High Magnesium and Sirolimus on Rabbit Vascular Cells—An In Vitro Proof of Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Wound Assay
2.3. Fluorescence Microscopy
2.4. Enzyme-Linked Immunosorbent Assay–ELISA
2.5. Cyclic GMP Measurement
2.6. Statistical Analysis
3. Results
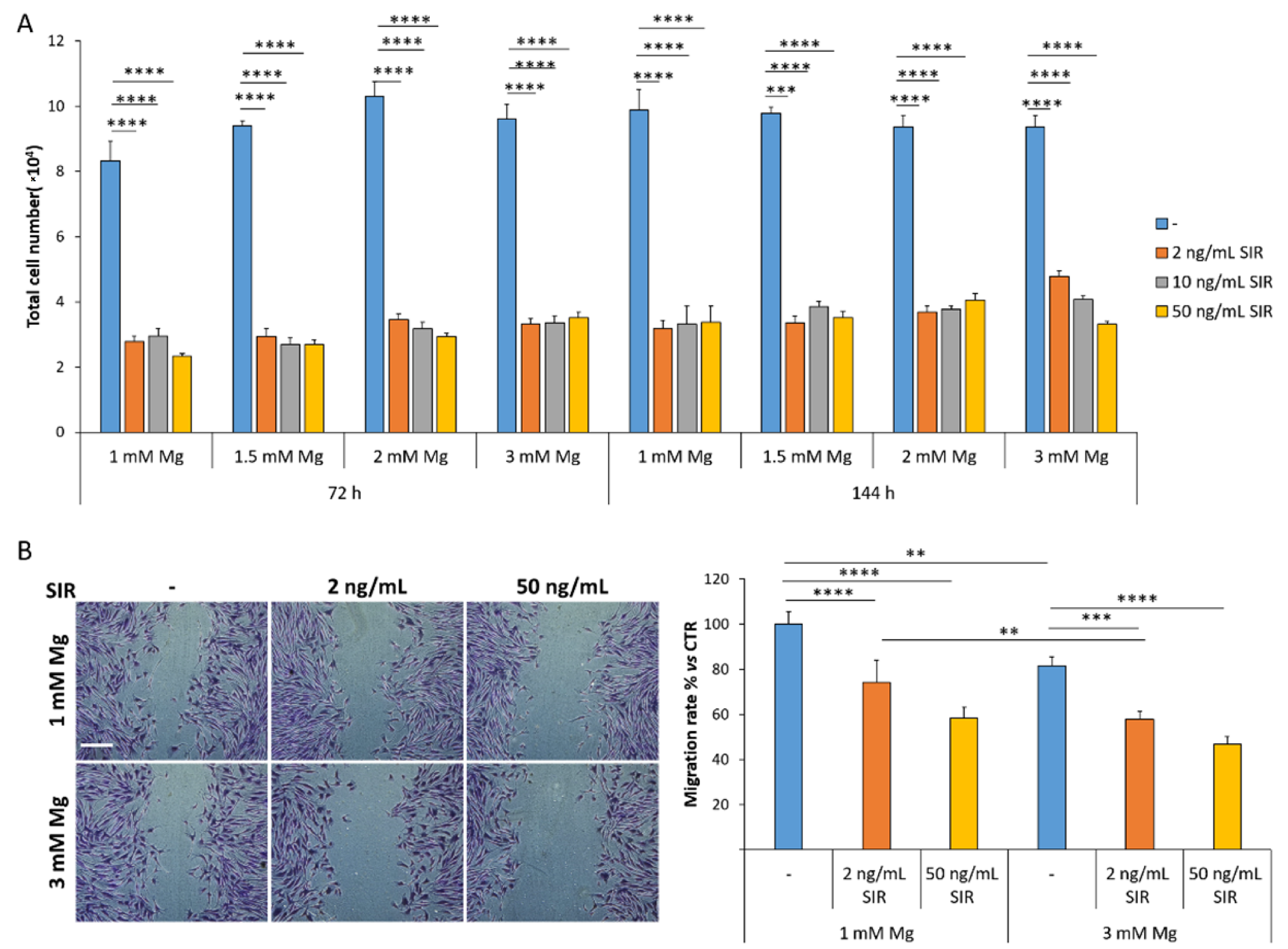
3.1. The Effect of Sirolimus and Mg on rCAEC Growth, Migration and Morphology
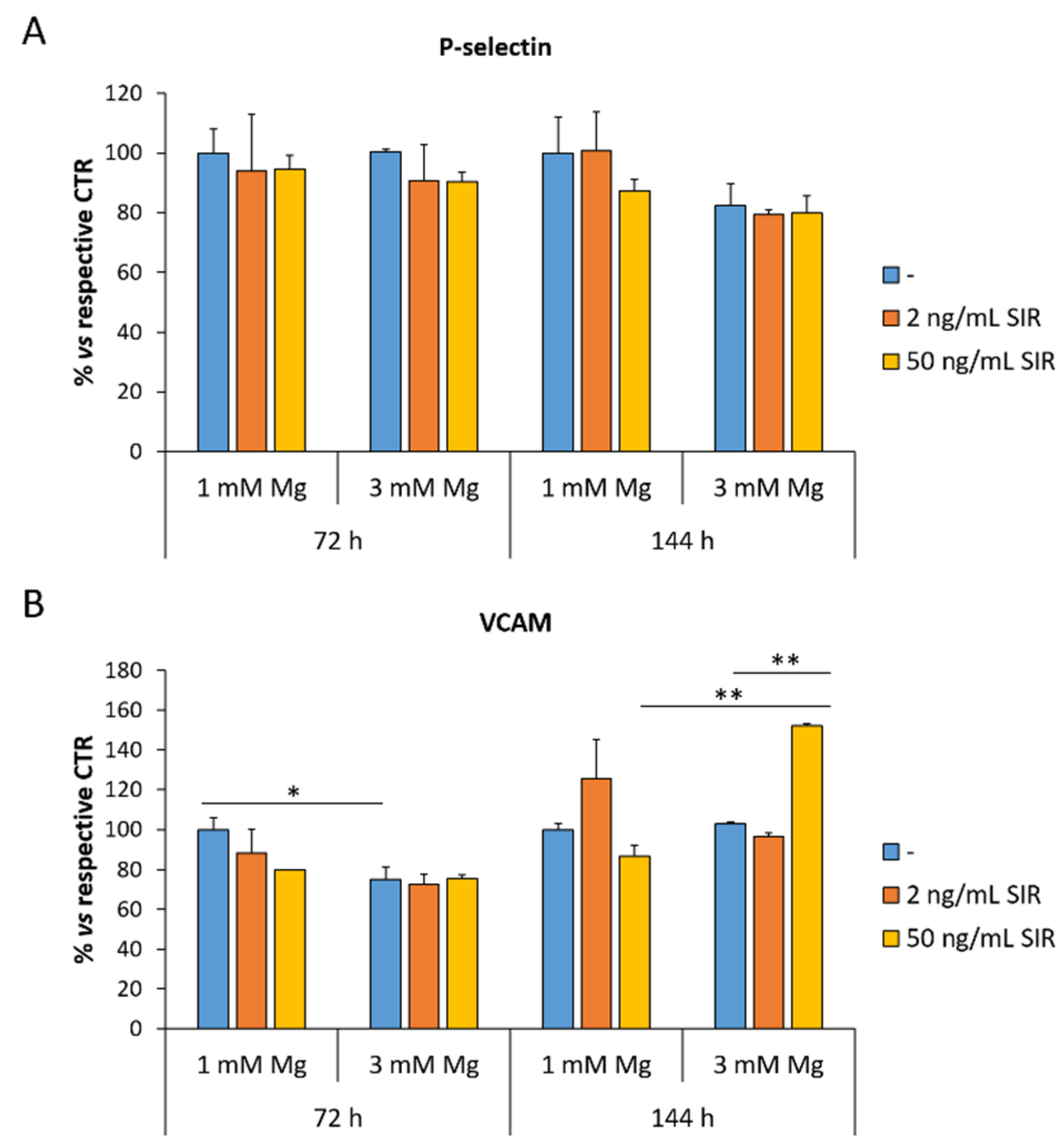
3.2. The Effect of Sirolimus and Mg on the Amounts of VCAM and P-Selectin in rCAEC
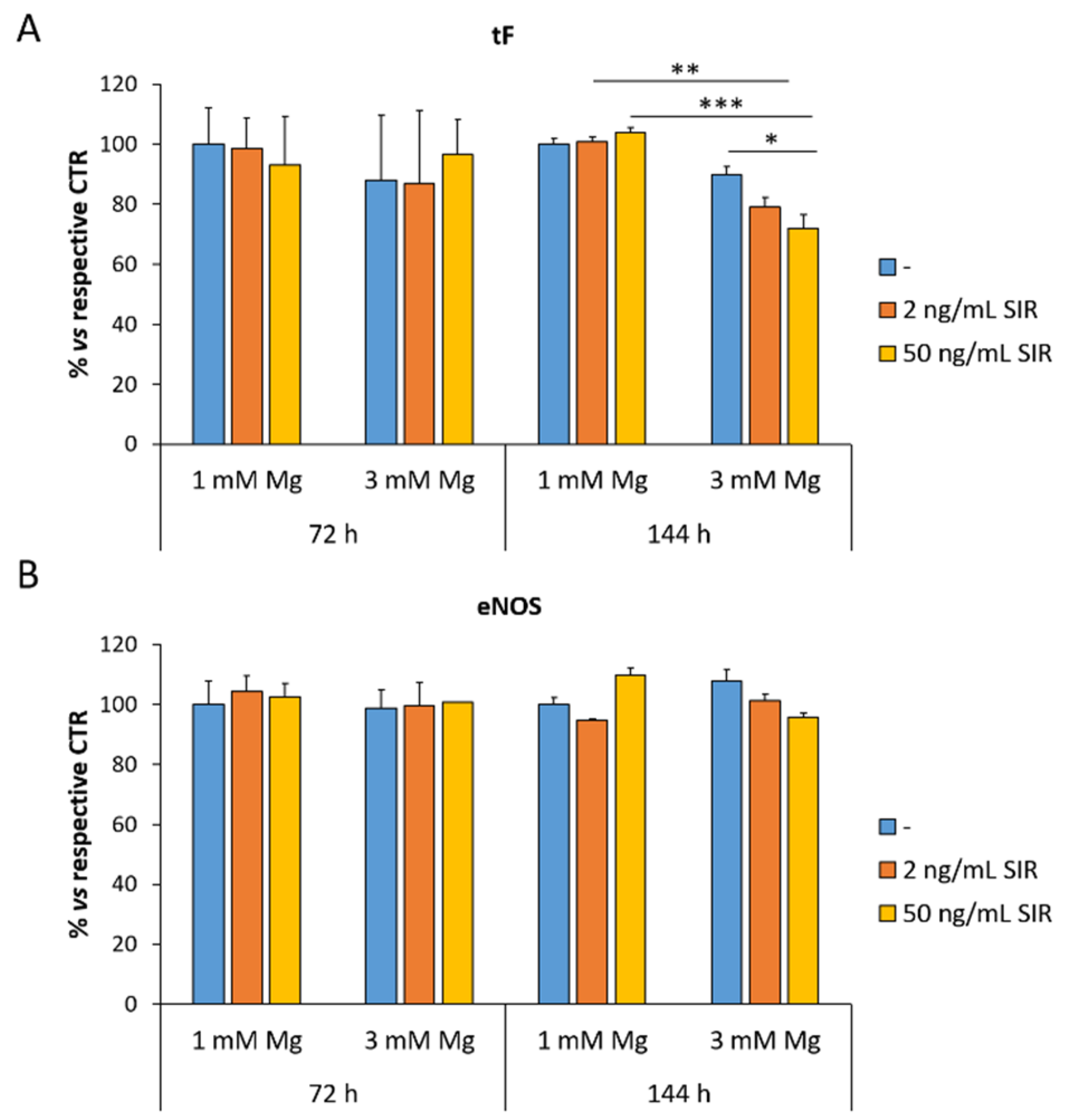
3.3. The Effect of Sirolimus and Mg on the Amounts of tF and eNOS in rCAEC
3.4. The Effect of Sirolimus and Mg on rSMC Growth and Migration
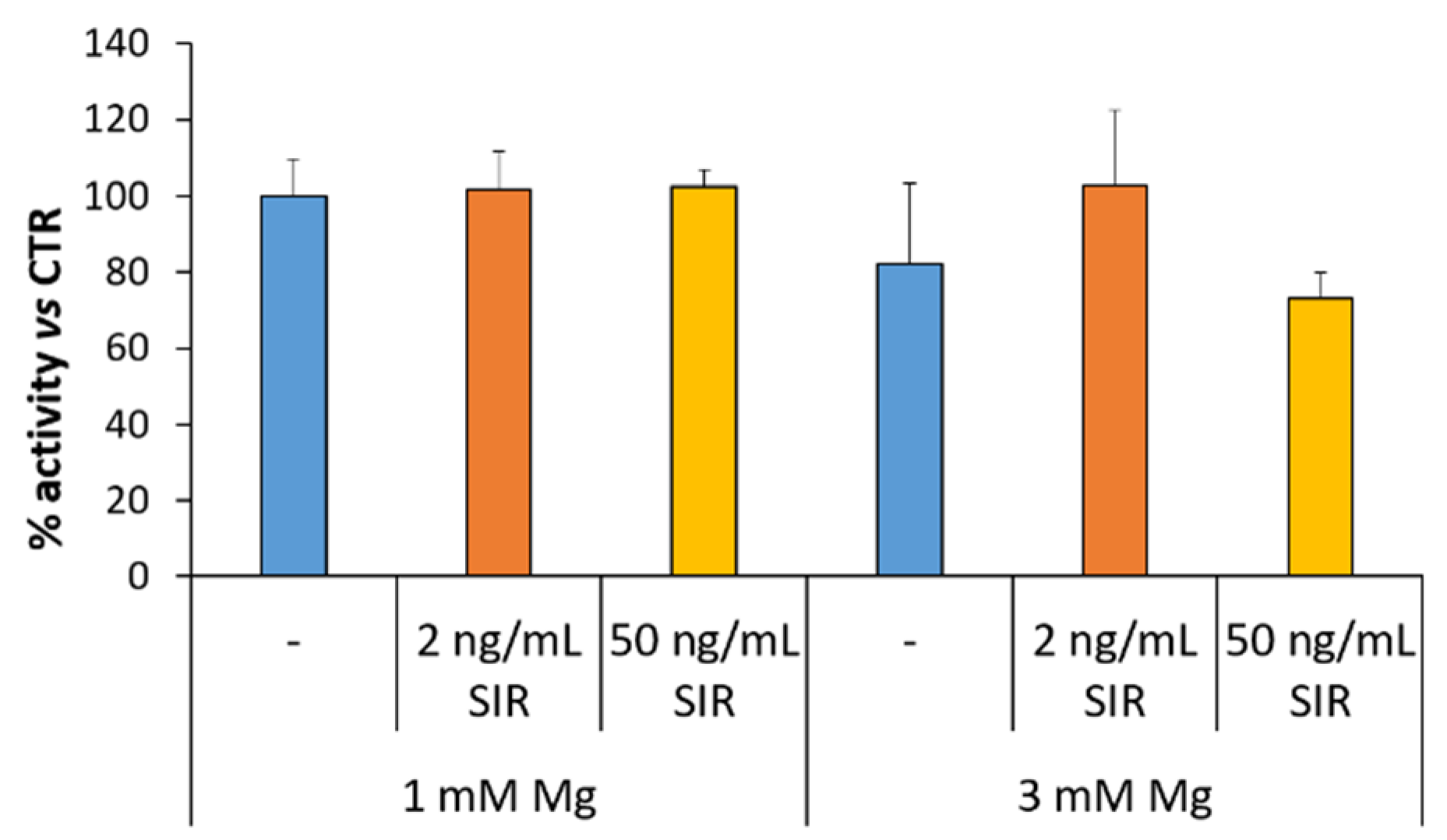
3.5. The Effect of Sirolimus and Mg on the Response of rSMC Growth to NO Donor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- Nicol, P.; Bulin, A.; Castellanos, M.I.; Stöger, M.; Obermeier, S.; Lewerich, J.; Lenz, T.; Hoppmann, P.; Baumgartner, C.; Fischer, J.; et al. Preclinical investigation of neoatherosclerosis in magnesium-based bioresorbable scaffolds versus thick-strut drug-eluting stents. EuroIntervention J. Eur. Collab. with Work. Gr. Interv. Cardiol. Eur. Soc. Cardiol. 2020, 16, e922–e929. [Google Scholar] [CrossRef]
