In Vitro and In Vivo Evaluation of Silver Nanoparticles Phytosynthesized Using Raphanus sativus L. Waste Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extracts
2.2. Evaluation of Chemical Composition of Radish Leaf Extracts
2.2.1. Evaluation of Total Phenolic Content of the Extracts
2.2.2. HPLC Analyses
2.3. Phytosynthesis of Silver Nanoparticles
2.4. Nanoparticles Characterization
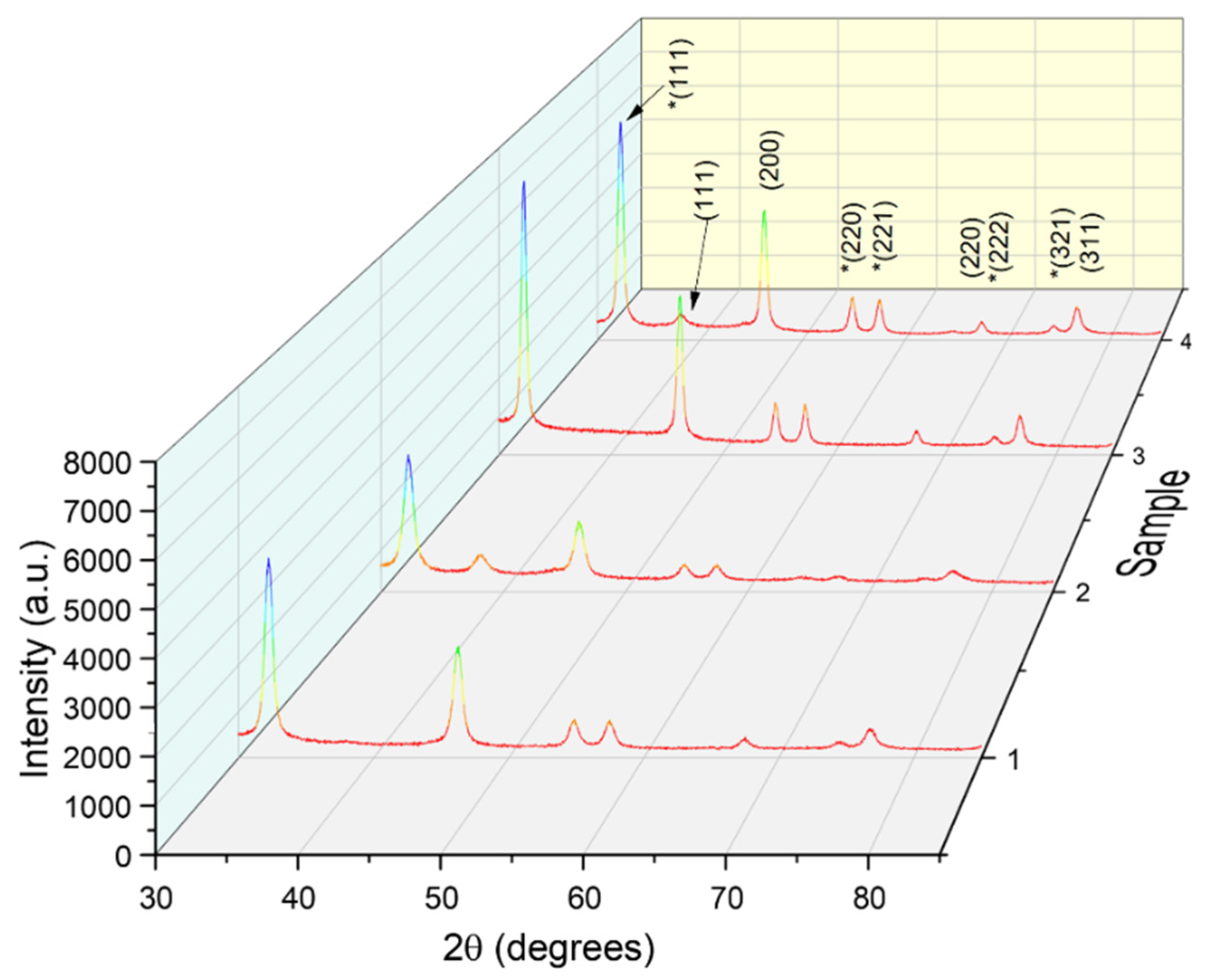
2.4.1. X-ray Diffraction
2.4.2. Morphological Observations
2.5. Antioxidant Activity
2.5.1. DPPH Assay
2.5.2. ABTS•+ Radical Cation Scavenging Assay
2.5.3. Ferric Reducing Power Assay
2.6. In Vitro Antimicrobial Activity
2.7. In Vivo Antifungal Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Chemical Composition of Leaf Extracts
3.2. Phytosynthesized Nanoparticles Characterization
3.3. Antioxidant Screening
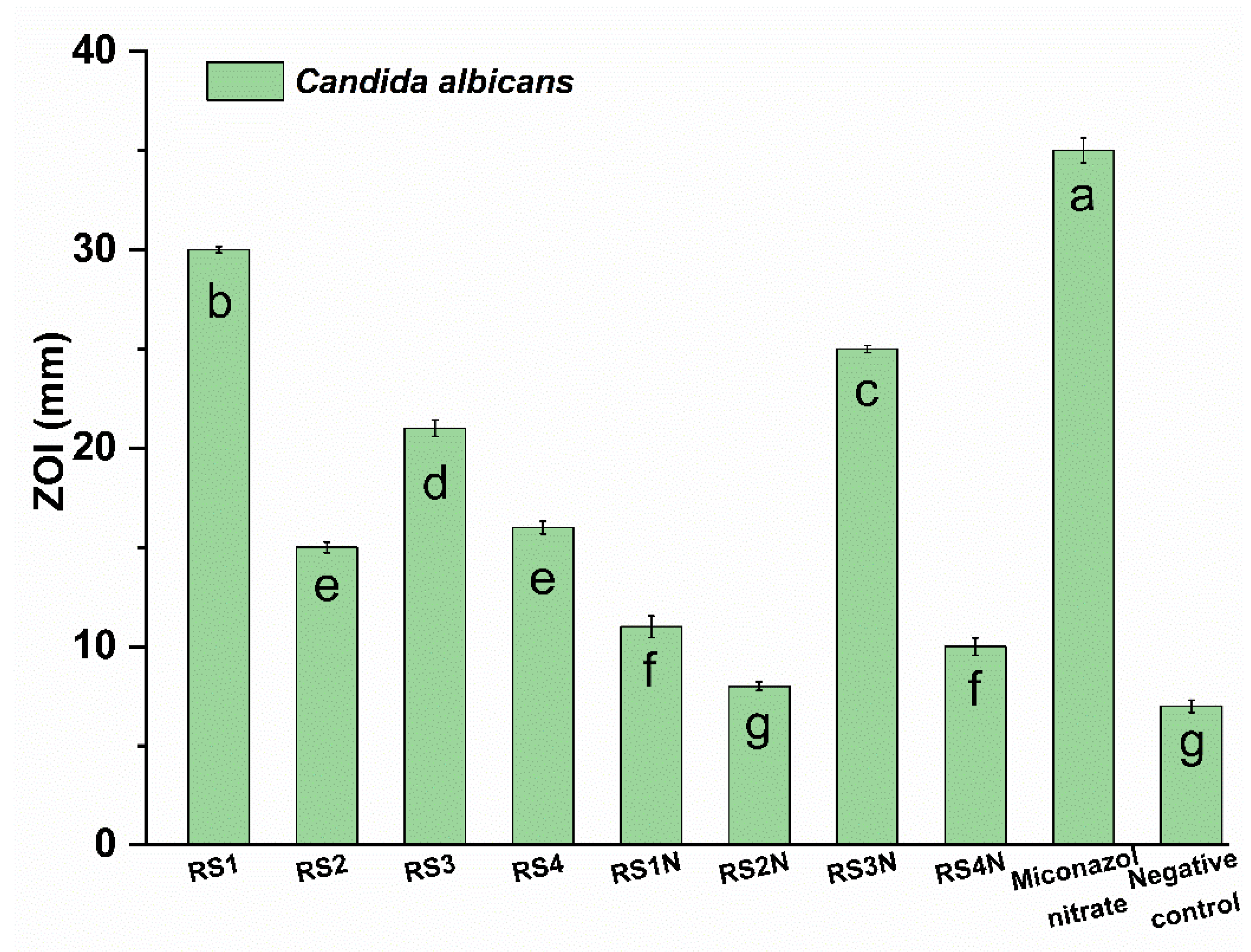
3.4. In Vitro Antimicrobial Activity
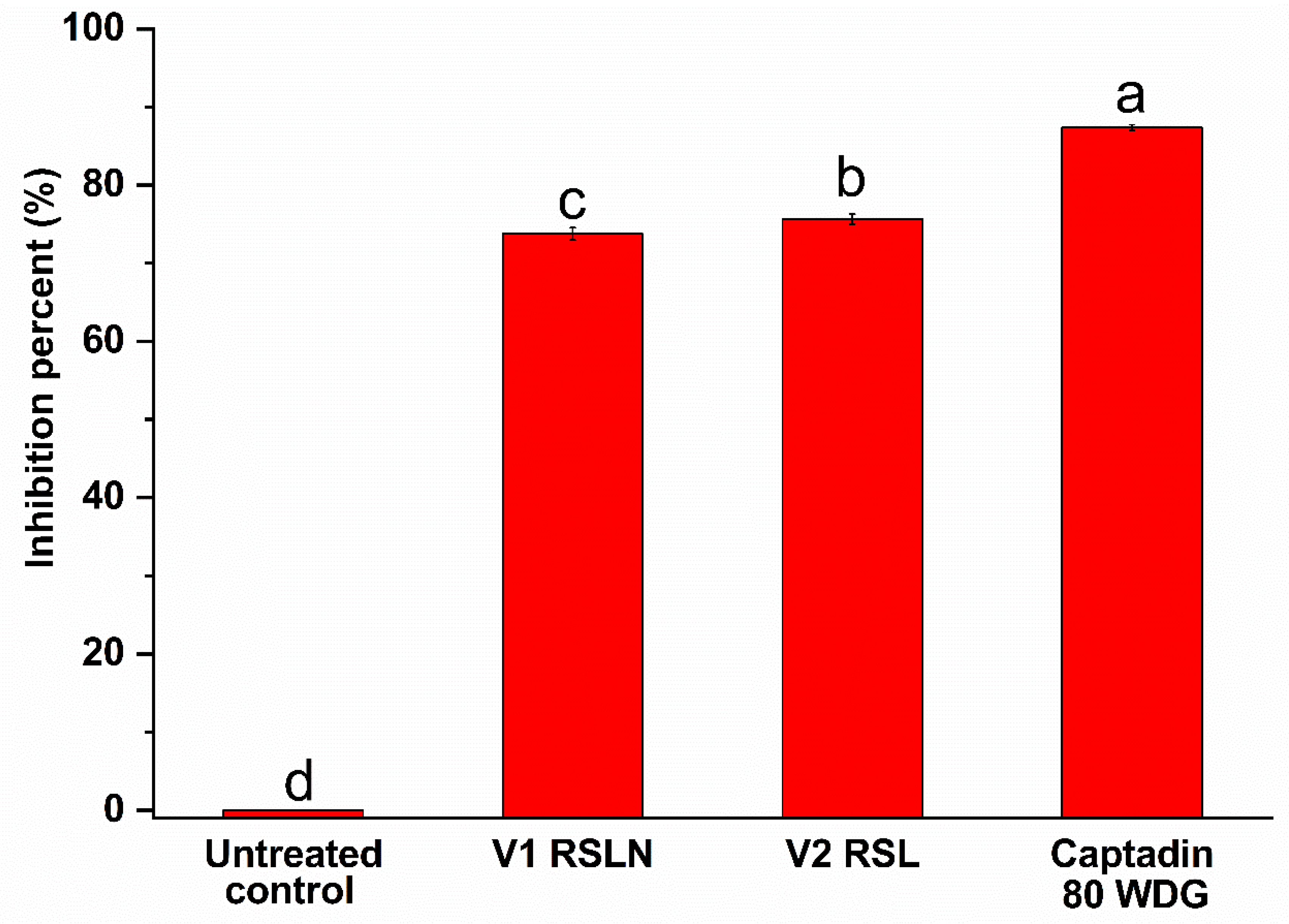
3.5. In Vivo Antifungal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zamora-Gálvez, A.; Morales-Narváez, E.; Mayorga-Martinez, C.C.; Merkoçi, A. Nanomaterials connected to antibodies and molecularly imprinted polymers as bio/receptors for bio/sensor applications. Appl. Mater. Today 2017, 9, 387–401. [Google Scholar] [CrossRef]
- Chan, K.; Shaw, D.; Simmonds, M.S.; Leon, C.J.; Xu, Q.; Lu, A.; Sutherland, I.; Ignatova, S.; Zhu, Y.P.; Verpoorte, R.; et al. Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. J. Ethnopharmacol. 2012, 140, 469–475. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Ortan, A.; Avramescu, S.M.; Fierascu, I. Phyto-nanocatalysts: Green synthesis, characterization, and applications. Molecules 2019, 24, 3418. [Google Scholar] [CrossRef] [Green Version]
