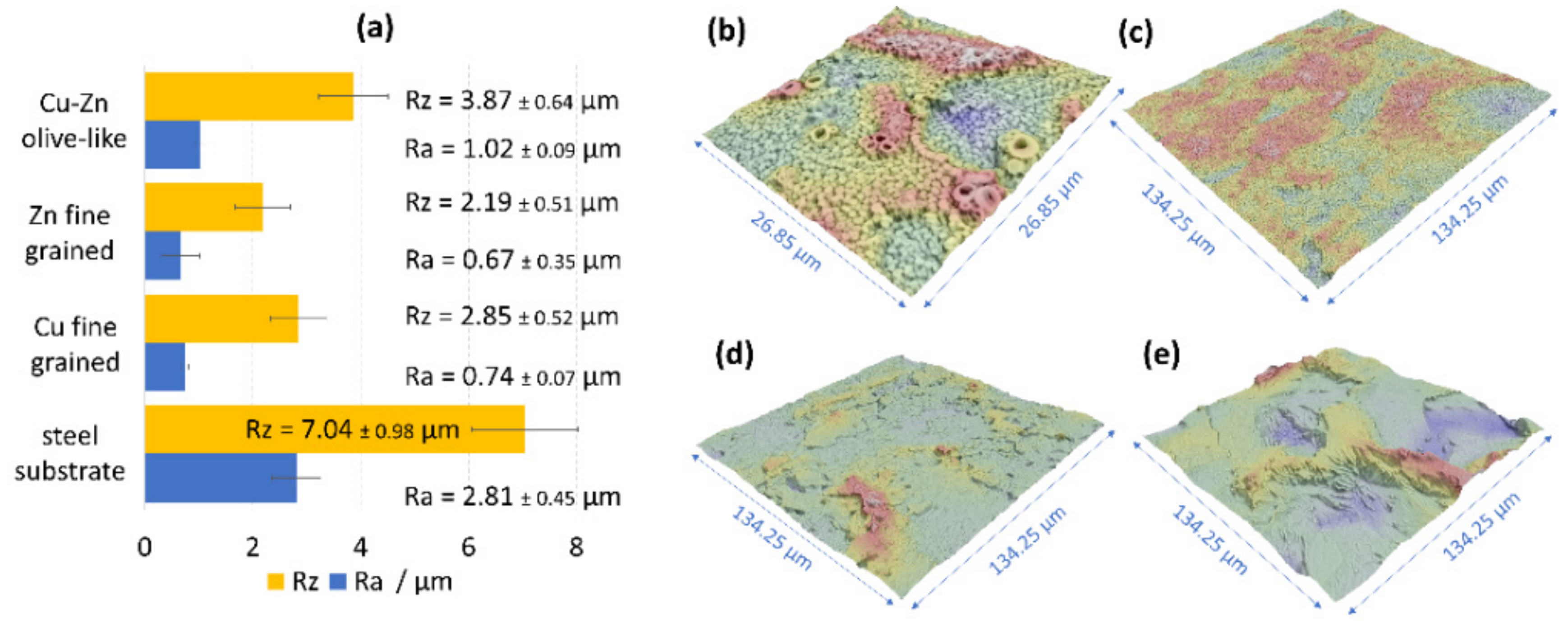
3.5. Roughness of the Olive-Like-Structured Brass Coatings
3.6. Corrosion Resistance of the Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, H.H.; Lu, L.; Fuh, J.Y. Development and characterisation of direct laser sintering Cu-based metal powder. J. Mater. Process. Technol. 2003, 140, 314–317. [Google Scholar] [CrossRef]
- Lesnikov, V.P.; Kuznetsov, V.P.; Goroshenko, Y.O.; Rozhko, A.L. Diffusion saturation of nickel alloys with aluminum and chromium from the gas phase by the circulation method. Met. Sci. Heat Treat. 1998, 40, 412–416. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Sivin, D.O.; Bozhko, I.A.; Stephanov, I.B.; Shevelev, A.E. Microstructure of titanium alloy modified by high-intensity implantation of low and high-energy aluminium ions. Surf. Coat. Technol. 2020, 391, 125722. [Google Scholar] [CrossRef]
- Ustinov, A.I.; Polishchuk, S.S.; Demchenkov, S.A.; Melnychenko, T.V.; Skorodzievskii, V.S. Formation of thin foils of high-entropy CrFeCoNiCu alloys by EB-PVD process. Surf. Coat. Technol. 2020, 403, 126440. [Google Scholar] [CrossRef]
- Shimizu, H.; Sakoda, K.; Shimigaki, Y. CVD of cobalt–tungsten alloy film as a novel copper diffusion barrier. Microelectron. Eng. 2013, 106, 91–95. [Google Scholar] [CrossRef]
- Gamburg, Y.D.; Zangari, G. Electrodeposition of alloys. In Theory and Practice of Metal Electrodeposition; Springer: New York, NY, USA, 2011; pp. 205–232. [Google Scholar]
- Musa, A.Y.; Slaiman, Q.J.M.; Kadhum, A.A.H.; Takriff, M.S. Effects of agitation, current density and cyanide concentration on Cu-Zn alloy electroplating. Eur. J. Sci. Res. 2008, 22, 517–524. [Google Scholar]
- Haciibrahimoglu, M.Y.; Yavuz, A.; Oztas, M.; Bedir, M. Electrochemical and structural study of Zn-rich brass deposited from py-rophosphate electrolyte onto the carbon steel. Dig. J. Nanomater. Biostruct. 2016, 11, 251–262. [Google Scholar]
- Vivegnis, S.; Krid, M.; Delhalle, J.; Mekhalif, Z.; Renner, F.U. Use of pyrophosphate and boric acid additives in the copper-zinc alloy electrodeposition and chemical dealloying. J. Electroanal. Chem. 2019, 848, 113310. [Google Scholar] [CrossRef]
- Silva, F.L.G.; do Lago, D.C.B.; Delia, E.; Senna, L.F. Electrodeposition of Cu-Zn alloy coating from citrate baths containing ben-zotriazole and cysteine as additives. J. Appl. Electrochem. 2010, 40, 2013–2022. [Google Scholar] [CrossRef]
- Tintelecan, M.; Iluțiu-Varvara, D.A.; Alabanda, O.R.; Sas-Boca, I.M. A technical version of achieving a brass coated surface on steel wires. Procedia Manuf. 2020, 46, 12–18. [Google Scholar] [CrossRef]
- Rossi, A. A tartrate-based alloy bath for brass plated steel wire production. J. Appl. Electrochem. 1992, 22, 64–72. [Google Scholar]
- Barros, K.S.; Ortega, E.M.; Pérez-Herranz, V.; Espinosa, D.C.R. Evaluation of brass electrodeposition at RDE from cyanide-free bath using EDTA as a complexing agent. J. Electroanal. Chem. 2020, 865, 1141. [Google Scholar] [CrossRef]
