The Effect of Oxygen Content in Binderless Cokes for High-Density Carbon Blocks from Coal Tar Pitch
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of High-Density Carbon Blocks
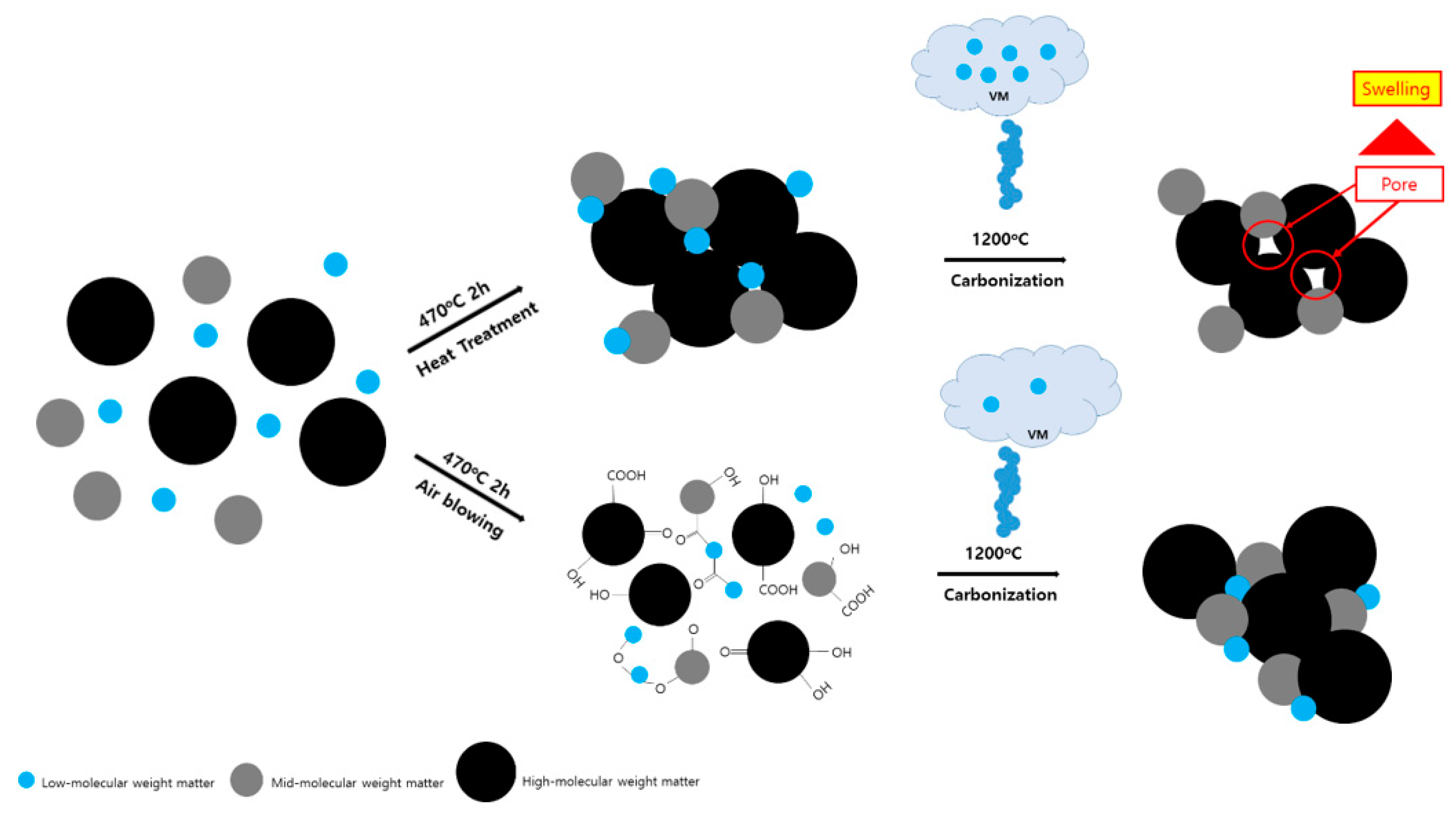
2.2.1. Preparation of Binderless Coke
2.2.2. Green Body Manufacturing and Carbonization
2.3. Analysis
2.4. Mechanical Properties
3. Results and Discussion
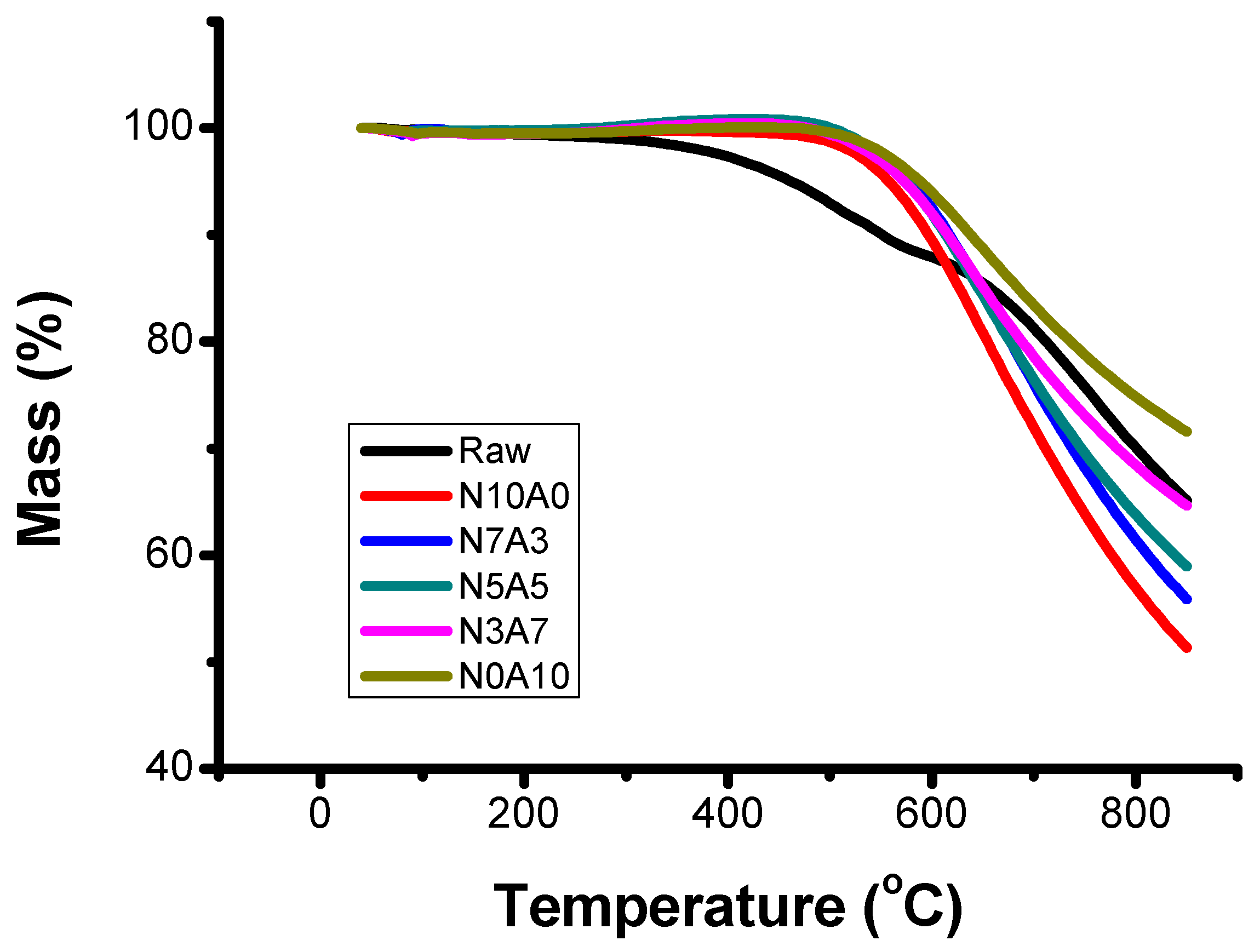
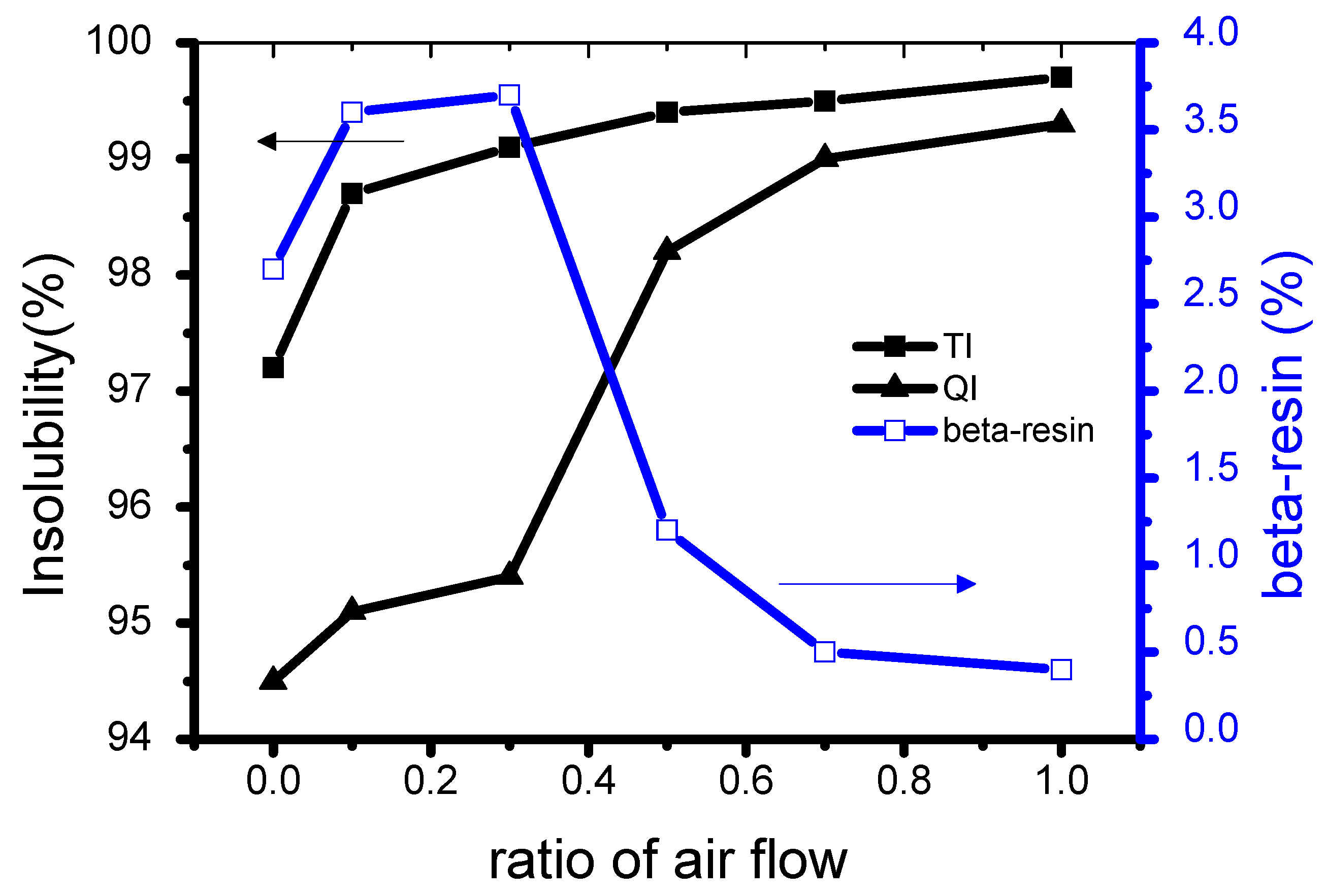
3.1. Chemical Properties of Binderless Cokes According to Air Flow Ratio
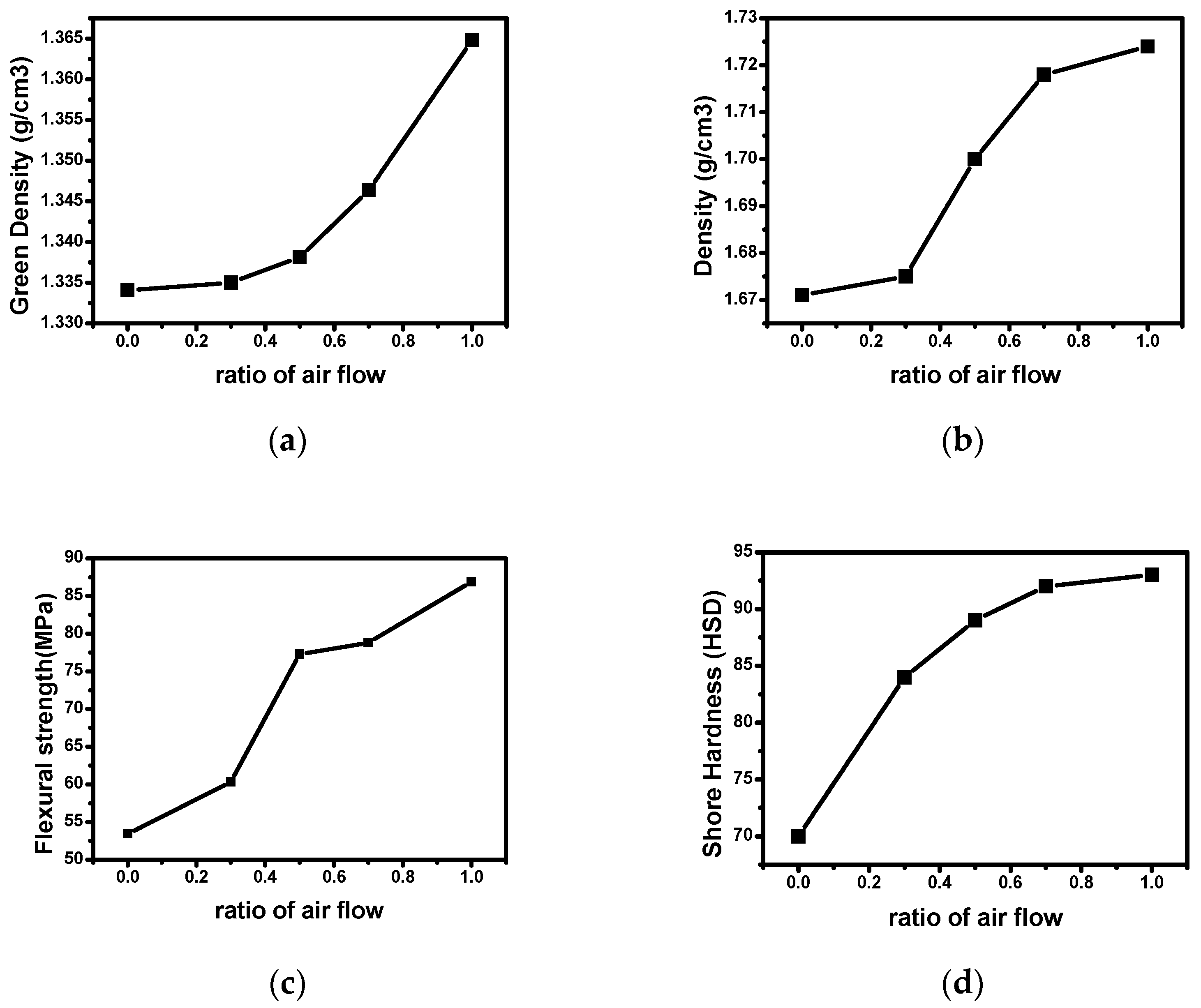
3.2. Mechanical Properties
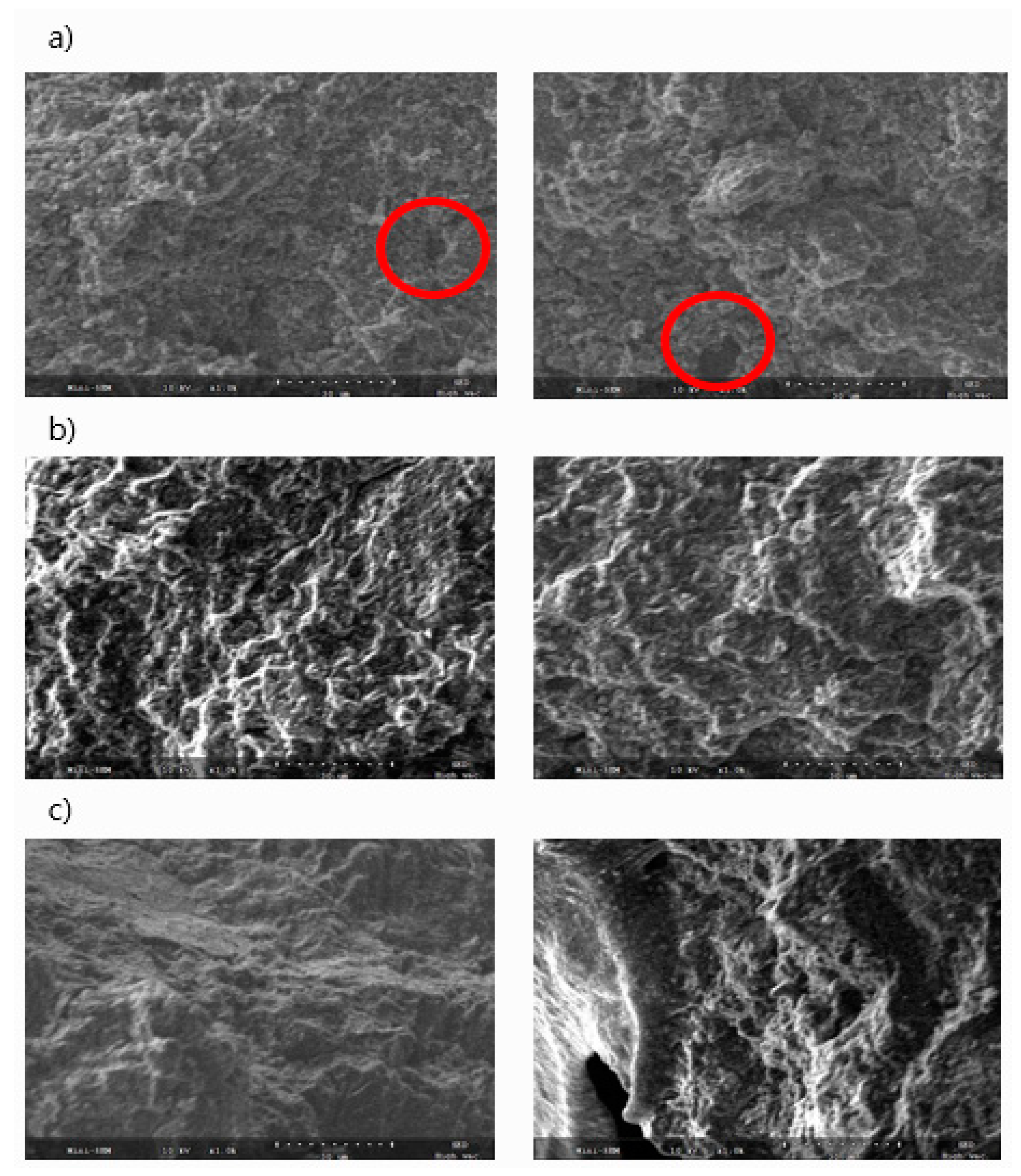
3.3. SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higinbotham, H. Colloida graphite as an adjunct lubricant for automobile engines. Proc. Inst. Automob. Eng. 1934, 29, 412–441. [Google Scholar]
- Bevilacqua, M.; Babutskyi, A.; Chrysanthou, A. A review of the catalytic oxidation of carbon-carbon composite aircraft brakes. Carbon 2015, 95, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, K.; Hagio, T.; Kobayashi, K. Graphite and boron carbide composites made by hot-pressing. J. Mater. Sci. 1981, 16, 752–762. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, D.S.; Kim, W.S.; Roh, J.S. Fabrication of isotropic bulk graphite using artificial graphite scrap. Carbon Lett. 2014, 15, 142–145. