Preparation and Thermal Conductivity of Epoxy Resin/Graphene-Fe3O4 Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
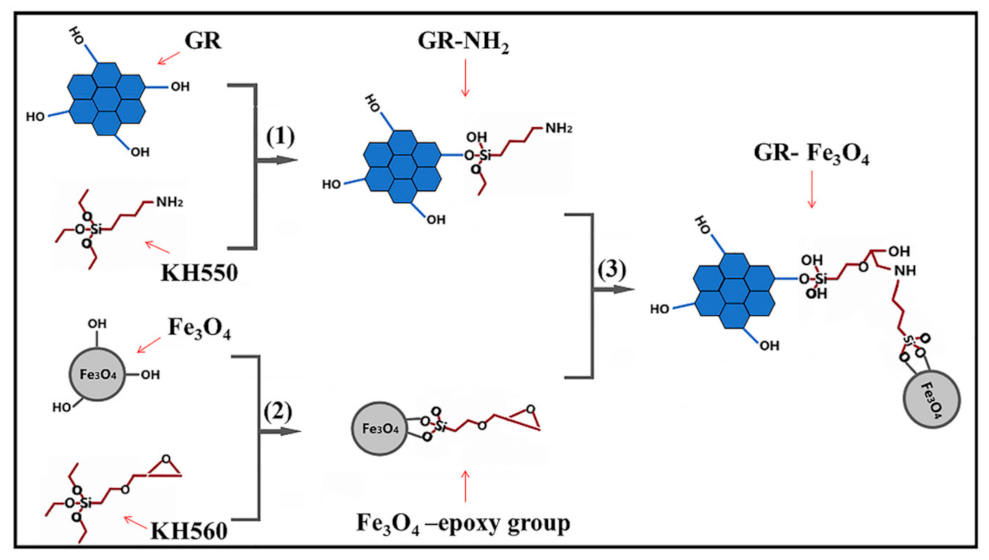