- Simard, T.; Hibbert, B.; Ramirez, F.D.; Froeschl, M.; Chen, Y.X.; O’Brien, E.R. The Evolution of Coronary Stents: A Brief Review. Can. J. Cardiol. 2014, 30, 35–45. [Google Scholar] [CrossRef]
- Canfield, J.; Totary-Jain, H. 40 years of percutaneous coronary intervention: History and future directions. J. Pers. Med. 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Testa, L.; Latib, A.; Montone, R.A.; Colombo, A.; Bedogni, F. Coronary Bioresorbable Vascular Scaffold Use in the Treatment of Coronary Artery Disease. Circ. Cardiovasc. Interv. 2016, 9. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, L. Research into biodegradable polymeric stents: A review of experimental and modelling work. Vessel Plus 2018, 2, 12. [Google Scholar] [CrossRef]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Castiglioni, S.; Maier, J.A.M. Magnesium alloys for vascular stents: The biological bases: A focus on the effects of magnesium on vascular cells. BioNanoMaterials 2015, 16, 23–29. [Google Scholar] [CrossRef][Green Version]
- Maier, J.A.M.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Haude, M.; Ince, H.; Kische, S.; Toelg, R.; Van Mieghem, N.M.; Verheye, S.; von Birgelen, C.; Christiansen, E.H.; Barbato, E.; Garcia-Garcia, H.M.; et al. Sustained Safety and Performance of the Second-Generation Sirolimus-Eluting Absorbable Metal Scaffold: Pooled Outcomes of the BIOSOLVE-II and -III Trials at 3 Years. Cardiovasc. Revasc. Med. 2020, 21, 1150–1154. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.-M.; Dutzmann, J.; Brunsch, H.; Bauersachs, J.; Braun-Dullaeus, R.; Sedding, D.G. Systemic application of sirolimus prevents neointima formation not via a direct anti-proliferative effect but via its anti-inflammatory properties. Int. J. Cardiol. 2017, 238, 79–91. [Google Scholar] [CrossRef]
- Jabs, A.; Göbel, S.; Wenzel, P.; Kleschyov, A.L.; Hortmann, M.; Oelze, M.; Daiber, A.; Münzel, T. Sirolimus-Induced Vascular Dysfunction. Increased Mitochondrial and Nicotinamide Adenosine Dinucleotide Phosphate Oxidase-Dependent Superoxide Production and Decreased Vascular Nitric Oxide Formation. J. Am. Coll. Cardiol. 2008, 51, 2130–2138. [Google Scholar] [CrossRef]
- Reineke, D.C.; Müller-Schweinitzer, E.; Winkler, B.; Kunz, D.; Konerding, M.A.; Grussenmeyer, T.; Carrel, T.P.; Eckstein, F.S.; Grapow, M.T.R. Rapamycin impairs endothelial cell function in human internal thoracic arteries. Eur. J. Med. Res. 2015, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Barilli, A.; Visigalli, R.; Sala, R.; Gazzola, G.C.; Parolari, A.; Tremoli, E.; Bonomini, S.; Simon, A.; Closs, E.I.; Dall’Asta, V.; et al. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc. Res. 2008, 78, 563–571. [Google Scholar] [CrossRef]
