Simulation of Leather Visco-Elastic Behavior Based on Collagen Fiber-Bundle Properties and a Meso-Structure Network Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Tensile Experiments on Leathers and Fiber-Bundles
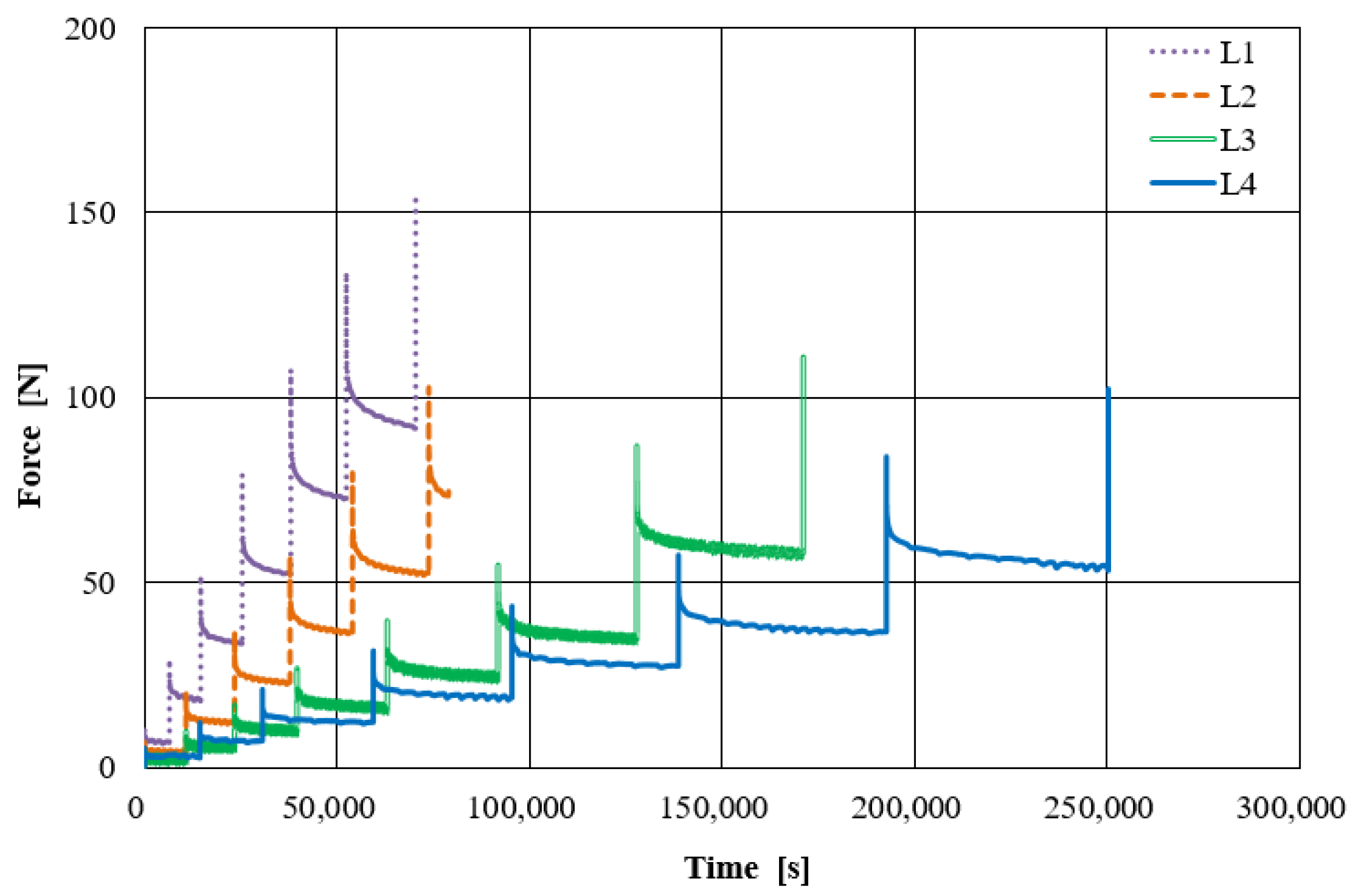
2.1.1. Tests on Fiber-Bundles
2.1.2. Tests on Leather Samples
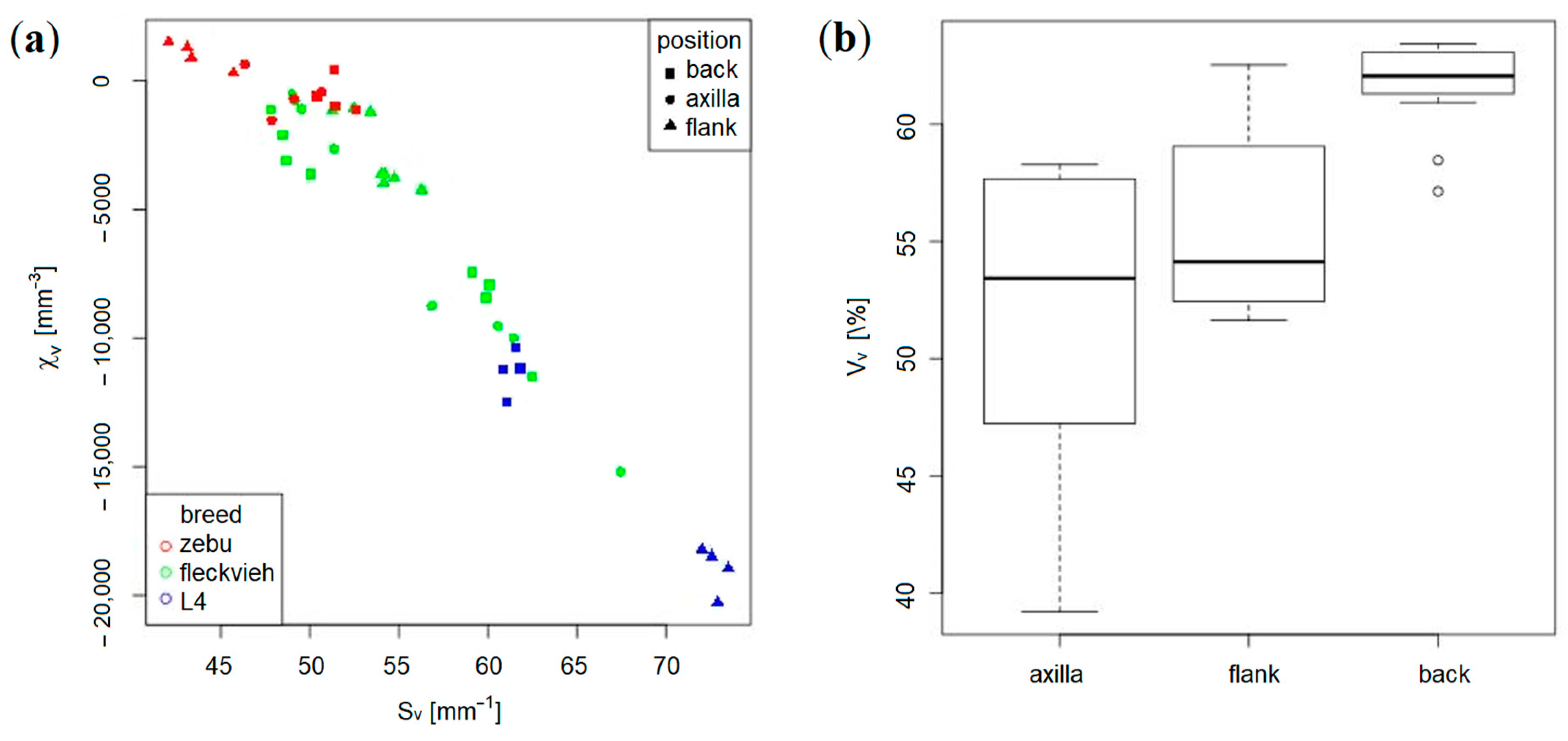
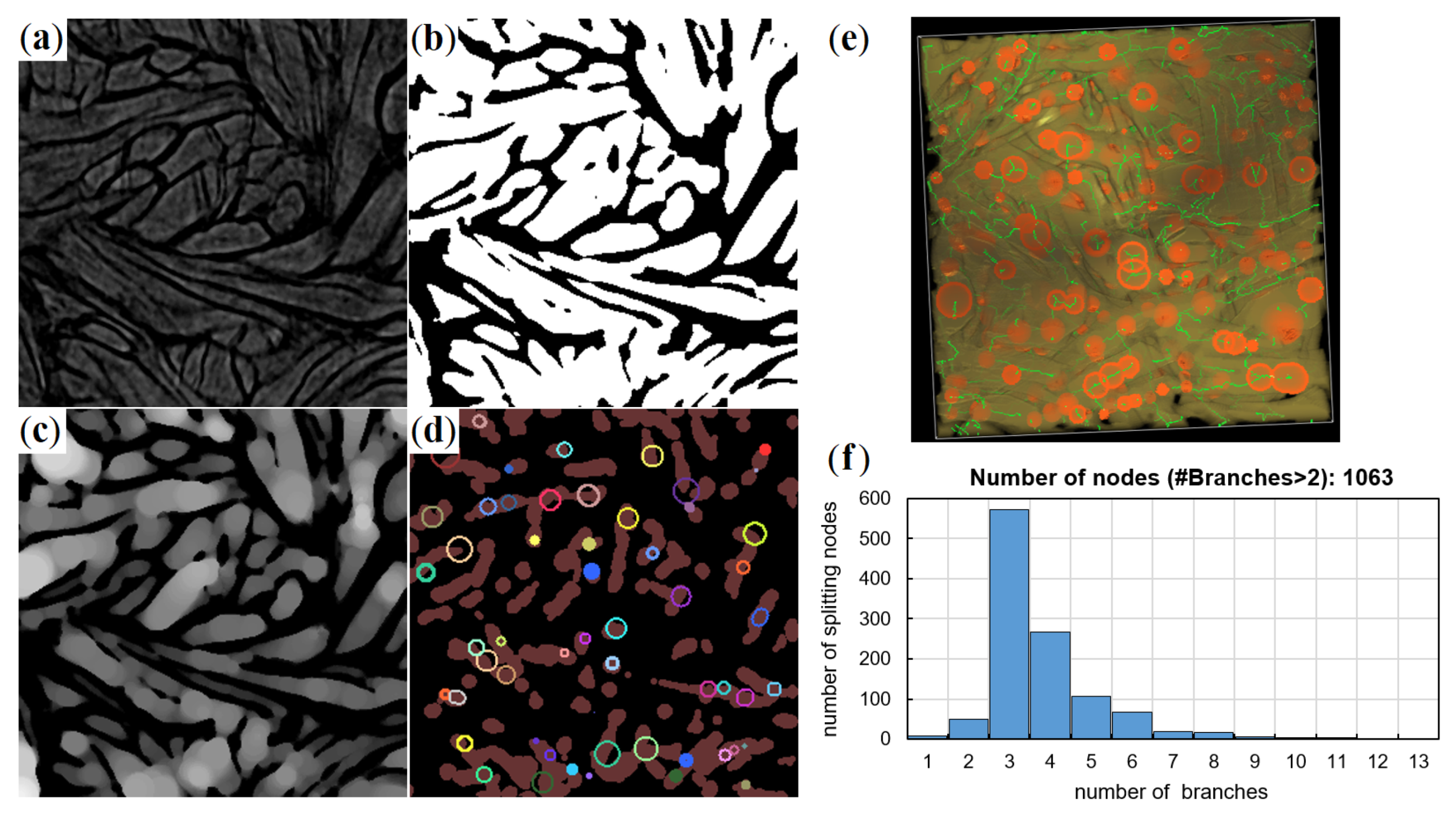
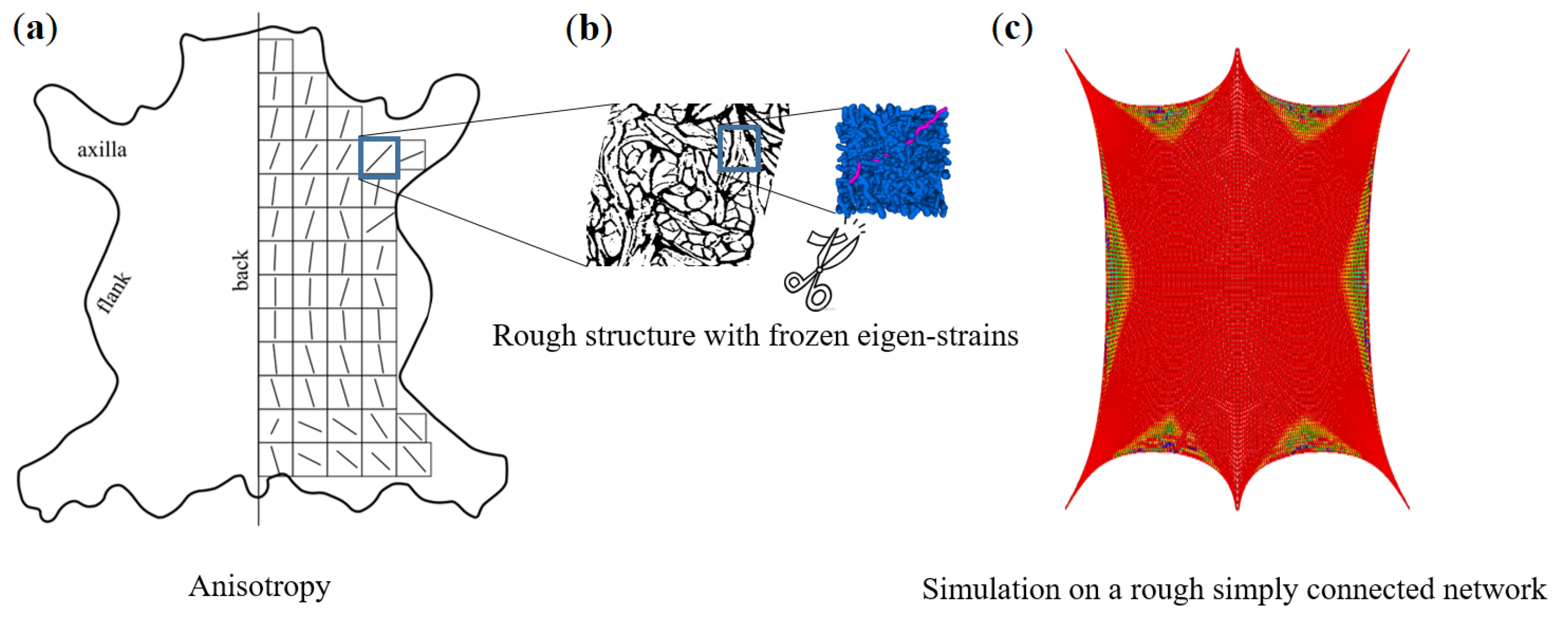
2.2. Micro-Computed Tomography of the Meso-Scale Collagen Fiber-Bundle Networks
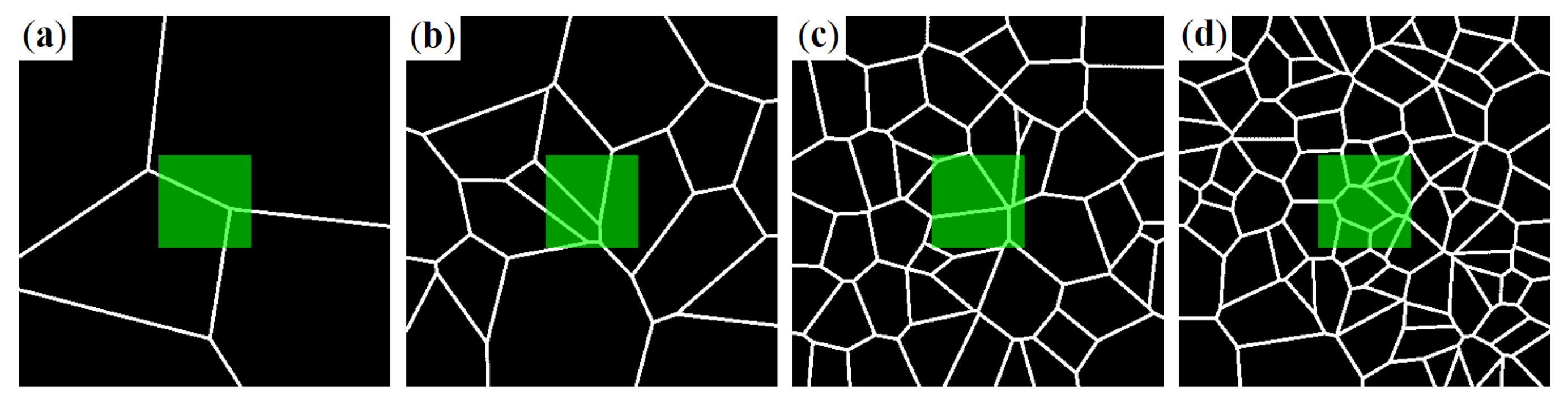
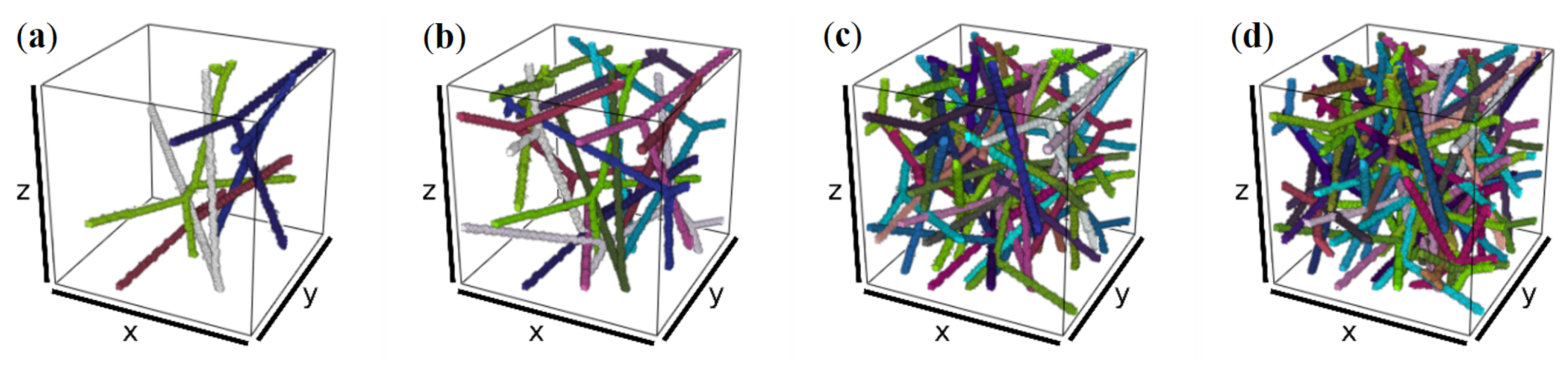
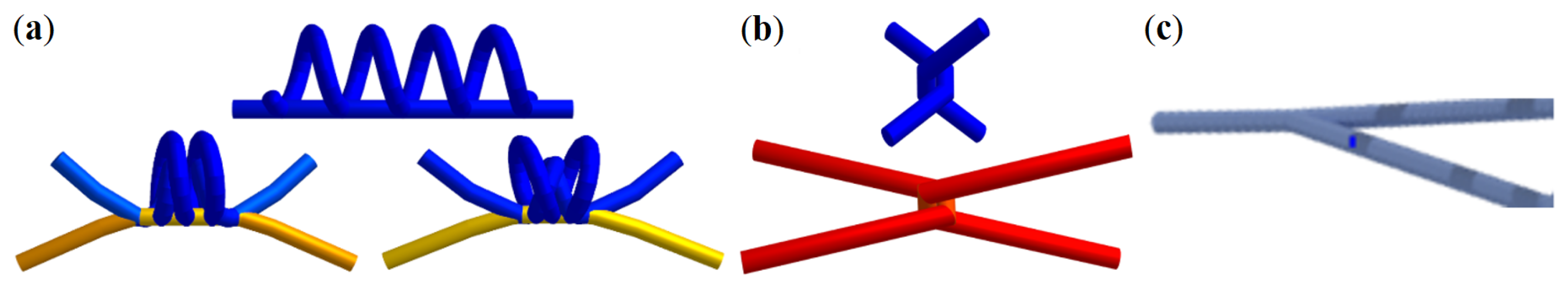
2.3. Network Model
2.4. Simulation Tool
2.5. Simulation on a Meso-Structure of Non-Linear Elastic Fibers on the Scale of Several Millimeters
3. Results and Discussion
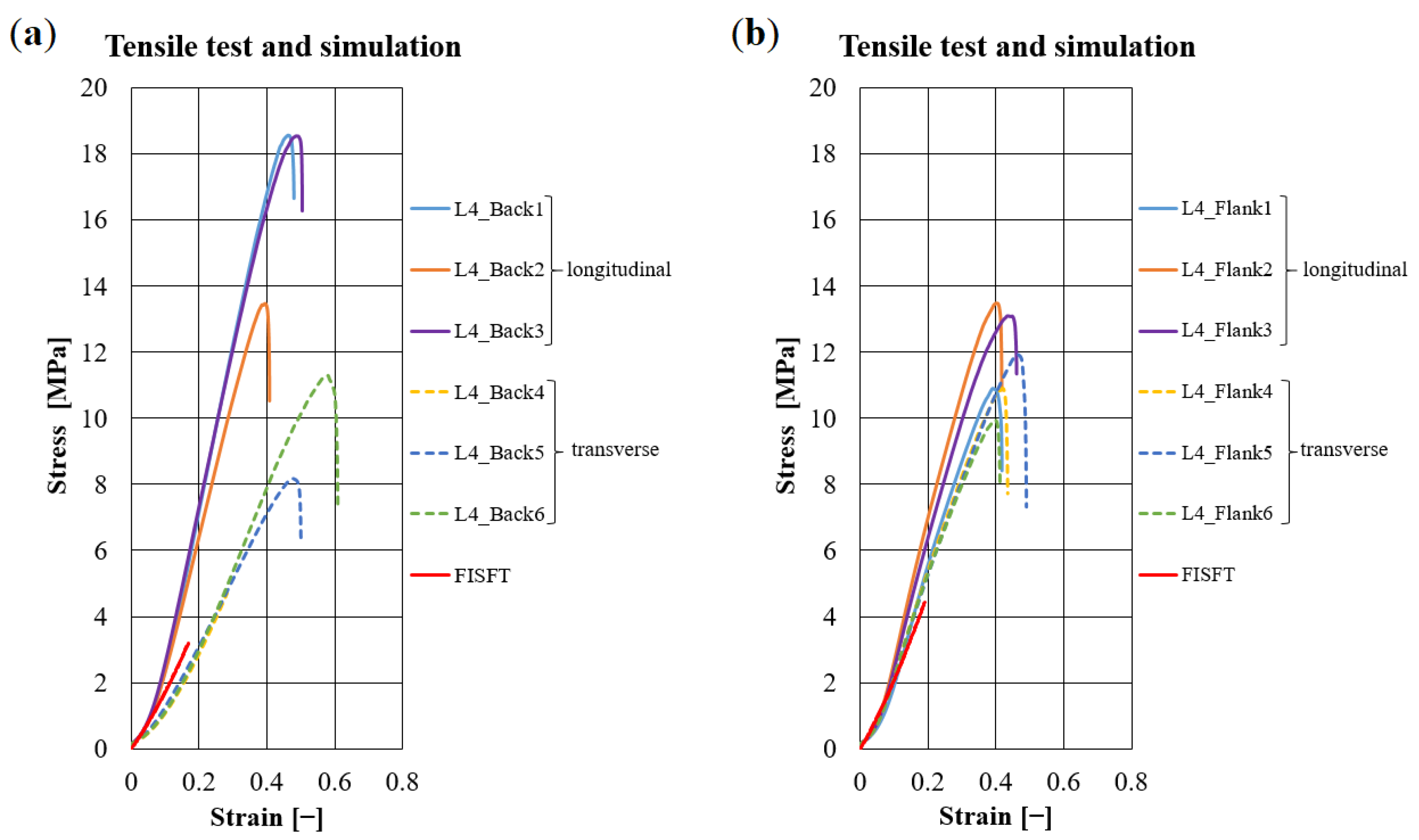
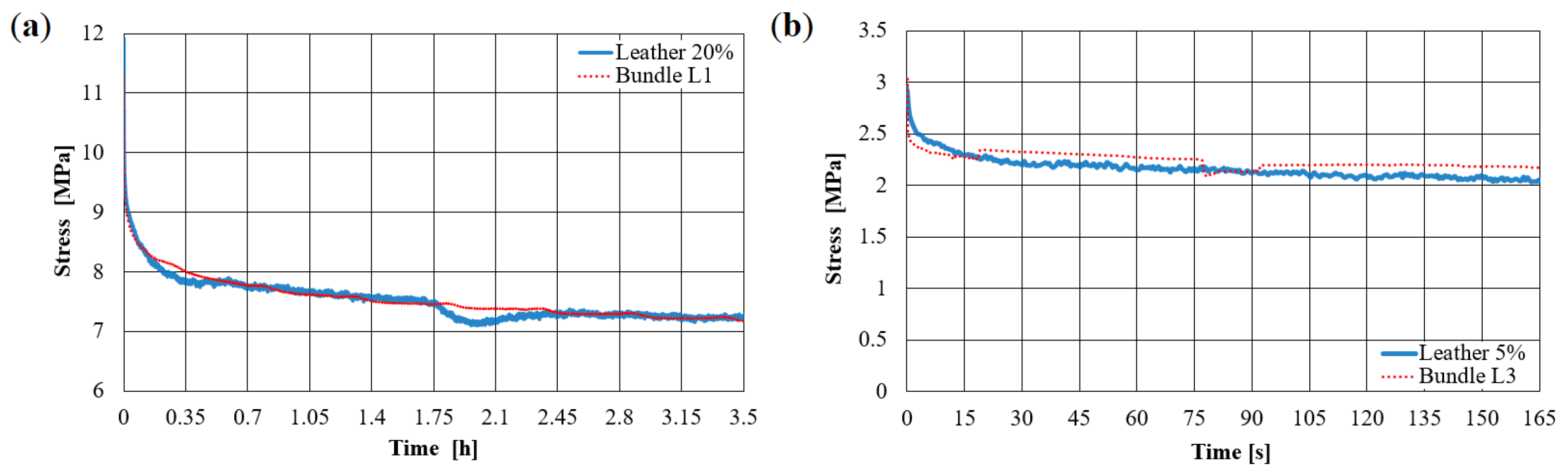
3.1. Validation Based on Synthetically Tanned Leather and Discussion
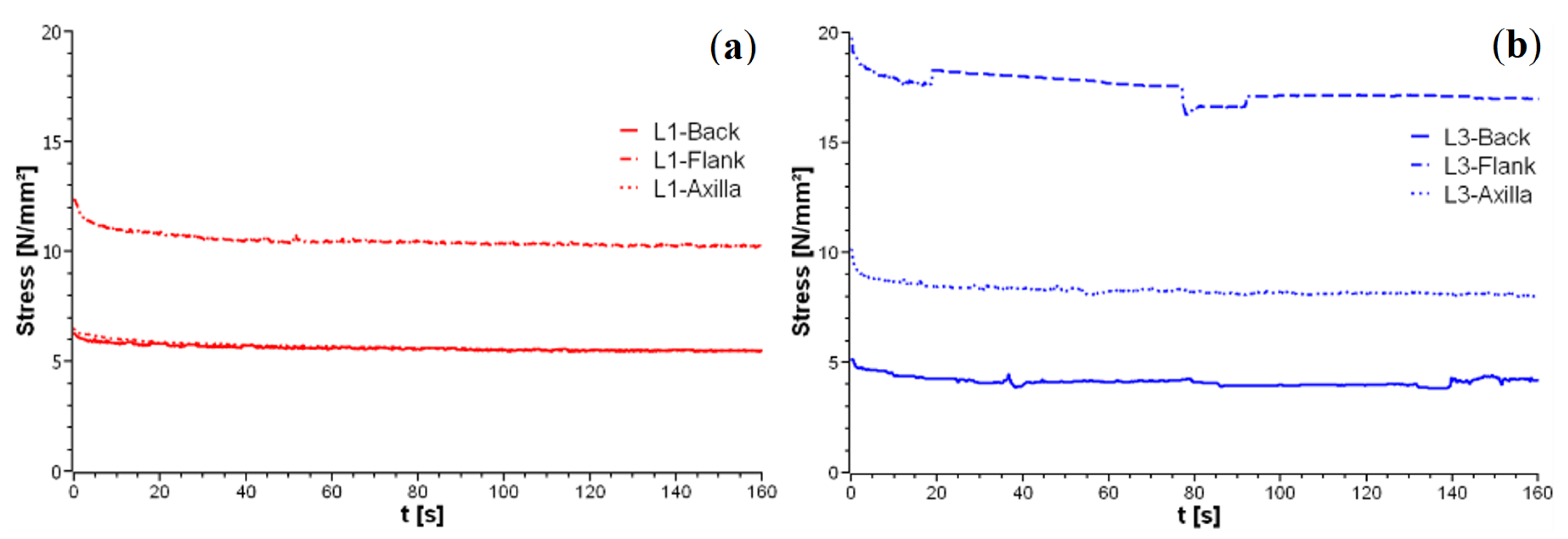
3.2. Prediction of Visco-Elastic Properties of Leather Samples and Relaxation Tests
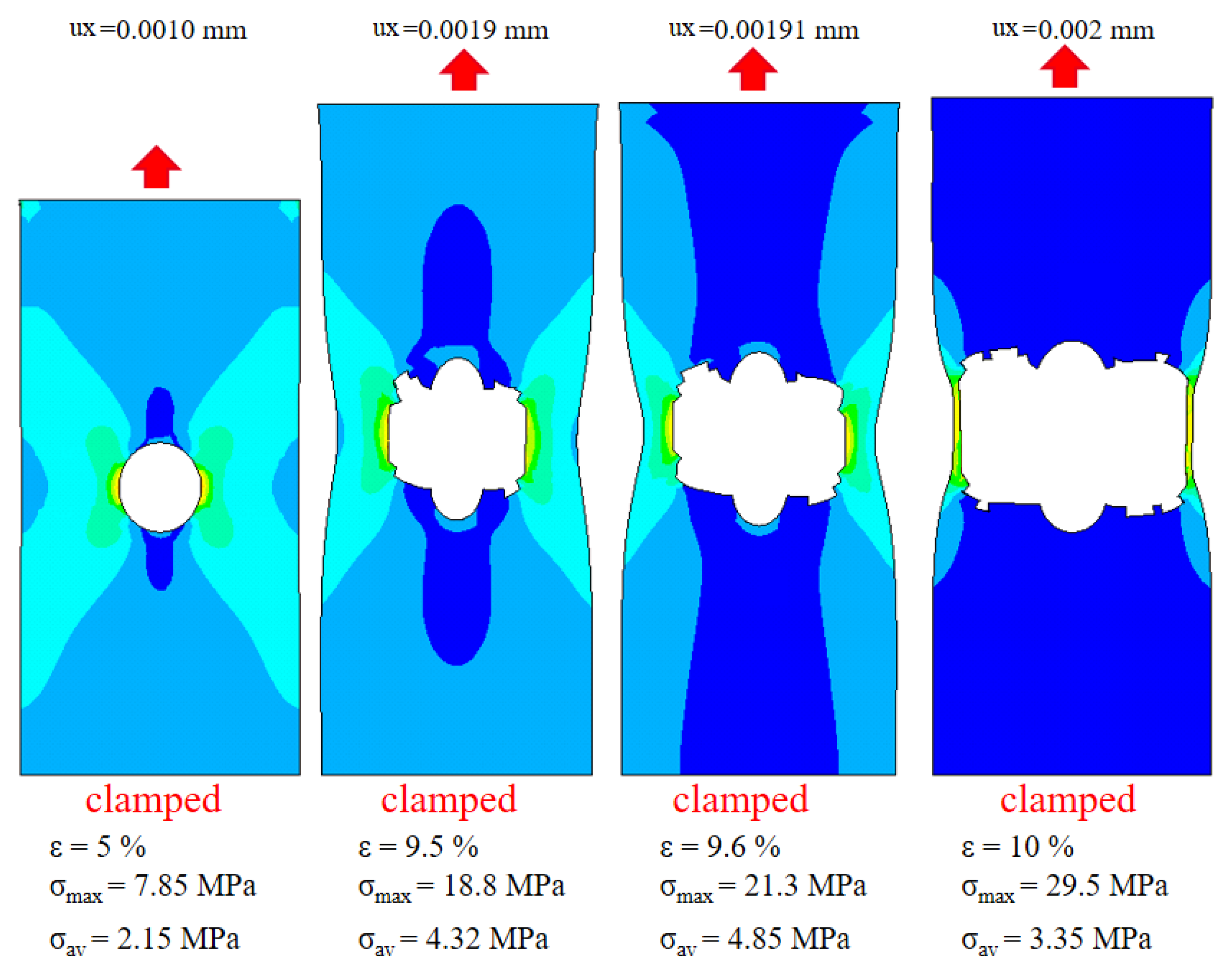
3.3. Macro-Structural Simulation of Leather Damage
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pissarenko, A.; Yang, W.; Quan, H.; Brown, K.A.; Williams, A.; Proud, W.G.; Meyers, M.A. Tensile behavior and structural characterization of pig dermis. Acta Biomater. 2019, 86, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Paudecerf, D.; Yang, J. Mechanical behaviour of natural cow leather in tension. Acta Mech. Solida Sin. 2009, 22, 37–44. [Google Scholar] [CrossRef]
