Positive Influence of Liquid Sodium Silicate on the Setting Time, Polymerization, and Strength Development Mechanism of MSWI Bottom Ash Alkali-Activated Mortars
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.1.1. Bottom Ash
2.1.2. Ground Granulated Blast Furnace Slag
2.1.3. Initiator
2.1.4. Others
2.2. Methods
2.2.1. Mix Proportions of Bottom Ash Alkali-Activated Mortars
2.2.2. Preparation and Curing Process of Samples
2.2.3. Compressive Strength Tests
2.2.4. Initial and Final Setting Time Tests
2.2.5. Microstructural Analysis
3. Results and Discussion
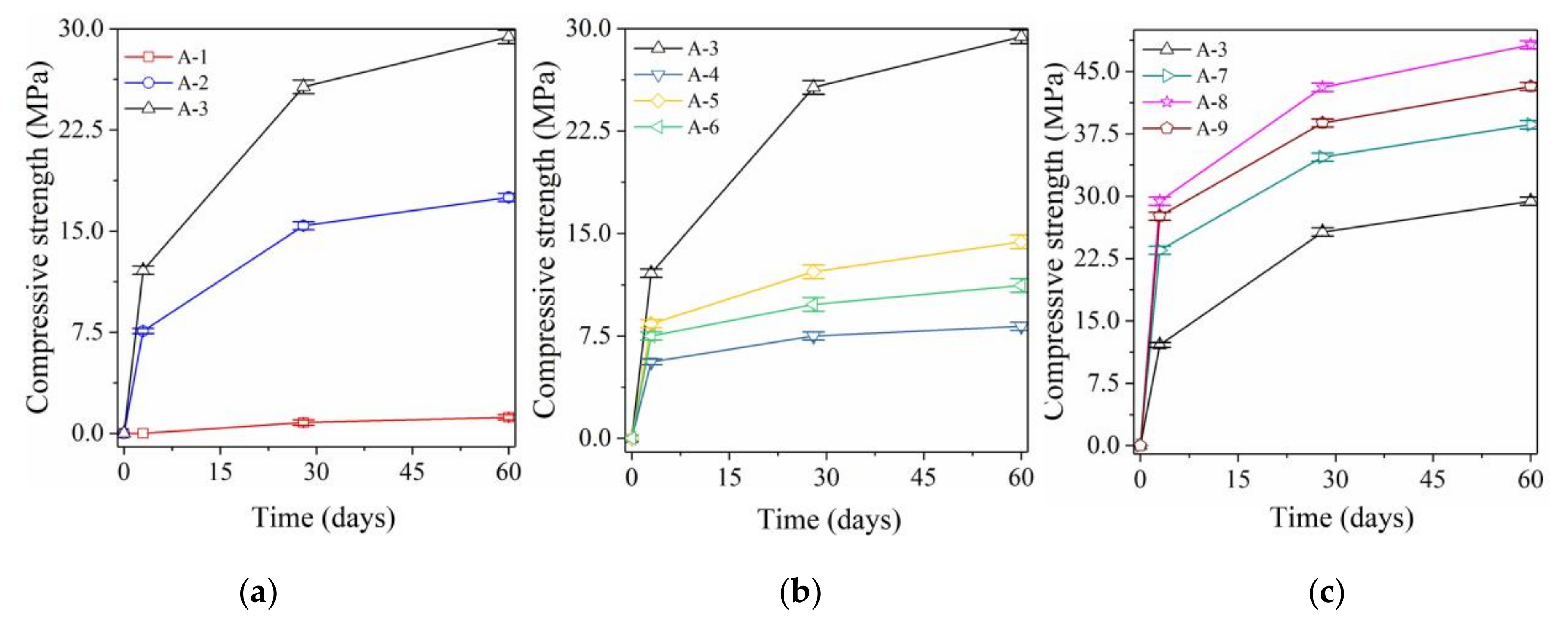
3.1. Mechanical Properties of Bottom Ash Alkali-Activated Mortars
3.1.1. Effect of NaOH on Compressive Strength
3.1.2. Effect of Liquid Sodium Silicate on Compressive Strength
3.1.3. Effect of Both NaOH and Sodium Silicate on Compressive Strength
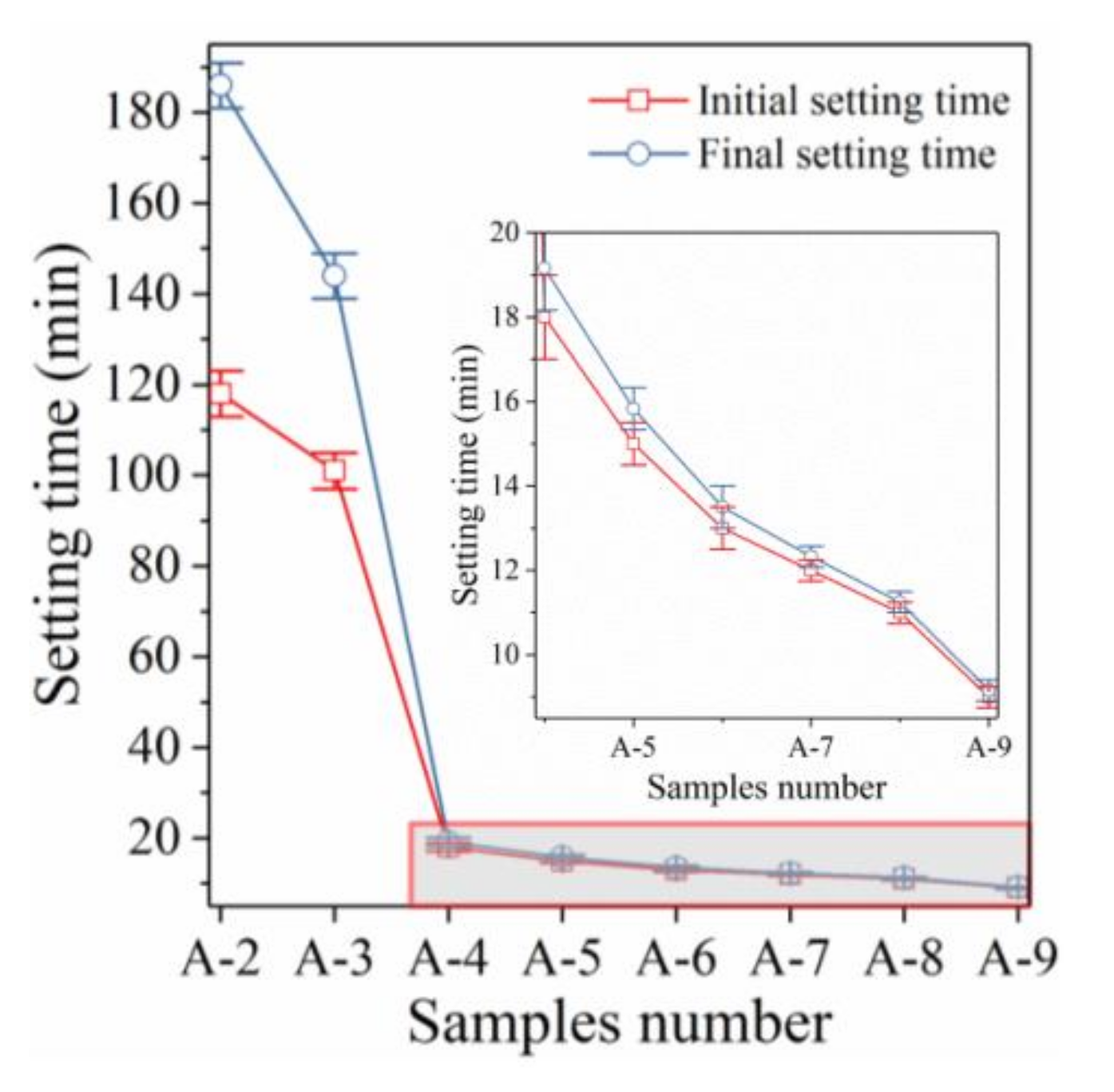
3.2. Effects of Different Initiators on the Setting Time
3.2.1. Effect of NaOH on the Setting Time
3.2.2. Effect of Sodium Silicate on the Setting Time
3.2.3. Effect of Both NaOH and Sodium Silicate on the Setting Time
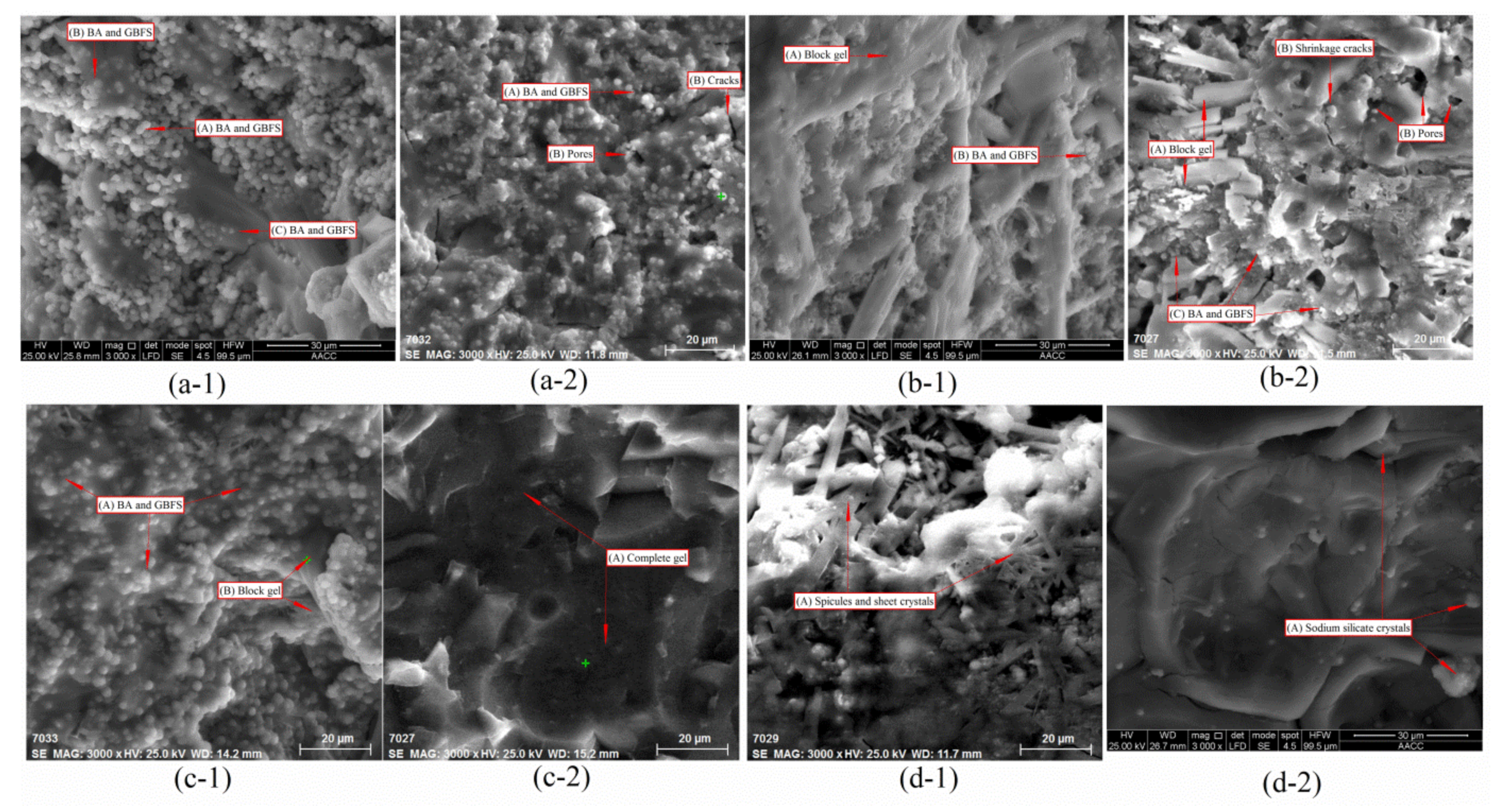