- Wang, C.; Qin, L.; Manes, T.D.; Kirkiles-Smith, N.C.; Tellides, G.; Pober, J.S. Rapamycin antagonizes TNF induction of VCAM-1 on endothelial cells by inhibiting mTORC2. J. Exp. Med. 2014, 211, 395–404. [Google Scholar] [CrossRef]
- Sternberg, K.; Gratz, M.; Koeck, K.; Mostertz, J.; Begunk, R.; Loebler, M.; Semmling, B.; Seidlitz, A.; Hildebrandt, P.; Homuth, G.; et al. Magnesium used in bioabsorbable stents controls smooth muscle cell proliferation and stimulates endothelial cells in vitro. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2012, 100 B, 41–50. [Google Scholar] [CrossRef]
- Cerrato, E.; Barbero, U.; Gil Romero, J.A.; Quadri, G.; Mejia-Renteria, H.; Tomassini, F.; Ferrari, F.; Varbella, F.; Gonzalo, N.; Escaned, J. MagmarisTM resorbable magnesium scaffold: State-of-art review. Future Cardiol. 2019, 15, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Baldoli, E.; Castiglioni, S.; Maier, J.A.M. Regulation and Function of TRPM7 in Human Endothelial Cells: TRPM7 as a Potential Novel Regulator of Endothelial Function. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef]