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, Z.; Wang, H.; Shi, J.; Zhang, J.; Tao, Z.; Guo, Q.; Liu, L. Microstructure and thermal/mechanical properties of short carbon fiber-reinforced natural graphite flake composites with mesophase pitch as the binder. Carbon 2013, 53, 313–320. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Q.; Shi, J.; Zhai, G.; Liu, L. Graphite blocks with high thermal conductivity derived from natural graphite flake. Carbon 2008, 46, 414–421. [Google Scholar] [CrossRef]
- Cho, J.H.; Im, J.S.; Kim, M.I.; Lee, Y.S.; Bai, Y.C. Preparation of petroleum-based binder pitch for manufacturing thermally conductive carbon molded body and comparison with commercial coal-based binder pitch. Carbon Lett. 2020, 30, 373–379. [Google Scholar] [CrossRef]
- Wang, Y.G.; Korai, Y.; Mochida, I. Carbon disc of high density and strength prepared from synthetic pitch-derived mesocarbon microbeads. Carbon 1999, 37, 1049–1057. [Google Scholar] [CrossRef]
- Gao, Y.; Song, H.; Chen, X. Self-sinterability of mesocarbon microbeads (MCMB) for preparation of high-density isotropic carbon. J. Mater. Sci. 2003, 38, 2209–2213. [Google Scholar] [CrossRef]
- Zhou, C.; McGinn, P.J. The effect of oxygen on the processing of mesocarbon microbeads to high-density carbon. Carbon 2006, 44, 1673–1681. [Google Scholar] [CrossRef]