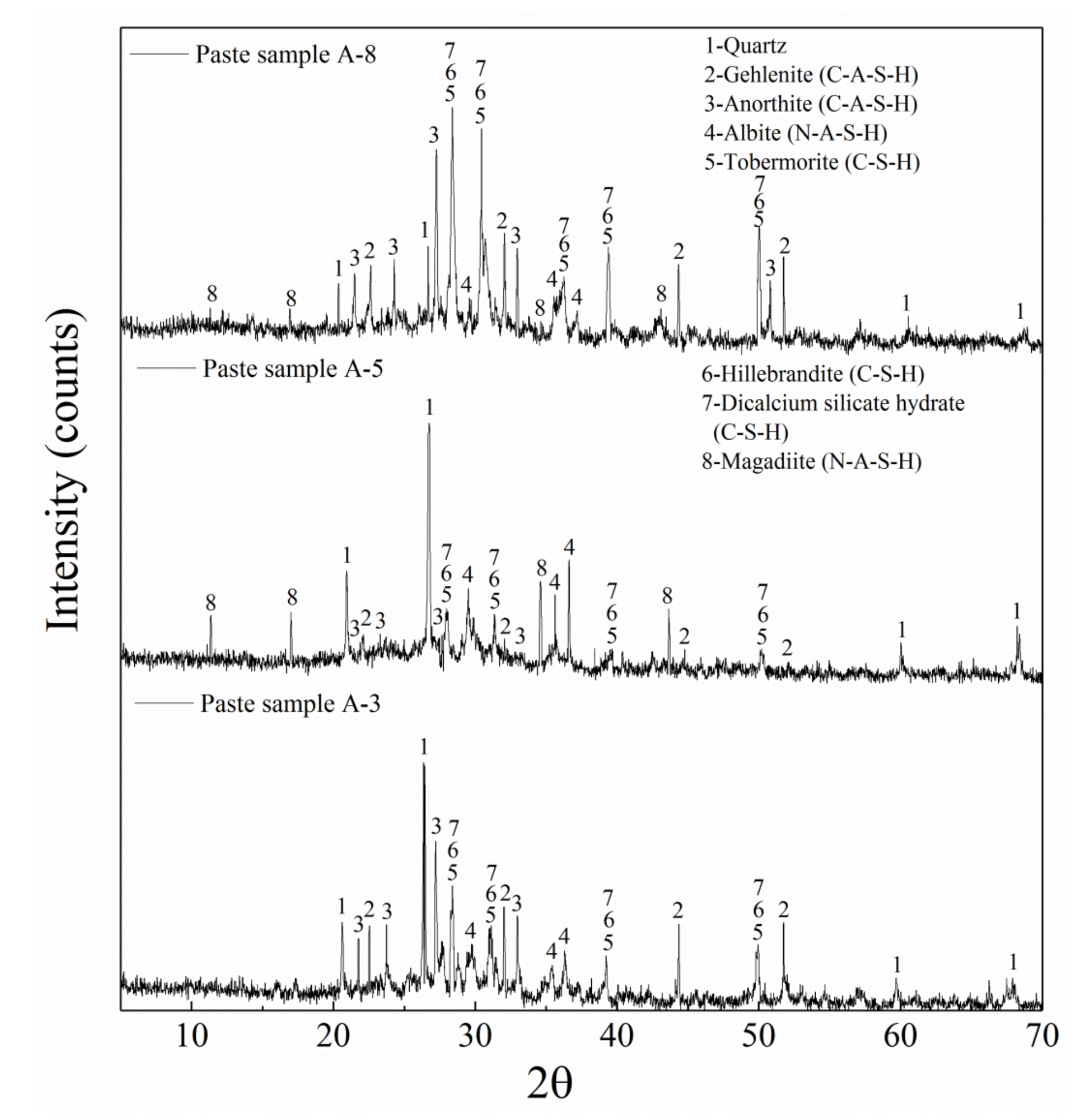
3.3. Results of Microanalysis
3.3.1. SEM Analysis
3.3.2. X-ray Diffraction Analysis
3.4. Discussion
3.4.1. Polymerization Mechanism Underlying NaOH Excitation
3.4.2. Polymerization Mechanism Underlying Liquid Sodium Silicate Excitation
3.4.3. Polymerization Mechanism Underlying Mixed Initiator Excitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, W.; Chen, X.; Zhao, A.; Struble, L.J.; Yang, E.-H. Synthesis of high strength binders from alkali activation of glass materials from municipal solid waste incineration bottom ash. J. Clean. Prod. 2019, 212, 261–269. [Google Scholar] [CrossRef]
- Aubert, J.E.; Husson, B.; Sarramone, N. Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement. Part 1: Processing and characterization of MSWI fly ash. J. Hazard. Mater. 2006, 136, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Aubert, J.; Husson, B.; Sarramone, N. Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement: Part 2. J. Hazard. Mater. 2007, 146, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Chen, L.; Lu, Z.; Sun, Y.; Zhang, X.; Feng, Y.; Ji, Y.; Xu, Z. Use of pretreatment to prevent expansion and foaming in high-performance MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 245, 118471. [Google Scholar] [CrossRef]