- Hong, F.-F.; Liang, X.-Y.; Liu, W.; Lv, S.; He, S.-J.; Kuang, H.-B.; Yang, S.-L. Roles of eNOS in atherosclerosis treatment. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2019, 68, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Kraehling, J.R.; Sessa, W.C. Contemporary approaches to modulating the nitric oxide-cGMP pathway in cardiovascular disease. Circ. Res. 2017, 120, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Behrens, P.; Brandt-Wunderlich, C.; Siewert, S.; Grabow, N.; Schmitz, K.-P. In vitro performance investigation of bioresorbable scaffolds—Standard tests for vascular stents and beyond. Cardiovasc. Revasc. Med. 2016, 17, 375–383. [Google Scholar] [CrossRef]
- Rapetto, C.; Leoncini, M. Magmaris: A new generation metallic sirolimus-eluting fully bioresorbable scaffold: Present status and future perspectives. J. Thorac. Dis. 2017, 9, S903–S913. [Google Scholar] [CrossRef]
- Farruggia, G.; Castiglioni, S.; Sargenti, A.; Marraccini, C.; Cazzaniga, A.; Merolle, L.; Iotti, S.; Cappadone, C.; Maier, J.A.M. Effects of supplementation with different Mg salts in cells: Is there a clue? Magnes. Res. 2014, 27, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Lipinski, M.J.; Acampado, E.; Cheng, Q.; Adams, L.; Torii, S.; Gai, J.; Torguson, R.; Hellinga, D.M.; Westman, P.C.; et al. Comparison of Acute Thrombogenicity for Metallic and Polymeric Bioabsorbable Scaffolds: Magmaris Versus Absorb in a Porcine Arteriovenous Shunt Model. Circ. Cardiovasc. Interv. 2017, 10. [Google Scholar] [CrossRef]