- Oh, J.S.; Kim, D.Y.; Kim, H.Y.; Lee, C.A.; Bang, J.H.; Choi, K.Y. Wrinkle prediction of seat cover considering cyclic loading-unloading with viscoelastic characteristics. Mater. Des. 2016, 109C, 270–281. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. BioMed. Eng. OnLine 2019, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Haines, B. Fiber structure and physical properties of leather. J. Am. Leather Chem. Assoc. 1974, 69, 96–111. [Google Scholar]
- Reich, R. Der Zusammenhang zwischen Kollagenstruktur und Ledereigenschaften. In Proceedings of the 2. Freiberger Kollagensymposium, Freiberg, Germany, 25–26 May 2000; FILK: Freiberg, Germany, 2000. [Google Scholar]
- Daniels, R. The scientific and commercial significance of variations in strength and stretch in leather. World Leather 2007, 20, 20–25. [Google Scholar]
- Basil-Jones, M.M.; Edmonds, R.L.; Allsop, T.F.; Cooper, S.M.; Holmes, G.; Norris, G.E.; Cookson, D.J.; Kirby, N.; Haverkamp, R.G. Leather structure determination by small-angle x-ray scattering (SAXS): Cross sections of ovine and bovine leather. J. Agric. Food Chem. 2010, 58, 5286–5291. [Google Scholar] [CrossRef]
- Wells, H.C.; Holmes, G.; Haverkamp, R.G. Looseness in bovine leather: Microstructural characterization. J. Sci. Food Agric. 2016, 96, 2731–2736. [Google Scholar] [CrossRef]
- Bittrich, E.; Schladitz, K.; Meyndt, R.; Schulz, H.; Godehardt, M. Micro-computed Tomography Studies for Three-dimensional Leather Structure Analysis. J. Am. Leather Chem. Assoc. 2014, 109, 367–371. [Google Scholar]