- Li, X.-G.; Lv, Y.; Ma, B.-G.; Chen, Q.-B.; Yin, X.-B.; Jian, S.-W. Utilization of municipal solid waste incineration bottom ash in blended cement. J. Clean. Prod. 2012, 32, 96–100. [Google Scholar] [CrossRef]
- Keulen, A.; van Zomeren, A.; Harpe, P.; Aarnink, W.; Simons, H.; Brouwers, H. High performance of treated and washed MSWI bottom ash granulates as natural aggregate replacement within earth-moist concrete. Waste Manag. 2016, 49, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Lynn, C.J.; Dhir, R.; Ghataora, G.S. Municipal incinerated bottom ash characteristics and potential for use as aggregate in con-crete. Constr. Build. Mater. 2016, 127, 504–517. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Shi, C.; Fernández-Jiménez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Puertas, F.; González-Fonteboa, B.; González-Taboada, I.; Alonso, M.M.; Torres-Carrasco, M.; Rojo, G.; Martínez-Abella, F. Alkali-activated slag concrete: Fresh and hardened behavior. Cem. Concr. Compos. 2018, 85, 22–31. [Google Scholar] [CrossRef]
- Tang, P.; Florea, M.V.A.; Spiesz, P.; Brouwers, H.J.H. Characteristics and application potential of municipal solid waste incineration (MSWI) bottom ashes from two waste-to-energy plants. Constr. Build. Mater. 2015, 83, 77–94. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Redmond, R.R.; Provis, J.; van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar]
- Bernal, S.A.; Mejía de Gutiérrez, R.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutierrez, R. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Bernal, S.A.; Nicolas, R.S.; Myers, R.J.; de Gutiérrez, R.M.; Puertas, F.; van Deventer, J.S.; Provis, J.L. MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Shi, C.; Wan, S.; Li, N.; Zhang, Z. Effect of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Zhang, J.; Wan, S.; Zhang, Z.; Ou, Z. Alkali-silica reaction in waterglass-activated slag mortars incorporating fly ash and metakaolin. Cem. Concr. Res. 2018, 108, 10–19. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z.; Gao, X.; Zhang, C.; Wu, Q. Effect of fly ash microsphere on the rheology and microstructure of alkaliactivated fly ash/slag pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Zhang, L.; Liu, X.; Liu, B. Effect of activated silica on polymerization mechanism and strength development of MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2019, 201, 90–99. [Google Scholar] [CrossRef]
- Bergold, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Interaction of silicate and aluminate reaction in a synthetic cement system: Implications for the process of alite hydration. Cem. Concr. Res. 2017, 93, 32–44. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China. Ground Granulated Blast Furnace Slag Used for Cement and Concrete; GB/T18046-2008; General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China: Beijing, China, January 2008.
- General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China. Method of Testing Cements—Determination of Strength–ISO China; GB/T17671-1999; General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China: Beijing, China, August 1999.
- National Standardization Technical Committee Cement. Apparatus for Determining Normal Consistency and Setting Time of Cement Paste; JC/T 727-2005; National Standardization Technical Committee Cement: Beijing, China, July 2005.