- Blanco, C.G.; Dominguez, A.; Iglesias, M.J.; Guillen, M.D. Relation between solubility of coal tar pitches and composition of their volatile fraction. Fuel 1994, 73, 510–514. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.M.; An, D.H.; Lee, Y.S. Effects of Oxidation Process on Thermal Properties of Petroleum-based Isotropic Pitch. Appl. Chem. Eng. 2017, 28, 432–436. [Google Scholar]
- Mochida, I.; Shimizu, K.; Korai, Y.; Otsuka, H.; Sakai, Y.; Fujiyama, S. Preparation of mesophase pitch from aromatic hydrocarbons by the aid of HFBF3. Carbon 1990, 28, 311–319. [Google Scholar] [CrossRef]
- Ragan, S.; Marsh, H.J. Use of oxidized needle-coke in the preparation of carbon artifacts. Mater. Sci. 1983, 18, 3705–3711. [Google Scholar] [CrossRef]
- Fernández, J.J.; Figueiras, A.; Granda, M.; Bermejo, J.; Parra, J.B.; Menéndez, R. Modification of coal-tar pitch by air-blowing II. Influence on coke structure and properties. Carbon 1995, 33, 1235–1245. [Google Scholar] [CrossRef]
- Im, U.S.; Kim, J.Y.; Lee, B.R.; Peck, D.H.; Jung, D.H. Mechanical and electrical properties of MCMB/Chopped carbon fiber composite with different bead size. Sci. Rep. 2019, 9, 7065. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Li, T.; Fang, C.; Liu, P.; Yu, R.; Hu, J. In situ preparation and mechanical properties of CNTs/MCMBs composites. Compos. Part B 2013, 47, 290–297. [Google Scholar] [CrossRef]