- Godehardt, M.; Schladitz, K.; Dietrich, S.; Meyndt, R.; Schulz, H. Segmentation of collagen fiber bundles in 3D by waterfall on orientations. In Mathematical Morphology and Its Applications to Signal and Image Processing; ISMM 2017; Lecture Notes in Computer Science; Angulo, J., Velasco-Forero, S., Meyer, F., Eds.; Speringer: Berlin, Germany, 2017; Volume 10225. [Google Scholar] [CrossRef]
- Dobrovolskij, D.; Persch, J.; Schladitz, K.; Steidl, G. Structure detection with second order Riesz transforms. Image Anal. Stereol. 2019, 38, 107–119. [Google Scholar] [CrossRef]
- Ehret, A.E.; Itskov, M. Modelling of anisotropic softening phenomena: Application to soft biological tissues. Int. J. Plast. 2008, 25, 901–919. [Google Scholar] [CrossRef]
- Damlamian, A.; Cioranescu, D.; Orlik, J. Two-scale analysis for homogenization of multi-scale contact problems in elasticity. Asymptot. Anal. 2013, 82. [Google Scholar] [CrossRef]
- Griso, G.; Orlik, J. Homogenization of contact problem with Coulomb’s friction on periodic cracks. Math. Methods Appl. Sci. 2019, 42. [Google Scholar] [CrossRef]
- Fillep, S.; Orlik, J.; Bare, Z.; Steinmann, P. Homogenization in periodically heterogeneous elastic bodies with multiple micro contact. Math. Mech. Solids 2013. [Google Scholar] [CrossRef]
- Griso, G.; Orlik, J.; Wackerle, S. Asymptotic Behavior for Textiles in von-Karman regime. J. Math. Pures Appl. 2020, 144, 164–193. [Google Scholar] [CrossRef]
- Griso, G.; Khilkova, L.; Orlik, J.; Sivak, O. Homogenization of perforated elastic structures. J. Elast. 2020. [Google Scholar] [CrossRef]