- Sutan, N.A.; Manolescu, D.S.; Fierascu, I.; Neblea, A.M.; Sutan, C.; Ducu, C.; Soare, L.C.; Negrea, D.; Avramescu, S.M.; Fierascu, R.C. Phytosynthesis of gold and silver nanoparticles enhance in vitro antioxidant and mitostimulatory activity of Aconitum toxicum Reichenb. rhizomes alcoholic extracts. Mater. Sci. Eng. C 2018, 93, 746–758. [Google Scholar] [CrossRef]
- Rashid, S.; Azeem, M.; Khan, S.A.; Shah, M.M.; Ahmad, R. Characterization and synergistic antibacterial potential of green synthesized silver nanoparticles using aqueous root extracts of important medicinal plants of Pakistan. Colloids Surf. B Biointerfaces 2019, 179, 317–325. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The role of calix[n]arenes and pillar[n]arenes in the design of silver nanoparticles: Self-assembly and application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef] [Green Version]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Ehiri, R.C.; Nnaji, N.J. Phytosynthesis of silver nanoparticles using aqueous leaf extracts of Lippia citriodora: Antimicrobial, larvicidal and photocatalytic evaluations. Mater. Sci. Eng. C 2017, 75, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Li, C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf. B Biointerfaces 2017, 158, 408–415. [Google Scholar] [CrossRef]
- Sangaonkar, G.M.; Pawar, K.D. Garcinia indica mediated biogenic synthesis of silver nanoparticles with antibacterial and antioxidant activities. Colloids Surf. B Biointerfaces 2018, 164, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Onitsuka, S.; Hamada, T.; Okamura, H. Preparation of antimicrobial gold and silver nanoparticles from tea leaf extracts. Colloids Surf. B Biointerfaces 2019, 173, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Hernández, D.; Fornes, F.; Belda, R.M. Compost and vermicompost of horticultural waste as substrates for cutting rooting and growth of rosemary. Sci. Horticult. 2014, 178, 192–202. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [Green Version]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ojo, S.A.; Gueguim-Kana, E.B.; Beukes, L.S. Cocoa pod husk extract-mediated biosynthesis of silver nanoparticles: Its antimicrobial, antioxidant and larvicidal activities. J. Nanostruct. Chem. 2016, 6, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Li, W.H.; Hao, L. Biosynthesis of Au nanoparticles using agricultural waste mango peel extract and its in vitro cytotoxic effect on two normal cells. Mater. Lett. 2014, 134, 67–70. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Su, H.; Gu, L.; Han, T.; Meng, F.; Liu, C. Making good use of food wastes: Green synthesis of highly stabilized silver nanoparticles from grape seed extract and their antimicrobial activity. Food Biophys. 2015, 10, 12–18. [Google Scholar] [CrossRef]
- Ullah, N.; Li, D.; Xiaodong, C.; Yasin, S.; Muhammed Umair, M.; Van Eede, S.S.; Wei, Q. Photo-irradiation based biosynthesis of silver nanoparticles by using an ever green shrub and its antibacterial study. Dig. J. Nanomater. Biostruct. 2015, 10, 95–105. [Google Scholar]
- Ullah, N.; Yasin, S.; Abro, Z.; Liu, L.; Wei, Q. Mechanically robust and antimicrobial cotton fibers loaded with silver nanoparticles: Synthesized via Chinese holly plant leaves. Int. J. Text. Sci. 2014, 3, 1–5. [Google Scholar]
- Yuvakkumar, R.; Suresh, J.; Nathanael, A.J.; Sundrarajan, M.; Hong, S.I. Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater. Sci. Eng. C 2014, 41, 17–27. [Google Scholar] [CrossRef]
- Nishanthi, R.; Malathi, S.; Paul, J.S.; Palani, P. Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Mater. Sci. Eng. C 2019, 96, 693–707. [Google Scholar]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Fierascu, I.; Lungulescu, E.M.; Nicula, N.; Somoghi, R.; Diţu, L.M.; Ungureanu, C.; Sutan, A.N.; Drăghiceanu, O.A.; Paunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2020, 55, 1915–1932. [Google Scholar] [CrossRef]