- Altura, B.M.; Altura, B.T.; Carella, A.; Gebrewold, A.; Murakawa, T.; Nishio, A. Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can. J. Physiol. Pharmacol. 1987, 65, 729–745. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedele, G.; Castiglioni, S.; Maier, J.A.; Locatelli, L. High Magnesium and Sirolimus on Rabbit Vascular Cells—An In Vitro Proof of Concept. Materials 2021, 14, 1970. https://doi.org/10.3390/ma14081970
Fedele G, Castiglioni S, Maier JA, Locatelli L. High Magnesium and Sirolimus on Rabbit Vascular Cells—An In Vitro Proof of Concept. Materials. 2021; 14(8):1970. https://doi.org/10.3390/ma14081970
Chicago/Turabian StyleFedele, Giorgia, Sara Castiglioni, Jeanette A. Maier, and Laura Locatelli. 2021. "High Magnesium and Sirolimus on Rabbit Vascular Cells—An In Vitro Proof of Concept" Materials 14, no. 8: 1970. https://doi.org/10.3390/ma14081970
APA StyleFedele, G., Castiglioni, S., Maier, J. A., & Locatelli, L. (2021). High Magnesium and Sirolimus on Rabbit Vascular Cells—An In Vitro Proof of Concept. Materials, 14(8), 1970. https://doi.org/10.3390/ma14081970