- Griso, G.; Orlik, J.; Wackerle, S. Asymptotic behavior for textiles. SIAM J. Math. Anal. 2020, 52, 1639–1689. [Google Scholar] [CrossRef]
- Shiryaev, V.; Orlik, J. A one-dimensional computational model for hyperelastic string structures with Coulomb friction. Math. Methods Appl. Sci. 2017, 40, 741–756. [Google Scholar] [CrossRef]
- Shiryaev, V.; Neusius, D.; Orlik, J. Extension of One-Dimensional Models for Hyperelastic String Structures under Coulomb Friction with Adhesion. Lubricants 2018, 6, 33. [Google Scholar] [CrossRef]
- Bare, D.; Orlik, J.; Panasenko, G. Asymptotic dimension reduction of a Robin type elasticity boundary value problem in thin beams. Appl. Anal. 2014, 93, 1217–1238. [Google Scholar] [CrossRef]
- Orlik, J.; Panasenko, G.; Shiryaev, V. Optimization of textile-like materials via homogenization and beam approximations. Multiscale Model. Simul. 2016, 14, 637–667. [Google Scholar] [CrossRef]
- Griso, G.; Khilkova, L.; Orlik, J.; Sivak, O. Asymptotic behavior of stable structures made of beams. J. Elast. 2021. [Google Scholar] [CrossRef]
- Orlik, J.; Pietsch, K.; Fassbender, A.; Sivak, O.; Steiner, K. Simulation and Experimental Validation of Spacer Fabrics Based on their Structure and Yarn’s Properties. Appl. Compos. Mater. 2018, 25, 709–724. [Google Scholar] [CrossRef]
- Orlik, J.; Andrae, H.; Argatov, I.; Staub, S. Does the weaving and knitting pattern of a fabric determine its relaxation time? Q. J. Mech. Appl. Math. 2017, 70, 337–361. [Google Scholar] [CrossRef]