- Lee, O.N.; Park, H.Y. Assessment of genetic diversity in cultivated radishes (Raphanus sativus) by agronomic traits and SSR markers. Sci. Horticult. 2017, 223, 19–30. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Perez, R. Raphanus sativus (radish): Their chemistry and biology. Sci. World J. 2004, 4, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Park, M.; Suh, J.H.; Choi, H.S. Protective effects of an extract of young radish (Raphanus sativus L) cultivated with sulfur (sulfur-radish extract) and of sulforaphane on carbon tetrachloride-induced hepatotoxicity. Biosci. Biotechnol. Biochem. 2008, 72, 1176–1182. [Google Scholar] [CrossRef] [Green Version]
- Goyeneche, R.; Fanovich, A.; Rodrigues, C.R.; Nicolao, M.C.; Di Scala, K. Supercritical CO2 extraction of bioactive compounds from radish leaves: Yield, antioxidant capacity and cytotoxicity. J. Supercrit. Fluids 2018, 135, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Akbar, S.; Haleem, K.S.; Tauseef, I.; Rehman, W.; Ali, N.; Hasan, M. Raphanus sativus mediated synthesis, characterization and biological evaluation of zinc oxide nanoparticles. Nanosci. Nanotechnol. Lett. 2017, 9, 2005–2012. [Google Scholar] [CrossRef]
- Kumar, A.B.V.K.; Saila, E.S.; Narang, P.; Aishwarya, M.; Raina, R.; Gautam, M.; Shankar, E.G. Biofunctionalization and biological synthesis of the ZnO nanoparticles: The effect of Raphanus sativus (white radish) root extract on antimicrobial activity against MDR strain for wound healing applications. Inorg. Chem. Commun. 2019, 100, 101–106. [Google Scholar] [CrossRef]
- Williams, S.D.; Boehm, M.J.; Mitchell, T.K. Fungal and Fungal-Like Diseases of Plants. 2017. Available online: https://ohioline.osu.edu/factsheet/plpath-gen-7 (accessed on 2 December 2019).