- Ramírez, C.; Calderón, J.A. Study of the effect of triethanolamine as a chelating agent in the simultaneous electrodeposition of copper and zinc from non-cyanide electrolytes. J. Electroanal. Chem. 2016, 765, 132–139. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; Bakdash, R.S. New cyanide-free ammonia bath for brass alloy coatings on steel substrate by electrodeposition. Inter. J. Electrochem. Sci. 2015, 10, 9666–9677. [Google Scholar]
- Fujiwara, Y.; Enomoto, H. Electrodeposition of Cu–Zn alloys from glucoheptonate baths. Surf. Coat. Technol. 1988, 35, 101–111. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; Bakdash, R.S. Copper-rich Cu–Zn alloy coatings prepared by electrodeposition from glutamate complex elec-trolyte: Morphology, structure, microhardness and electrochemical studies. Surf. Interfaces 2020, 18, 100404. [Google Scholar] [CrossRef]
- Rashwan, S.M. Electrodeposition of Zn–Cu coatings from alkaline sulphate bath containing glycine. Trans. IMF 2007, 85, 217–224. [Google Scholar] [CrossRef]
- Ghorbani, M.; Mazaheria, M.; Khangholi, K.; Kharazi, Y. Electrodeposition of graphite-brass composite coatings and characterization of the tribological properties. Surf. Coat. Technol. 2001, 148, 71–76. [Google Scholar] [CrossRef]
- Das, S.; Jena, S.; Banthia, S.; Mitra, A.; Das, S.; Das, K. Novel pulse potentiostatic electrodeposition route for obtaining pure intermetallic Cu5Zn8-CuZn composite coating using glycerol-NaOH based electrolyte with advanced scratch resistance and anti-corrosive properties. J. Alloys Compd. 2019, 792, 770–779. [Google Scholar] [CrossRef]
- Freemantle, M. An Introduction to Ionic Liquids, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 1–18. [Google Scholar]
- Endres, F.; Abbott, A.P.; MacFarlane, D.R. Electrodeposition from Ionic Liquids, 1st ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–13. [Google Scholar]
- Liu, Z.; Zein El Abedin, S.; Endres, F. Electrodeposition of zinc films from ionic liquids and ionic liquid/water mixtures. Electrochim. Acta 2013, 89, 635–643. [Google Scholar] [CrossRef]
- Yang, W.; Cang, H.; Tang, T.; Wang, J.; ShiNi, Y. Electrodeposition of tin and antimony in 1-ethyl-3-methylimidazolium tetrafluoroborate ionic liquid. J. Appl. Electrochem. 2008, 38, 537–542. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, X.; Luo, Y.; Yu, P. Electrodeposition of nickel in air- and water-stable 1-butyl-3-methylimidazolium dibutylphosphate ionic liquid. RSC Adv. 2020, 28, 16576–16583. [Google Scholar] [CrossRef]
- Surviliene, S.; Eugénio, S.; Vilar, R. Chromium electrodeposition from [BMim][BF4] ionic liquid. J. Appl. Electrochem. 2011, 41, 107–114. [Google Scholar] [CrossRef]
- Mukhopadhyay, I.; Aravinda, C.; Borissov, D.; Freyland, W. Electrodeposition of Ti from TiCl4 in the ionic liquid l-methyl-3-butyl-imidazolium bis (trifluoro methyl sulfone) imide at room temperature: Study on phase formation by in situ electrochemical scanning tunneling microscopy. Electrochim. Acta 2005, 50, 1275–1281. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. A new method for practical electrodeposition of aluminium from ionic liquids. Electrochem. Commun. 2015, 51, 113–116. [Google Scholar] [CrossRef]
- Cheek, G.; O’Grady, W.; El Abedin, S.; Moustafa, E.; Endres, F. Studies on the electrodeposition of magnesium in ionic liquids. J. Electrochem. Soc. 2008, 155, D91–D95. [Google Scholar] [CrossRef]