- Schladitz, K.; Godehardt, M.; Orlik, J.; Dietrich, S.; Meyndt, R.; Schulz, H. Micro-structural Analysis of Leather based on 3D Image Data. In Proceedings of the 6th Freiberg Collagen Symposium, Freiberg, Germany, 14–15 September 2016. [Google Scholar]
- Dietrich, S.; Schulz, H.; Hauch, K.; Schladitz, K.; Godehardt, M.; Orlik, J.; Neusius, D. 3D Image Based Structural Analysis of Leather for Macroscopic Structure-Property Simulation. In Proceedings of the XXXV International IULTCS Congress, Dresden, Germany, 25–28 June 2019. [Google Scholar]
- Okabe, A.; Boots, B.; Sugihara, K.; Chiu, S.N. Spatial Tessellations: Concepts and Applications of Voronoi Diagrams; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2000; ISBN 978-0-471-98635-5. [Google Scholar]
- Ohser, J.; Schladitz, K. 3D Images of Materials Structures—Processing and Analysis; Wiley-VCH: Weinheim, Germany; Berlin, Germany, 2009. [Google Scholar]
- Nakahara, H.; Matsuda, A. Finite element computation with anisotropic hyperelastic model considering distributed fibers for artificial and natural leather used in sports. Bull. Mech. Eng. J. 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Cheng, X.; Föhst, S.; Redenbach, C.; Schladitz, K. Detecting Branching Nodes of Multiply Connected 3D Structures. In Mathematical Morphology and Its Applications to Signal and Image Processing; ISMM 2019; Burgeth, B., Kleefeld, A., Naegel, B., Passat, N., Perret, B., Eds.; Springer: Cham, Switzerland, 2019; Volume 11564. [Google Scholar] [CrossRef]
- Couprie, M.; Zrour, R. Discrete bisector function and Euclidean skeleton. In Proceedings of the 12th International Conference on Discrete Geometry for Computer Imagery, DGCI 2005, Poitiers, France, 11–13 April 2005; Volume 3429. [Google Scholar]