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Boxi, S.S.; Mukherjee, K.; Paria, S. Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology 2016, 27, 085103. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.J.; Paria, S. Use of sulfur nanoparticles as a green pesticide on Fusarium solani and Venturia inaequalis phytopathogens. RSC Adv. 2013, 3, 10471–10478. [Google Scholar] [CrossRef]
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process. Biochem. 2016, 51, 1306–1313. [Google Scholar] [CrossRef]
- Lu, Y.; Du, Y.; Qin, X.; Wu, H.; Huang, Y.; Cheng, Y.; Wei, Y. Comprehensive evaluation of effective polyphenols in apple leaves and their combinatory antioxidant and neuroprotective activities. Ind. Crops Prod. 2019, 129, 242–252. [Google Scholar] [CrossRef]
- Stojiljković, D.; Arsić, I.; Tadić, V. Extracts of wild apple fruit (Malus sylvestris (L.) Mill. Rosaceae) as a source of antioxidant substances for use in production of nutraceuticals and cosmeceuticals. Ind. Crops Prod. 2016, 80, 165–176. [Google Scholar] [CrossRef]
- Sutan, N.A.; Fierascu, I.; Fierascu, R.C.; Manolescu, D.S.; Soare, L.C. Comparative analytical characterization and in vitro citogenotoxic activity evaluation of Asplenium scolopendrium L. leaves and rhizome extracts prior to and after Ag nanoparticles phytosynthesis. Ind. Crops Prod. 2016, 83C, 379–386. [Google Scholar] [CrossRef]
- Maurya, S.; Singh, D. Quantitative analysis of flavonoids in Adhatoda vasica Nees extracts. Der Pharma Chem 2010, 2, 242–246. [Google Scholar]
- Ortan, A.; Fierascu, I.; Ungureanu, C.; Fierascu, R.C.; Avramescu, S.M.; Dumitrescu, O.; Dinu-Pirvu, C.E. Innovative phytosynthesized silver nanoarchitectures with enhanced antifungal and antioxidant properties. Appl. Surf. Sci. 2015, 358, 540–548. [Google Scholar] [CrossRef]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Fierascu, R.C.; Ortan, A.; Soare, L.C.; Paunescu, A. In vitro antioxidant and antifungal properties of Achillea millefolium L. Rom. Biotechnol. Lett. 2015, 20, 10626–10636. [Google Scholar]
- Ohnishi, M.; Morishita, H.; Toda, S.; Shirataki, Y.; Kimura, M. Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. Phytochemistry 1994, 36, 579–583. [Google Scholar] [CrossRef]
- Costea, T.; Vlase, L.; Istudor, V.; Gîrd, C.E.; Popescu, M.L. Researches upon indigenous herbal products for therapeutic valorification in metabolic diseases. Note II. Polyphenols content, antioxidant activity and cytoprotective effect of Betulae folium dry extract. Farmacia 2014, 62, 961–970. [Google Scholar]
- Costea, T.; Lupu, A.R.; Vlase, L.; Nencu, I.; Gîrd, C.E. Phenolic content and antioxidant activity of a raspberry leaf dry extract. Rom. Biotechnol. Lett. 2016, 21, 11345–11356. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Popescu, M.L.; Costea, T.; Nencu, I.; Duţu, L.E.; Gîrd, C.E. Polyphenols contents and antioxidant activity of some Romanian wild edible mushrooms. Farmacia 2016, 64, 231–236. [Google Scholar]
- Wassermann, B.; Müller, H.; Berg, G. An apple a day: Which bacteria do we eat with organic and conventional apples? Front. Microbiol. 2019, 10, 1629. [Google Scholar] [CrossRef] [Green Version]
- Niessen, L. PCR-based diagnosis and quantification of mycotoxin producing fungi. Int. J. Food Microbiol. 2007, 119, 38–46. [Google Scholar] [CrossRef]
- Ungureanu, C.; Călinescu, M.; Ferdes, M.; Soare, L.; Vizitiu, D.; Fierascu, I.; Fierascu, R.C.; Raileanu, S. Isolation and cultivation of some pathogen fungi from apple and grapevines grown in Arges county. Rev. Chim. 2019, 70, 3913–3916. [Google Scholar] [CrossRef]