- Haerens, K.; Matthijs, E.; Chmielarz, A.; Van der Bruggen, B. The use of ionic liquids based on choline chloride for metal deposition: A green alternative? J. Environ. Manag. 2009, 90, 3245–3252. [Google Scholar] [CrossRef]
- Cao, X.; Xu, L.; Shi, Y.; Wang, Y.; Xue, X. Electrochemical behavior and electrodeposition of cobalt from choline chloride-urea deep eutectic solvent. Electrochim. Acta. 2019, 295, 550–557. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Wang, Q.; Mou, H.; Cao, B.; Yu, D.; Wang, D.; Zhang, S.; Mu, T. Low temperature electrochemical deposition of aluminum in organic bases/thiourea-based deep eutectic solvents. ACS Sustain. Chem. Eng. 2018, 6, 15480–15486. [Google Scholar] [CrossRef]
- Alesary, H.F.; Ismail, H.K.; Shiltagh, N.M.; Alattar, R.A.; Ahmed, L.M.; Watkins, M.J.; Ryder, K.S. Effects of additives on the electrodeposition of Zn-Sn alloys from choline chloride/ethylene glycol-based deep eutectic solvent. J. Electroanal. Chem. 2020, 874, 114517. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Reddy, R.G. Cobalt electrodeposition using urea and choline chloride. Electrochim. Acta 2014, 123, 325–331. [Google Scholar] [CrossRef]
- Bernasconi, R.; Zebarjadi, M.; Magagnin, L. Copper electrodeposition from a chloride free deep eutectic solvent. J. Electroanal. Chem. 2015, 758, 163–169. [Google Scholar] [CrossRef]
- De Vreese, P.; Skoczylas, A.; Matthijs, E.; Fransaer, J.; Binnemans, K. Electrodeposition of copper–zinc alloys from an ionic liquid-like choline acetate electrolyte. Electrochim. Acta 2013, 108, 788–794. [Google Scholar] [CrossRef]
- Xie, X.; Zou, X.; Lu, X.; Lu, C.; Cheng, H.; Xu, Q.; Zhou, Z. Electrodeposition of Zn and Cu–Zn alloy from ZnO/CuO precursors in deep eutectic solvent. Appl. Surf. Sci. 2016, 386, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Rousse, C.; Beaufils, S.; Fricoteaux, P. Electrodeposition of Cu–Zn thin films from room temperature ionic liquid. Electrochim. Acta 2013, 107, 624–631. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Zhang, J.; Yang, P.; An, M.; Li, Q. Electrodeposition of Cu-Zn alloy from EMImTfO ionic liquid/ethanol mixtures for replacing the cyanide zincate layer on Al alloy. J. Alloys Compd. 2019, 806, 79–88. [Google Scholar] [CrossRef]
- Maciej, A.; Kądziela, M.; Michalska, J.; Dercz, G. Electrodeposition of Zn, Cu, Cu-Zn alloy coatings from the galvanic baths based on 2-hydroxyethyl-trimethylammonium acetate. Ochr. Przed Koroz. 2019, 62, 112–115. [Google Scholar] [CrossRef]
- Guo, L.; Oskam, G.; Radisic, A.; Hoffman, P.M.; Searson, P.C. Island growth in electrodeposition. J. Phys. D Appl. Phys. 2011, 44, 443001. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Kacalak, W.; Tandecka, K.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Malorny, W.; Matýsek, D.; Dudek, Ł.; et al. Characterization of porous phosphate coatings enriched with calcium, magnesium, zinc and copper created on CP titanium Grade 2 by plasma electrolytic oxidation. Metals 2018, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Rokosz, K.; Hryniewicz, T.; Pietrzak, K.; Dudek, Ł. Porous PEO coatings on titanium, obtained under DC regime, enriched in magnesium, calcium, zinc, and copper. World Sci. News 2018, 94, 99–114. [Google Scholar]