- Mecke, J. Isoperimetric Properties of Stationary Random Mosaics. Mathematische Nachrichten 1984, 117, 75–82. [Google Scholar] [CrossRef]
- Osaki, S. Distribution Map of Collagen Fiber Orientation in a Whole Calf Skin. Anat. Rec. 1999, 254, 147–152. [Google Scholar] [CrossRef]
- Sharphouse, J.H. Leather Technician’s Handbook, 2nd ed.; Shoe Trades Pub: Montreal, QC, Canada, 1971. [Google Scholar]
- ANSYS Inc. ANSYS 2018 Release 2; Documentation; ANSYS Inc.: Canonsburg, PA, USA, 2018. [Google Scholar]

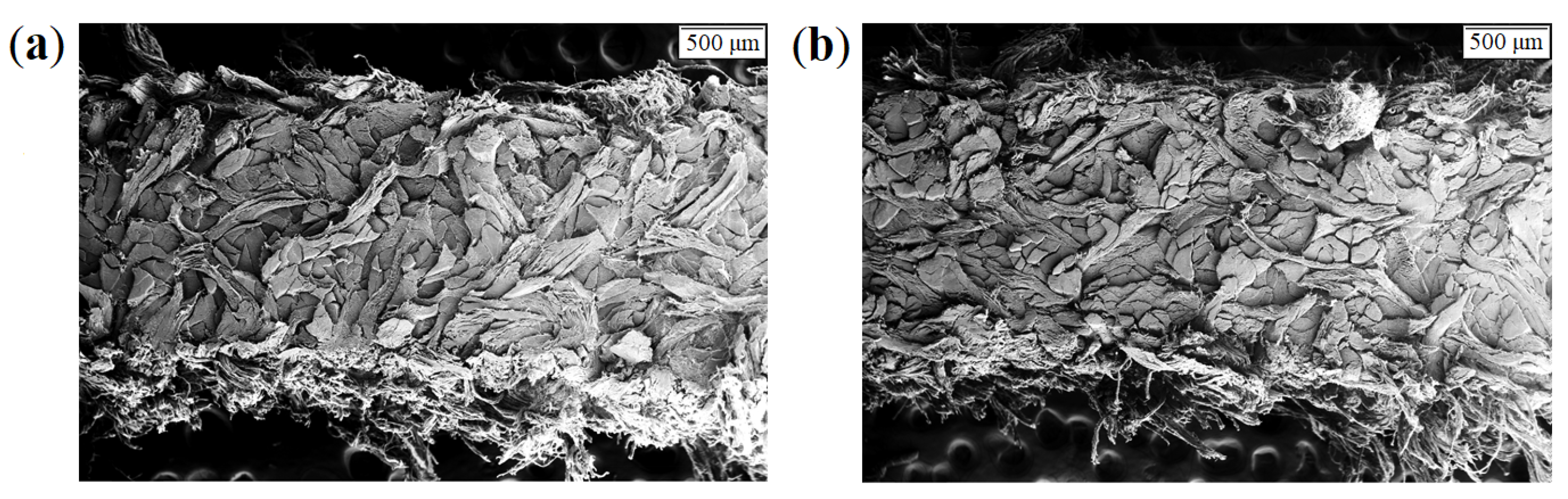
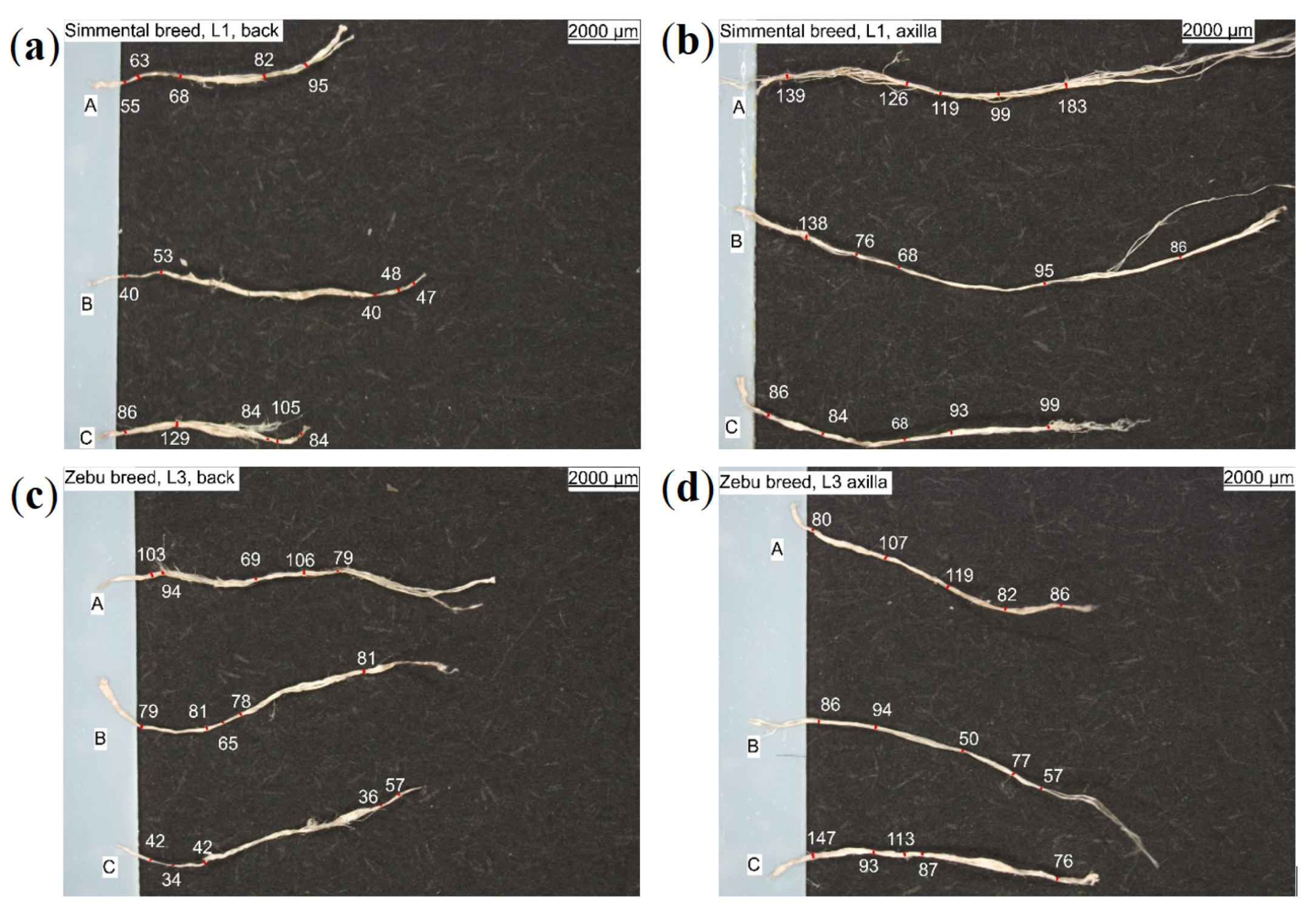
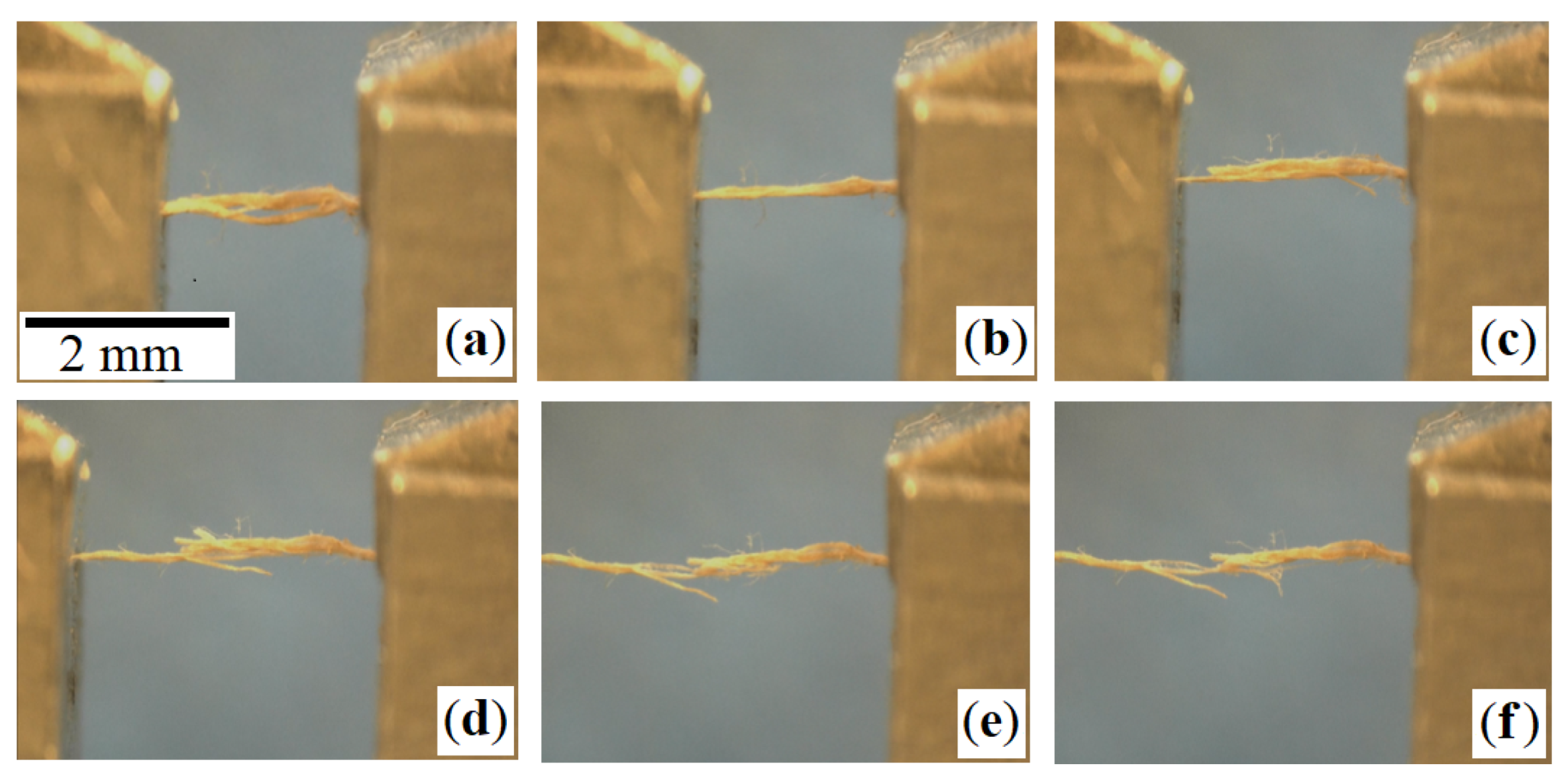

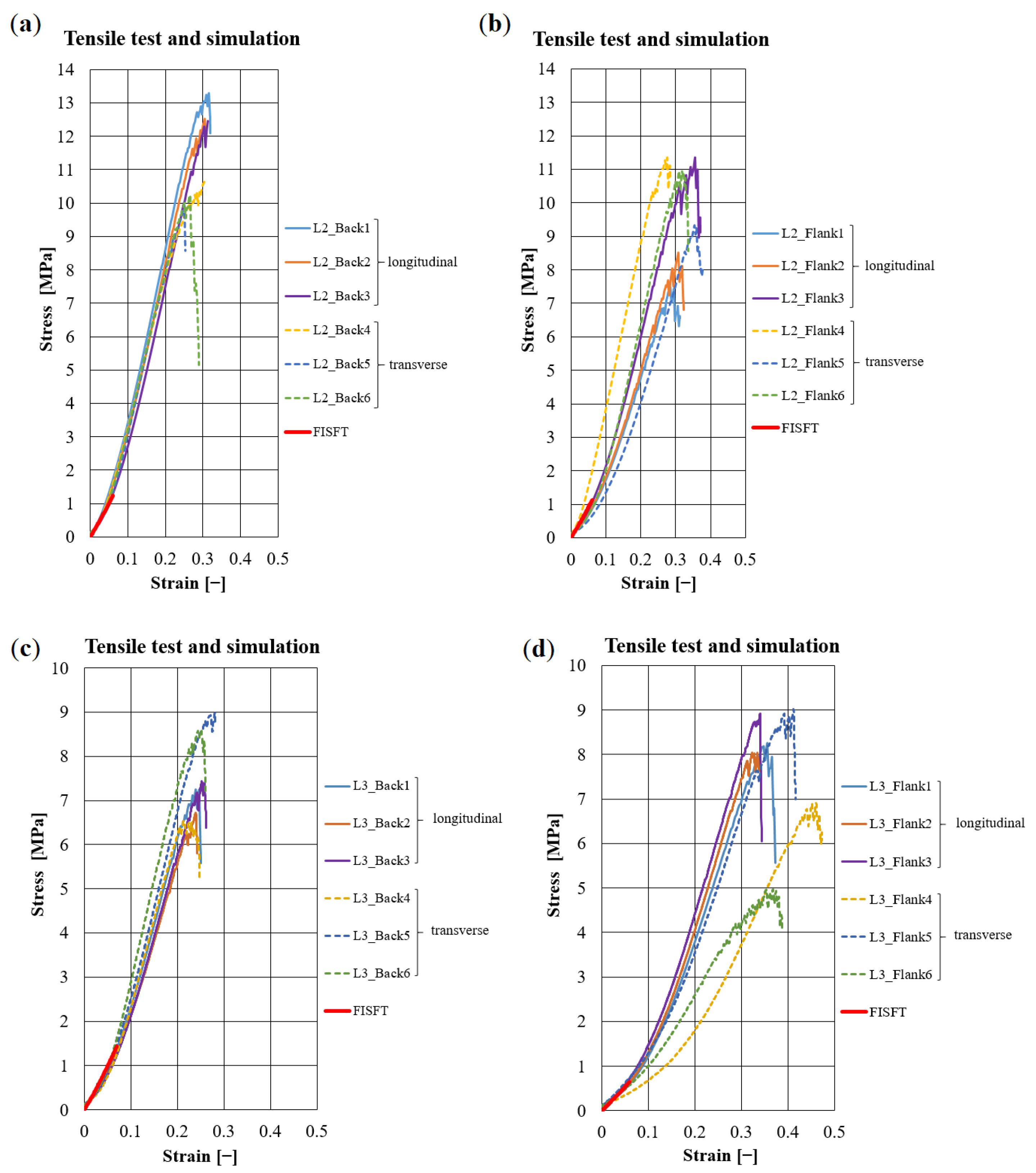
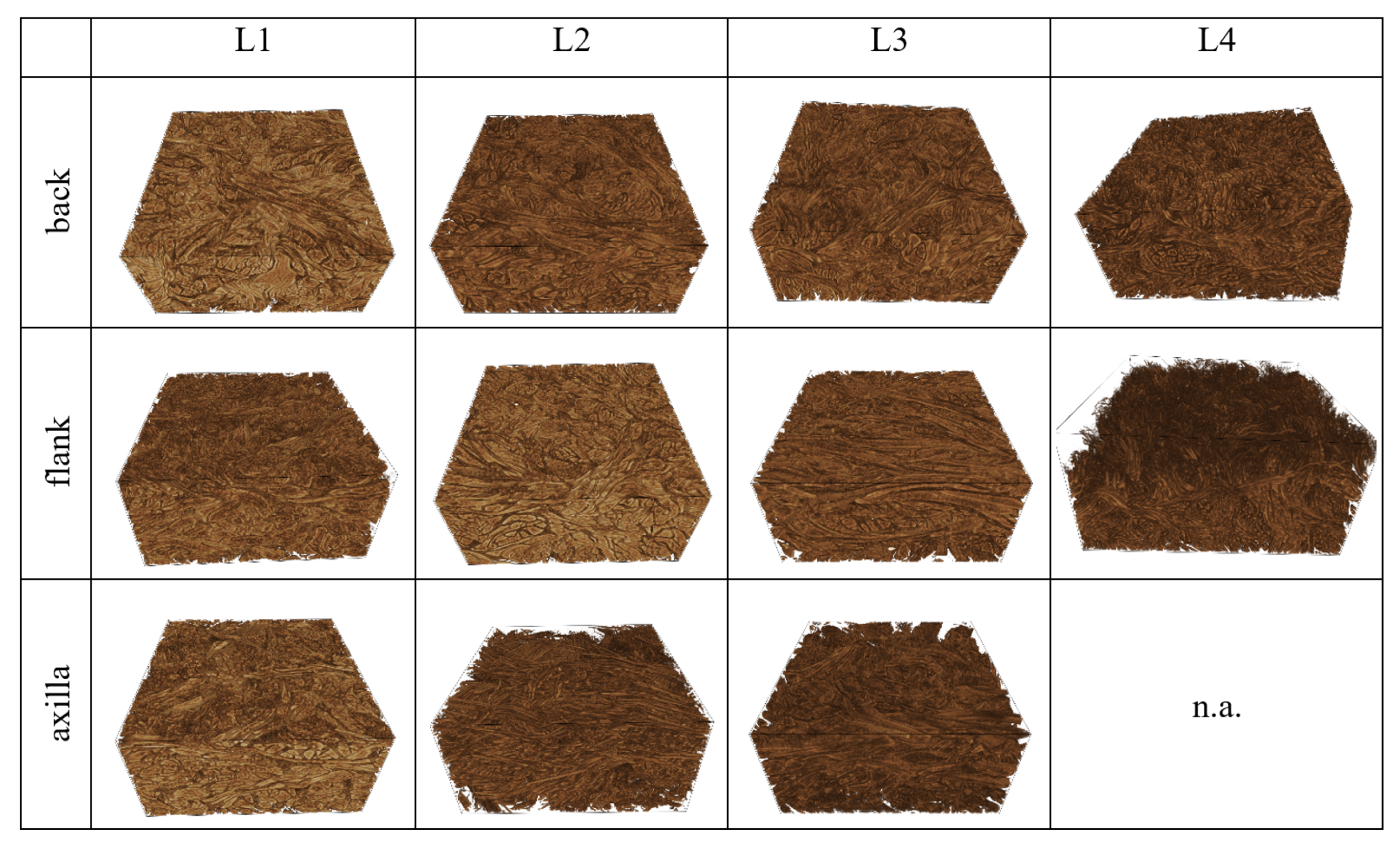



| Leather | Part | Thickness, mm | Mass per Area, g/ | CT Based Volume Fraction, % | , N | E, MPa | , MPa | , % |
|---|---|---|---|---|---|---|---|---|
| L1 | back | 1.60 | 1095 | 61 | 178 | 53.0 | 10.8 | 26.3 |
| flank | 1.56 | 893 | 52 | 102 | 24.1 | 5.82 | 29.4 | |
| axilla | 1.82 | 905 | 58 | 89 | 30.6 | 5.59 | 22.5 | |
| L2 | back | 1.66 | 1130 | 63 | 196 | 49.4 | 11.5 | 29.3 |
| flank | 1.76 | 1103 | 62 | 174 | 38.5 | 9.83 | 31.5 | |
| axilla | 1.74 | 881 | 61 | 143 | 32.1 | 9.19 | 38.9 | |
| L3 | back | 1.63 | 1069 | 63 | 126 | 39.9 | 7.59 | 24.5 |
| flank | 1.81 | 970 | 55 | 139 | 28.1 | 7.68 | 37.7 | |
| axilla | 1.86 | 978 | 54 | 123 | 29.6 | 7.51 | 34.6 | |
| L4 | back | 1.42 | 1004 | 66 | 185 | 34.3 | 13.3 | 49.1 |
| flank | 1.35 | 945 | 68 | 159 | 33.1 | 11.7 | 41.8 | |