- Huang, G.; Yang, K.; Sun, Y.; Lu, Z.; Zhang, X.; Zuo, L.; Feng, Y.; Qian, R.; Qi, Y.; Ji, Y.; et al. Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 248, 118582. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, L.; Li, J.; Hou, Z. The influence of curing methods on the strength of MSWI bottom ash-based alkali-activated mortars: The role of leaching of OH− and free alkali. Constr. Build. Mater. 2018, 186, 978–985. [Google Scholar] [CrossRef]
- Maraghechi, H.; Rajabipour, F.; Pantano, C.G.; Burgos, W.D. Effect of calcium on dissolution and precipitation reactions of amorphous silica at high alkalinity. Cem. Concr. Res. 2016, 87, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Escalante-Garcia, J.; Castro-Borges, P.; Gorokhovsky, A.; Rodriguez-Varela, F. Portland cement-blast furnace slag mortars activated using waterglass: Effect of temperature and alkali concentration. Constr. Build. Mater. 2014, 66, 323–328. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geo-polymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline initiators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali initiator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. Part B 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Cristelo, N.; Tavares, P.; Lucas, E.; Miranda, T.; Oliveira, D. Quantitative and qualitative assessment of the amorphous phase of a Class F fly ash dissolved during alkali activation reactions-Effect of mechanical activation, solution concentration and temperature. Compos. Part B 2016, 103, 1–14. [Google Scholar] [CrossRef]
- Angulo-Ramírez, D.E.; de Gutiérrez, R.M.; Puertas, F. Alkali-activated Portland blast-furnace slag cement: Mechanical properties and hydration. Constr. Build. Mater. 2017, 140, 119–128. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Hou, J.L.Z.; Jin, C. Use of slaked lime and Portland cement to improve the resistance of MSWI bottom ash-GGBFS geopolymer concrete against carbonation. Constr. Build. Mater. 2018, 166, 290–300. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, L.; Li, J.; Hou, Z. Advances in Understanding and Analyzing the Anti-diffusion Behavior in Complete Carbonation Zone of MSWI Bottom Ash-based Alkali-activated Concrete. Constr. Build. Mater. 2018, 186, 1072–1081. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect of Calcium Additions on N-A-S-H Cementitious Gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar] [CrossRef]
- Lee, T.-C.; Wang, W.-J.; Shih, P.-Y.; Lin, K.-L. Enhancement in early strengths of slag-cement mortars by adjusting basicity of the slag prepared from fly-ash of MSWI. Cem. Concr. Res. 2009, 39, 651–658. [Google Scholar] [CrossRef]
- Bernal, S.A. Effect of the initiator dose on the compressive strength and accelerated carbonation resistance of alkali silicate-activated slag/metakaolin blended materials. Constr. Build. Mater. 2015, 98, 217–226. [Google Scholar] [CrossRef]
- Lee, N.; Lee, H. Reactivity and reaction products of alkali-activated, fly ash/slag paste. Constr. Build. Mater. 2015, 81, 303–312. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Meller, N.; Kyritsis, K.; Hall, C. The mineralogy of the CaO-A12O3-SiO2-H2O (CASH) hydroceramie system from 200 to 350 °C. Cem. Concr. Res. 2009, 39, 45–53. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Brice, D.G.; Kilcullen, A.; Duxson, P.; van Deventer, J.S.J. Accelerated carbonation testing of alkali-activated binders significantly underestimates service life: The role of pore solution chemistry. Cem. Concr. Res. 2012, 42, 1317–1326. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Hatanaka, S.; Cao, T. High-Strength Geopolymer Using Fine High-Calcium Fly Ash. J. Mater. Civ. Eng. 2011, 23, 264–270. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Bernal, S.A.; van Deventer, J.S.J.; Provise, J.L. Structural evolution of synthetic alkali-activated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem. Concr. Res. 2017, 99, 155–171. [Google Scholar] [CrossRef]