- Komarova, E.G.; Kazantseva, E.A.; Chaikina, M.V.; Bulina, N.V.; Sedelnikova, M.B.; Sharkeev, Y.P. Morphological and structural features of micro-arc Zn-Si-containing calcium phosphate coatings. Mater. Today 2020, 25, 439–442. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys, 1st ed.; Academic Press Inc.: New York, NY, USA; London, UK, 1963. [Google Scholar]
- Fujiwara, Y.; Enomoto, H. Electrodeposition of β’-brass from cyanide baths with accumulative underpotential deposition of Zn. J. Electrochem. Soc. 2000, 147, 1840–1846. [Google Scholar] [CrossRef]
- El-Sherif, R.M.; Ismail, K.M.; Badawy, W.A. Effect of Zn and Pb as alloying elements on the electrochemical behavior of brass in NaCl solutions. Electrochim. Acta 2004, 49, 5139–5150. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Thampuran, R.; Chen, X.; Teoh, S.H. A comparison between the corrosion behaviour of sintered and unsintered porous titanium. Corr. Sci. 1995, 37, 1333–1340. [Google Scholar] [CrossRef]









| Desirable Type of Coating | Bath Symbol | Component | at. % of Cu in Bath | ||
|---|---|---|---|---|---|
| [EMIM][Ac] | CuAc, g·L−1 | ZnAc, g·L−1 | |||
| Copper coating | Cu-5 | Basic electrolyte (solvent) | 5 | - | 100 |
| Cu-10 | 10 | - | |||
| Cu-20 | 20 | - | |||
| Cu-25 | 25 | - | |||
| Cu-30 | 30 | - | |||
| Cu-35 | 35 | - | |||
| Cu-40 | 40 | - | |||
| Cu-60 | 60 | - | |||
| Cu-80 | 80 | - | |||
| Zinc coating | Zn-0 group | Basic electrolyte (solvent) | - | 5–370 (no deposit) | 0 |
| Zn-430 | - | 430 | |||
| Zn-480 | - | 480 | |||
| Zn-600 | - | 600 | |||
| Zn-800 | - | 800 | |||
| Zn-1000 | - | 1000 | |||
| Cu-Zn alloy coating | CuZn-1 | Basic electrolyte (solvent) | 60 | 480 | 12.6 |
| CuZn-2 | 60 | 600 | 10.1 | ||
| CuZn-3 | 60 | 800 | 7.5 | ||
| Cu-Zn-4 | 60 | 1000 | 6.1 | ||
| Coating Type | at. % of Cu in Coating | Structure of Coating | Ecorr vs. SCE (V) | jcorr (μA·cm−2) | Rp (kΩ·cm2) |
|---|---|---|---|---|---|
| Cu | 100 | olive-like | −0.148 | 3.1·10−3 | 4726.8 |
| Cu | 100 | fine-grained | −0.146 | 5.22 | 4.79 |
| Cu-Zn | 96 | olive-like | −0.156 | 5.3·10−3 | 4359.0 |
| Cu-Zn | 81 | porous | −0.364 | 1.97 | 13.65 |
| Cu-Zn | 56 | fine-grained | −0.587 | 6.50 | 3.67 |
| Cu-Zn | 51 | fine-grained | −0.684 | 5.54 | 3.76 |
| Cu-Zn | 28 | fine-grained | −0.858 | 5.48 | 4.58 |
| Zn | 0 | fine-grained | −1.082 | 5.60 | 3.97 |
| Steel (substrate) | - | - | −0.621 | 27.8 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciej, A.; Łatanik, N.; Sowa, M.; Matuła, I.; Simka, W. Electrodeposition of Copper and Brass Coatings with Olive-Like Structure. Materials 2021, 14, 1762. https://doi.org/10.3390/ma14071762
Maciej A, Łatanik N, Sowa M, Matuła I, Simka W. Electrodeposition of Copper and Brass Coatings with Olive-Like Structure. Materials. 2021; 14(7):1762. https://doi.org/10.3390/ma14071762
Chicago/Turabian StyleMaciej, Artur, Natalia Łatanik, Maciej Sowa, Izabela Matuła, and Wojciech Simka. 2021. "Electrodeposition of Copper and Brass Coatings with Olive-Like Structure" Materials 14, no. 7: 1762. https://doi.org/10.3390/ma14071762