- Shen, K.; Huang, Z.H.; Hu, K.; Shen, W.; Yu, S.; Yang, J.; Yang, G.; Kang, F. Advantages of natural microcrystalline graphite filler over petroleum coke in isotropic graphite preparation. Carbon 2015, 90, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Bruneton, E.; Narcy, B.; Oberlin, A. Carbon-carbon composites prepared by a rapid densification process I: Synthesis and physico-chemical data. Carbon 1997, 35, 1593–1598. [Google Scholar] [CrossRef]
- Im, U.S.; Kim, J.Y.; Lee, B.R.; Peck, D.H.; Jung, D.H. Manufacture of high density carbon blocks by self-sintering coke produced via a two-stage heat treatment of coal tar. Heliyon 2019, 5, e01341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM D2318-15. Standard Test Method for Quinoline-Insoluble (QI) Content of Tar and Pitch; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM D4072-98. Standard Test Method for Toluene-Insoluble (TI) Content of Tar and Pitch; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM D790-17. Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM D2240-15e1. Standard Test Method for Rubber Property—Durometer Hardness; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Andrzejewska, E.; Bogachi, M.B.; Andrzejewski, M.; Janaszczyk, M. Termination mechanism during the photo-induced radical cross-linking polymerization in the presence and absence of oxygen. Phys. Chem. Chem. Phys. 2003, 5, 2635–2642. [Google Scholar] [CrossRef]
- Shen, K.; Huang, Z.H.; Yang, J.; Shen, W.; Kang, F. Effect of oxidative stabilization on the sintering of mesocarbon microbeads and a study of their carbonization. Carbon 2011, 49, 3200–3211. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z.; Hong, Q.M.; Song, H.H.; Liang, J. Fabrication and mechanical properties of in situ prepared mesocarbon microbead/carbon nanotube composites. Mater. Sci. Eng. 2008, 487, 271–277. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Lu, J.; Zhang, X.; Li, K. Effects of air oxidation on mesophase pitch-based carbon/carbon composites. Carbon 2011, 49, 1416–1422. [Google Scholar] [CrossRef]
- Plancher, H.; Agarwal, P.K.; Severns, R. Improving form coke briquette strength. Fuel Process. Technol. 2002, 79, 83–92. [Google Scholar] [CrossRef]
- Zeng, S.M.; Maeda, T.; Tokumitsu, K.; Mondori, J.; Mochida, I. Preparation of isotropic pitch precursors for general purpose carbon fibers (GPCF) by air blowing—II. Air blowing of coal tar, hydrogenated coal tar, and petroleum pitches. Carbon 1993, 31, 413–419. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Wrabetz, S.; Blume, R.; Lerch, M.; McGregor, J.; Parrott, E.P.J.; Zeitler, J.A.; Gladden, L.F.; Gericke, A.K.; et al. Tuning the Acid/Base Properties of Nanocarbons by Functionalization via Amination. J. Am. Chem. Soc. 2010, 132, 9616–9630. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yan, X.; Yu, Y.; Zhao, G.J. Sustainable activated carbon fibers from liquefied wood with controllable porosity for high-performance supercapacitors. Mater. Chem. A 2014, 2, 11706–11715. [Google Scholar] [CrossRef]
- Gupta, V.K.; Atar, N.; Yola, M.L.; Eryılmaz, M.; Torul, H.; Tamer, U.; Boyacı, I.H.; Üstündag˘, Z. A novel glucose biosensor platform based on Ag@AuNPs modified graphene oxide nanocomposite and SERS application. J. Colloid Interface Sci. 2013, 406, 231–237. [Google Scholar] [CrossRef]
- Yun, S.M.; Kim, J.W.; Jung, M.J.; Nho, Y.C.; Kang, P.H.; Lee, Y.S. An XPS Study of Oxyfluorinated Multiwalled Carbon Nano Tubes. Carbon Lett. 2007, 8, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Brand, J.; Sloof, W.G.; Terryn, H.; de Wit, J.H.W. Correlation between hydroxyl fraction and O/Al atomic ratio as determined from XPS spectra of aluminium oxide layers. Surf. Interface Anal. 2004, 36, 81–88. [Google Scholar] [CrossRef]