- Irbe, L.; Beddoe, R.E.; Heinz, D. The role of aluminium in C-A-S-H during sulfate attack on concrete. Cem. Concr. Res. 2019, 116, 71–80. [Google Scholar] [CrossRef]
- L’Hôpital, E.; Lothenbach, B.; Scrivener, K.; Kulik, D. Alkali uptake in calcium alumina silicate hydrate (C-A-S-H). Cem. Concr. Res. 2016, 85, 122–136. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Swanton, S.W.; Heath, T.G.; Clacher, A. Leaching behaviour of low Ca:Si ratio CaO–SiO2–H2O systems. Cem. Concr. Res. 2016, 88, 82–95. [Google Scholar] [CrossRef]

| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | Na2O | Others | LOI | |
|---|---|---|---|---|---|---|---|---|---|
| GGBFS | 31.35 | 34.65 | 18.65 | 0.57 | 7.31 | 0.65 | 0.94 | 2.73 | 0.7 |
| BA | 53.82 | 14.44 | 14.18 | 6.18 | 3.26 | 2.52 | 2.24 | 0.61 | 1.62 |
| Portland cement | 26.55 | 59.34 | 7.77 | 1.62 | 2.68 | 1.5 | 0.31 | 1.25 | 3.2 |
| I BA (60%) | II GGBFS (40%) | III Water | IV Sodium Silicate | Liquid-Solid Ratio | V NaOH | Sand | pH of Initiator | |
|---|---|---|---|---|---|---|---|---|
| A-1 | 351.6 | 234.4 | 293 | 0 | 0.5 | 0 | 1758 | 10.02 |
| A-2 | 351.6 | 234.4 | 293 | 0 | 0.5 | 15.63 | 1758 | 14.12 |
| A-3 | 351.6 | 234.4 | 293 | 0 | 0.5 | 31.25 | 1758 | 14.43 |
| A-4 | 351.6 | 234.4 | 208 | 130.2 | 0.5 | 0 | 1758 | 11.55 |
| A-5 | 351.6 | 234.4 | 191 | 156.2 | 0.5 | 0 | 1758 | 11.55 |
| A-6 | 351.6 | 234.4 | 174 | 182.3 | 0.5 | 0 | 1758 | 11.55 |
| A-7 | 351.6 | 234.4 | 208 | 130.2 | 0.5 | 31.25 | 1758 | 14.49 |
| A-8 | 351.6 | 234.4 | 191 | 156.2 | 0.5 | 31.25 | 1758 | 14.49 |
| A-9 | 351.6 | 234.4 | 174 | 182.3 | 0.5 | 31.25 | 1758 | 14.49 |
| Samples Number | Sodium Silicate | NaOH | Initial Setting Time | Final Setting Time |
|---|---|---|---|---|
| A-1 | 0 | 0 | 600 min | 600 min |
| A-2 | 0 | 12 | 118 min | 186 min |
| A-3 | 0 | 24 | 101 min | 144 min |
| A-4 | 100 | 0 | 18 min | 19 min 10 s |
| A-5 | 120 | 0 | 15 min | 15 min 50 s |
| A-6 | 140 | 0 | 13 min | 13 min 30 s |
| A-7 | 100 | 24 | 12 min | 12 min 20 s |
| A-8 | 120 | 24 | 11 min | 11 min 15 s |
| A-9 | 140 | 24 | 9 min | 9 min 10 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Huang, G.; Li, Y.; Zhang, X.; Ji, Y.; Xu, Z. Positive Influence of Liquid Sodium Silicate on the Setting Time, Polymerization, and Strength Development Mechanism of MSWI Bottom Ash Alkali-Activated Mortars. Materials 2021, 14, 1927. https://doi.org/10.3390/ma14081927
Jin L, Huang G, Li Y, Zhang X, Ji Y, Xu Z. Positive Influence of Liquid Sodium Silicate on the Setting Time, Polymerization, and Strength Development Mechanism of MSWI Bottom Ash Alkali-Activated Mortars. Materials. 2021; 14(8):1927. https://doi.org/10.3390/ma14081927
Chicago/Turabian StyleJin, Lei, Guodong Huang, Yongyu Li, Xingyu Zhang, Yongsheng Ji, and Zhishan Xu. 2021. "Positive Influence of Liquid Sodium Silicate on the Setting Time, Polymerization, and Strength Development Mechanism of MSWI Bottom Ash Alkali-Activated Mortars" Materials 14, no. 8: 1927. https://doi.org/10.3390/ma14081927