| axilla | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Leather | Localization | Thickness, mm | , N | , MPa | E, MPa | , % |
|---|---|---|---|---|---|---|
| L1 | back | 0.156 | 258 | 13.6 | 304 | 7.90 |
| flank | 0.128 | 45.3 | 3.54 | 210 | 2.90 | |
| axilla | 0.147 | 3.5 * | 0.21 * | 4.96 * | 6.26 | |
| L2 | back | 0.107 | 51.7 | 6.46 | 308 | 5.20 |
| flank | 0.095 | 44.4 | 6.22 | 264 | 4.50 | |
| axilla | 0.039 | 33.5 | 27.7 | 517 | 2.38 | |
| L3 | back | 0.153 | 131 | 7.11 | 216 | 6.44 |
| flank | 0.134 | 434 | 30.8 | 233 | 11.8 | |
| axilla | 0.120 | 342 | 30.3 | 235 | 3.34 | |
| L4 | back | 0.118 | 652 | 23.7 | 294 | 14.6 |
| flank | 0.106 | 731 | 26.0 | 190 | 20.2 | |
| axilla | 0.116 | 323 | 12.8 | 130 | 21.4 |
| Back (Average Value) | Flank (Average Value) | ||||||
|---|---|---|---|---|---|---|---|
| Leather | Size, mm | Thickness, mm | E, MPa | Strength *, MPa | Thickness, mm | E, MPa | Strength *, MPa |
| L1 | 10 × 50 | 1.64 | 53.0 | 12 (15) | 1.69 | 24.1 | 5.5 * (8) |
| L2 | 10 × 50 | 1.71 | 49.4 | 9 * (13.2) | 1.78 | 38.5 | 6 * (11.5) |
| L3 | 10 × 50 | 1.66 | 39.9 | 6 * (9) | 1.82 | 28.1 | 4 * (9) |
| L4 | 10 × 50 | 1.38 | 34.3 | 10 ** (13.3) | 1.36 | 33.1 | 9 ** (11.7) |
| Hole Diam., mm | Size x, y, z, mm | /(), MPa | G, MPa | , - | Elongation, mm | Aver. Stress, MPa | Max. Stress (Concentr.), MPa |
|---|---|---|---|---|---|---|---|
| Simmental breed (limit strength 10 MPa) | |||||||
| 3 | 20 × 10 × 1.77 | 55/45 | 20 | 0.3 | 1.41 | 6.5 | 19.1 |
| - | 20 × 10 × 1.77 | 55/45 | 20 | 0.3 | 2.61 | 4.25 | 29.1 |