- Ungureanu, C.; Ferdes, M. Evaluation of antioxidant and antimicrobial activities of torularhodin. Adv. Sci. Lett. 2012, 18, 50–53. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Iordache, S.M.; Iordache, A.M.; Bunghez, I.R.; Ghiurea, M.; Badea, N.; Fierascu, R.C.; Stamatin, I. Eco-designed biohybrids based on liposomes, mint-nanosilver and carbon nanotubes for antioxidant and antimicrobial coating. Mater. Sci. Eng. C 2014, 39, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.G.; Fritz, R.; Del Valle, C.; Roura, S.I. Antimicrobial activity of essential oils on the native microflora of organic swiss chard. LWT Food Sci. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Hugo, W.B.; Russel, A.D. Pharmaceutical Microbiology, 6th ed.; Blackwell Science: London, UK, 1998; pp. 248–253. [Google Scholar]
- Pfaller, M.A.; Sheehan, D.J.; Rex, J.H. Determination of fungicidal activities against yeasts and molds: Lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 2004, 17, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Yuri, J.A.; Moggia, C.; Sepulveda, A.; Poblete-Echeverría, C.; Valdés-Gómez, H.; Torres, C.A. Effect of cultivar, rootstock, and growing conditions on fruit maturity and postharvest quality as part of a six-year apple trial in Chile. Sci. Horticult. 2019, 253, 70–79. [Google Scholar] [CrossRef]
- Chevalier, M.; Lespinasse, Y.; Renaudin, S. A microscopic study of the different classes of symptoms coded by the Vf gene in apple resistance to scab (Venturia inaequalis). Plant Pathol. 1991, 40, 249–256. [Google Scholar] [CrossRef]
- Kellerhals, M.; Fouillet, A.; Lespinasse, Y. Effect of the scab inoculum and the susceptible parent on resistance to apple scab (Venturia inaequalis) in the progenies of crosses to the scab resistant cv ‘Florina’. Agronomie 1993, 13, 631–636. [Google Scholar] [CrossRef]
- Spencer, D.M. Standardized methods for the evaluation of fungicides to control cucumber powdery mildew. In Crop Protection Agents—Their Biological Evaluation; McFarlane, N.R., Ed.; Academic Press: London, UK, 1977; pp. 455–464. [Google Scholar]
- Wurms, K.V.; Chee, A.A. Control of powdery mildew (Podosphaera leucotricha) on apple seedlings using anhydrous milk fat and soybean oil emulsions. N. Z. Plant Prot. 2011, 64, 201–208. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.C.; Jeon, G.E.; Kim, C.S.; Seo, J.H. Effect of the size and shape of silver nanoparticles on bacterial growth and metabolism by monitoring optical density and fluorescence intensity. Biotechnol. Bioproc. E 2017, 22, 210–217. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.G. Polyphenols content and antioxidant activities of Taraxacum officinale FH Wigg (dandelion) leaves. Int. J. Pharmacog. Phytochem. Res. 2019, 6, 889–893. [Google Scholar]
- Ilaiyaraja, N.; Likhith, K.R.; Babu, G.S.; Khanum, F. Optimisation of extraction of bioactive compounds from Feronia limonia (wood apple) fruit using response surface methodology (RSM). Food Chem. 2015, 173, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Bastola, K.P.; Guragain, Y.N.; Bhadriraju, V.; Vadlani, P.V. Evaluation of standards and interfering compounds in the determination of phenolics by Folin-Ciocalteu assay method for effective bioprocessing of biomass. Am. J. Anal. Chem. 2017, 8, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, A.; Petrović, P.; Ðorđević, V.; Zdunić, G.; Šavikin, K.; Bugarski, B. Polyphenols extraction from plant sources. Lek. Sirovine 2017, 37, 46–51. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Georgiev, M.I.; Sieniawska, E. Innovative approaches for recovery of phytoconstituents from medicinal/aromatic plants and biotechnological production. Molecules 2020, 25, 309. [Google Scholar] [CrossRef] [Green Version]