- Pilkenton, M.; Lewman, J.; Chartoff, R. Effect of oxygen on the crosslinking and mechanical properties of a thermoset formed by free-radical photocuring. J. Appl. Polym. Sci. 2011, 119, 2359–2370. [Google Scholar] [CrossRef]


| Sample Name | Proximate Analysis (wt%) | |||
|---|---|---|---|---|
| M a | VM b | Ash | FC c | |
| Special Pitch | 0.04 | 18.28 | 0.19 | 81.49 |
| Sample Name | Raw Material | N2/Air (cc/min) | Temperature (°C) | Treating Time (h) | Yield (wt%) |
|---|---|---|---|---|---|
| N10A0 | Special pitch | 100/0 | 470 | 2 | 94.4 |
| N7A3 | 70/30 | 91.3 | |||
| N5A5 | 50/50 | 90.9 | |||
| N3A7 | 30/70 | 91.6 | |||
| N0A10 | 0/100 | 91.0 |
| Proximate Analysis (wt%) | ||||
|---|---|---|---|---|
| Sample Name | M a | VM b | Ash | FC c |
| N10A0 d | 1.30 | 8.61 | 0.65 | 89.44 |
| N7A3 | 1.26 | 7.35 | 0.43 | 90.96 |
| N5A5 | 1.13 | 7.34 | 0.40 | 91.13 |
| N3A7 | 1.55 | 6.69 | 0.37 | 91.39 |
| N0A10 | 1.57 | 6.58 | 0.39 | 91.46 |
| Atomic% | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample Name | C–C (C1s) | O–C=O (C1s) | C–O (C1s) | C1s | C–O (O1s) | O–C=O (O1s) | O1s | O1s/C1s |
| Raw pitch | 67.6 | 2.16 | 24.41 | 94.17 | 5.59 | 0.24 | 5.83 | 6.19 |
| N10A0 | 64.66 | 2.93 | 25.88 | 93.47 | 5.89 | 0.64 | 6.53 | 6.97 |
| N7A3 | 58.93 | 2.87 | 31.39 | 93.19 | 5.29 | 1.52 | 6.81 | 7.31 |