| Zebu (limit strength 6.5 MPa) | |||||||
| 3 | 20 × 10 × 1.77 | 45/30 | 15 | 0.3 | 1.15 | 3.5 | 15.7 |
| - | 20 × 10 × 1.77 | 45/30 | 15 | 0.3 | 1.51 | 2.2 | 9.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, S.; Lykhachova, O.; Cheng, X.; Godehardt, M.; Kronenberger, M.; Meyer, M.; Neusius, D.; Orlik, J.; Schladitz, K.; Schulz, H.; et al. Simulation of Leather Visco-Elastic Behavior Based on Collagen Fiber-Bundle Properties and a Meso-Structure Network Model. Materials 2021, 14, 1894. https://doi.org/10.3390/ma14081894
Dietrich S, Lykhachova O, Cheng X, Godehardt M, Kronenberger M, Meyer M, Neusius D, Orlik J, Schladitz K, Schulz H, et al. Simulation of Leather Visco-Elastic Behavior Based on Collagen Fiber-Bundle Properties and a Meso-Structure Network Model. Materials. 2021; 14(8):1894. https://doi.org/10.3390/ma14081894
Chicago/Turabian StyleDietrich, Sascha, Olga Lykhachova, Xiaoyin Cheng, Michael Godehardt, Markus Kronenberger, Michael Meyer, David Neusius, Julia Orlik, Katja Schladitz, Haiko Schulz, and et al. 2021. "Simulation of Leather Visco-Elastic Behavior Based on Collagen Fiber-Bundle Properties and a Meso-Structure Network Model" Materials 14, no. 8: 1894. https://doi.org/10.3390/ma14081894
APA StyleDietrich, S., Lykhachova, O., Cheng, X., Godehardt, M., Kronenberger, M., Meyer, M., Neusius, D., Orlik, J., Schladitz, K., Schulz, H., Steiner, K., & Voigt, D. (2021). Simulation of Leather Visco-Elastic Behavior Based on Collagen Fiber-Bundle Properties and a Meso-Structure Network Model. Materials, 14(8), 1894. https://doi.org/10.3390/ma14081894