- Dudonné, S.; Vitrac, X.; Coutierré, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD and ORAC assays. J. Agric. Food Chem. 2009, 57, 1764–1778. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-mediated synthesis of silver nanoparticles: Their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Vasyliev, G.; Vorobyova, V.; Skiba, M.; Khrokalo, L. Green synthesis of silver nanoparticles using waste products (apricot and black currant pomace) aqueous extracts and their characterization. Adv. Mater. Sci. Eng. 2020, 2020, 4505787. [Google Scholar] [CrossRef]
- Ansar, S.; Tabassum, H.; Aladwan, N.S.M.; Ali, M.N.; Almaarik, B.; AlMahrouqi, S.; Abudawood, M.; Banu, N.; Alsubki, R. Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci. Rep. 2020, 10, 18564. [Google Scholar] [CrossRef]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro evaluation of antioxidant and antibacterial potential of greensynthesized silver nanoparticles using Prosopis farcta fruit extract. Iran. J. Pharm. Res. 2019, 18, 430–455. [Google Scholar] [PubMed]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of plant extracts on the characteristics of silver nanoparticles for topical application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Ortan, A.; Fierascu, I.C.; Fierascu, I. In vitro and in vivo evaluation of antioxidant properties of wild-growing plants. A short review. Curr. Opin. Food Sci. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Muthuswamy, S.; Rupasinghe, H.P.V. Fruit phenolics as natural antimicrobial agents: Selective antimicrobial activity of catechin, chlorogenic acid and phloridzin. J. Food Agric. Environ. 2007, 5, 81–85. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Merkl, R.; Hradkova, I.; Filip, V.; Smidrkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Evensen, N.A.; Braun, P.C. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 2009, 55, 1033–1039. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizur, M.A.R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Yu, L.; Memon, H.; Bhavsar, P.; Yasin, S. Fabrication of alginate fibers loaded with silver nanoparticles biosynthesized via dolcetto grape leaves (Vitis vinifera cv.): Morphological, antimicrobial characterization and in vitro release studies. Mater. Focus 2016, 5, 216–221. [Google Scholar] [CrossRef]
- Fierascu, I.; Fierascu, I.C.; Brazdis, R.I.; Baroi, A.M.; Fistos, T.; Fierascu, R.C. Phytosynthesized metallic nanoparticles-between nanomedicine and toxicology. A brief review of 2019′s findings. Materials 2020, 13, 574. [Google Scholar] [CrossRef] [Green Version]






| Crude Extracts | Total Phenols (mg GAE/g DW) | Chlorogenic Acid (μg/mL) | Ferulic Acid (μg/mL) | Rutin (μg/mL) | Rosmarinic Acid (μg/mL) | Quercetin (μg/mL) | Chalcone (μg/mL) |
|---|---|---|---|---|---|---|---|
| RS1 | 12.42 ± 0.72 a | 41.024 ± 0.031 b | 53.163 ± 0.022 b | 105.954 ± 0.03 b | 0.0993 ± 0.001 b | 6.585 ± 0.012 b | 12.749 ± 0.014 a |
| RS2 | 5.32 ± 0.21 c | - | 1.074 ± 0.003 d | 3.104 ± 0.013 d | - | - | 1.665 ± 0.009 d |