| N5A5 | 44.24 | 3.87 | 44.76 | 92.87 | 6.9 | 0.23 | 7.13 | 7.68 |
| N3A7 | 42.62 | 4.1 | 42.12 | 90.84 | 7.24 | 1.92 | 9.16 | 10.08 |
| N0A10 | 45.47 | 4.33 | 40.14 | 89.94 | 7.64 | 2.43 | 10.07 | 11.20 |
| Sample Name | Molding Pressure (MPa) | Green Density (g/cm3) | Density (g/cm3) | Flexural Strength (MPa) | Shore Hardness (HSD) |
|---|---|---|---|---|---|
| CB-N10A0 | 150 | 1.324 | 1.518 | 44 | 62 |
| CB-N7A3 | 1.335 | 1.675 | 60 | 84 | |
| CB-N5A5 | 1.338 | 1.700 | 77 | 89 | |
| CB-N3A7 | 1.346 | 1.718 | 79 | 92 | |
| CB-N0A10 | 1.365 | 1.724 | 87 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, S.H.; Lee, S.M.; Park, J.-W.; Kim, S.-S.; Jung, D.-H. The Effect of Oxygen Content in Binderless Cokes for High-Density Carbon Blocks from Coal Tar Pitch. Materials 2021, 14, 1832. https://doi.org/10.3390/ma14081832
Park S, Lee SH, Lee SM, Park J-W, Kim S-S, Jung D-H. The Effect of Oxygen Content in Binderless Cokes for High-Density Carbon Blocks from Coal Tar Pitch. Materials. 2021; 14(8):1832. https://doi.org/10.3390/ma14081832
Chicago/Turabian StylePark, Seungjoo, Seon Ho Lee, Song Mi Lee, Jin-Woo Park, Sung-Soo Kim, and Doo-Hwan Jung. 2021. "The Effect of Oxygen Content in Binderless Cokes for High-Density Carbon Blocks from Coal Tar Pitch" Materials 14, no. 8: 1832. https://doi.org/10.3390/ma14081832