| RS3 | 8.98 ± 0.37 b | 81.048 ± 0.053 a | 90.443 ± 0.041 a | 279.912 ± 0.04 a | 0.156 ± 0.001 a | 20.655 ± 0.015 a | 5.741 ± 0.011 b |
| RS4 | 4.49 ± 0.18 c | - | 50.632 ± 0.028 c | 11.12 ± 0.021 c | - | 2.718 ± 0.011 c | 2.710 ± 0.009 c |
| Scheme. | Analysed Peak | FWHM (Full Width at Half Maximum)—XRD | Crystallite Size (nm)—XRD | Average Diameter (nm)—TEM |
|---|---|---|---|---|
| RS1N | 200 | 0.784 | 11.51 | 12 |
| RS2N | 200 | 0.957 | 9.43 | 11 |
| RS3N | 200 | 0.525 | 17.16 | 16 |
| RS4N | 200 | 0.607 | 14.87 | 15 |
| Extract/Nanoparticles | Method—EC50 (mg/mL) | ||
|---|---|---|---|
| DPPH | ABTS | Ferric Reducing Power | |
| RS1 | 3.0368 ± 0.2536 e,f | 0.3972 ± 0.00277 d | 1.6028 ± 0.1250 c |
| RS1N | 1.9746 ± 0.1281 f | 0.3478 ± 0.02998 d | 1.3976 ± 0.1396 c |
| RS2 | 39.065 ± 0.4315 a | 49.85 ± 0.1296 a | 10.17 ± 0.8414 a |
| RS2N | 21.790 ± 0.8768 d | 12.37 ± 0.7484 c | 8.4650 ± 0.2333 b |
| RS3 | 3.7026 ± 0.1232 e | 0.8801 ± 0.1302 d | 1.9610 ± 0.0091 c |
| RS3N | 3.3596 ± 0.0738 e | 0.3582 ± 0.0273 d | 1.9565 ± 0.1851 c |
| RS4 | 29.35 ± 0.3676 b | 23.67 ± 2.0718 b | 10.34 ± 0.2404 a |
| RS4N | 27.015 ± 0.8280 c | 11.54 ± 1.47077 c | 10.20 ± 0.1838 a |
| Variant/Product Name | Replication | Assessed Leaves (no) | Attacked Leaves (no) | Frequency [F%] | Intensity (I) [Scale 1–6] | Damage Degree DD [%] | Inhibition Percent [%] |
|---|---|---|---|---|---|---|---|
| Untreated control | R1_R3 | 46 | 44 | 95.60 | 6.00 | 5.74 | 0.00 |
| V1 RS1n | R1_R3 | 48 | 22 | 46.76 | 3.70 | 1.73 | 69.86 |
| V2 RS1 | R1_R3 | 48 | 14 | 29.30 | 2.00 | 0.59 | 89.72 |
| Standard Sulfomat 80 PU | R1_R3 | 50 | 12 | 23.80 | 1.80 | 0.50 | 91.29 |
| AVG_v | 48.,67 | 16.00 | 33.29 | 2.50 | 0.94 | 83.62 | |
| Indicators | STDEV | 1.1547 | 5.2915 | 11.9879 | 1.0440 | 0.6856 | 11.9452 |
| VAR | 2.3727 | 33.0719 | 36.0142 | 41.7612 | 72.9403 | 14.2846 |
| Variant/Product Name | Replication | Assessed Leaves (no) | Attacked Leaves (no) | Frequency [F%] | Intensity (I) [Scale 1–6] | Damage Degree DD [%] | Inhibition Percent [%] |
|---|---|---|---|---|---|---|---|
| Untreated control | R1_R3 | 47 | 29 | 62.37 | 3.67 | 2.30 | 0.00 |
| V1 RS1N | R1_R3 | 51 | 16 | 21.94 | 2.50 | 0.60 | 73.76 |
| V2 RS1 | R1_R3 | 47 | 24 | 37.53 | 1.50 | 0.56 | 75.65 |
| Captadin 80 WDG | R1_R3 | 52 | 17 | 21.94 | 1.33 | 0.29 | 87.39 |
| AVG_v | 50.00 | 19.00 | 27.14 | 1.78 | 0.48 | 78.93 | |
| Indicators | STDEV | 2.6458 | 4.3589 | 9.0007 | 0.6322 | 0.1698 | 7.3844 |
| VAR | 5.2915 | 22.9416 | 33.1642 | 35.5816 | 35.0467 | 9.3552 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, C.; Fierascu, I.; Fierascu, R.C.; Costea, T.; Avramescu, S.M.; Călinescu, M.F.; Somoghi, R.; Pirvu, C. In Vitro and In Vivo Evaluation of Silver Nanoparticles Phytosynthesized Using Raphanus sativus L. Waste Extracts. Materials 2021, 14, 1845. https://doi.org/10.3390/ma14081845
Ungureanu C, Fierascu I, Fierascu RC, Costea T, Avramescu SM, Călinescu MF, Somoghi R, Pirvu C. In Vitro and In Vivo Evaluation of Silver Nanoparticles Phytosynthesized Using Raphanus sativus L. Waste Extracts. Materials. 2021; 14(8):1845. https://doi.org/10.3390/ma14081845
Chicago/Turabian StyleUngureanu, Camelia, Irina Fierascu, Radu Claudiu Fierascu, Teodora Costea, Sorin Marius Avramescu, Mirela Florina Călinescu, Raluca Somoghi, and Cristian Pirvu. 2021. "In Vitro and In Vivo Evaluation of Silver Nanoparticles Phytosynthesized Using Raphanus sativus L. Waste Extracts" Materials 14, no. 8: 1845. https://doi.org/10.3390/ma14081845