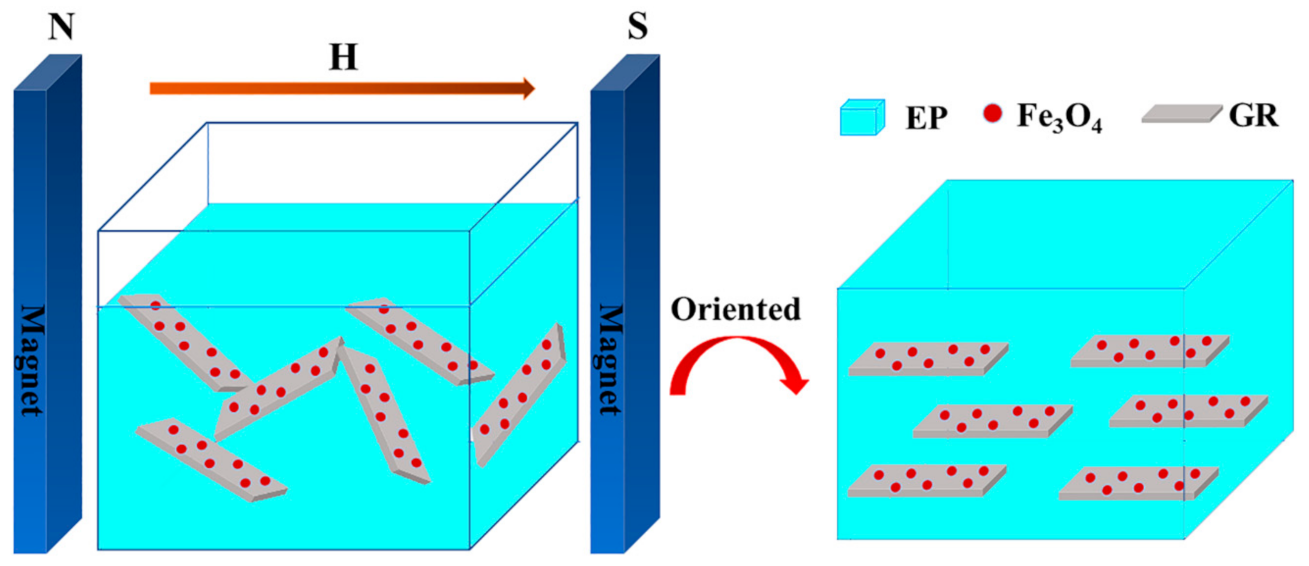
2.2. Preparation
2.3. Characterization
3. Results and Discussion
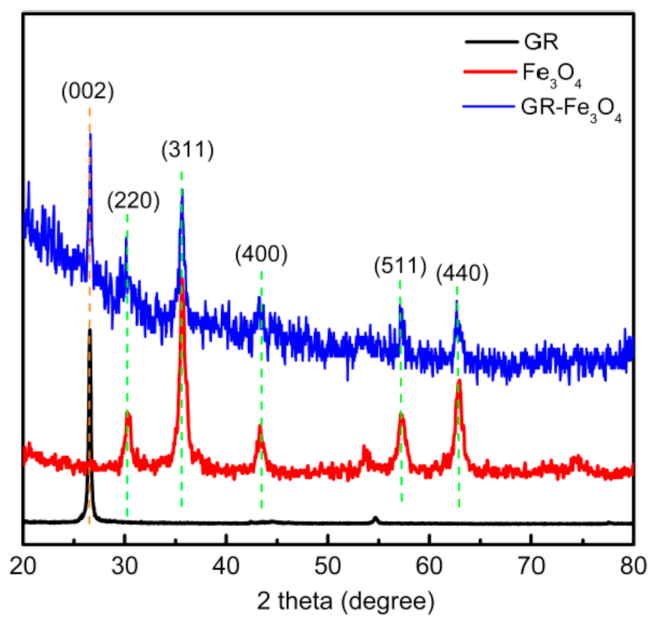
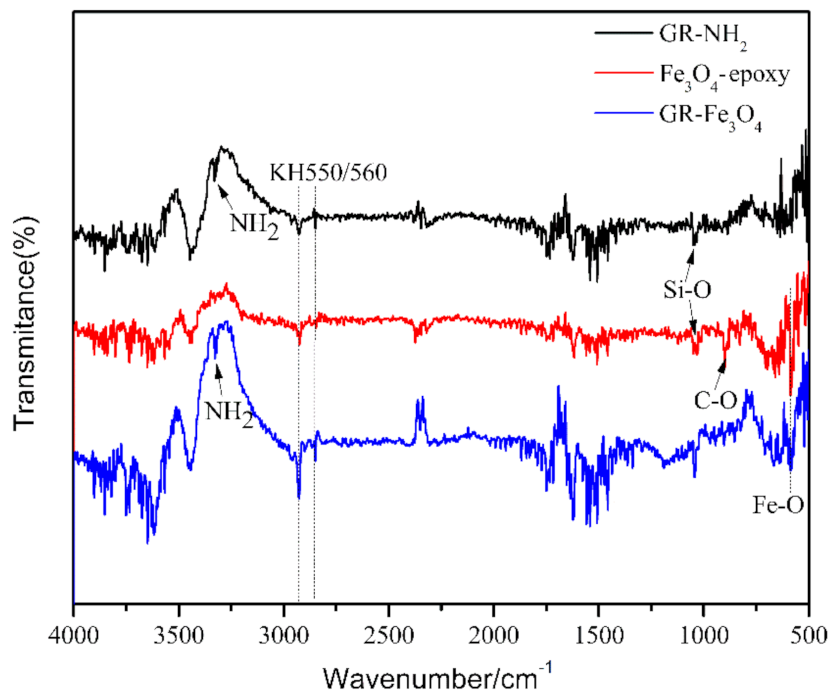
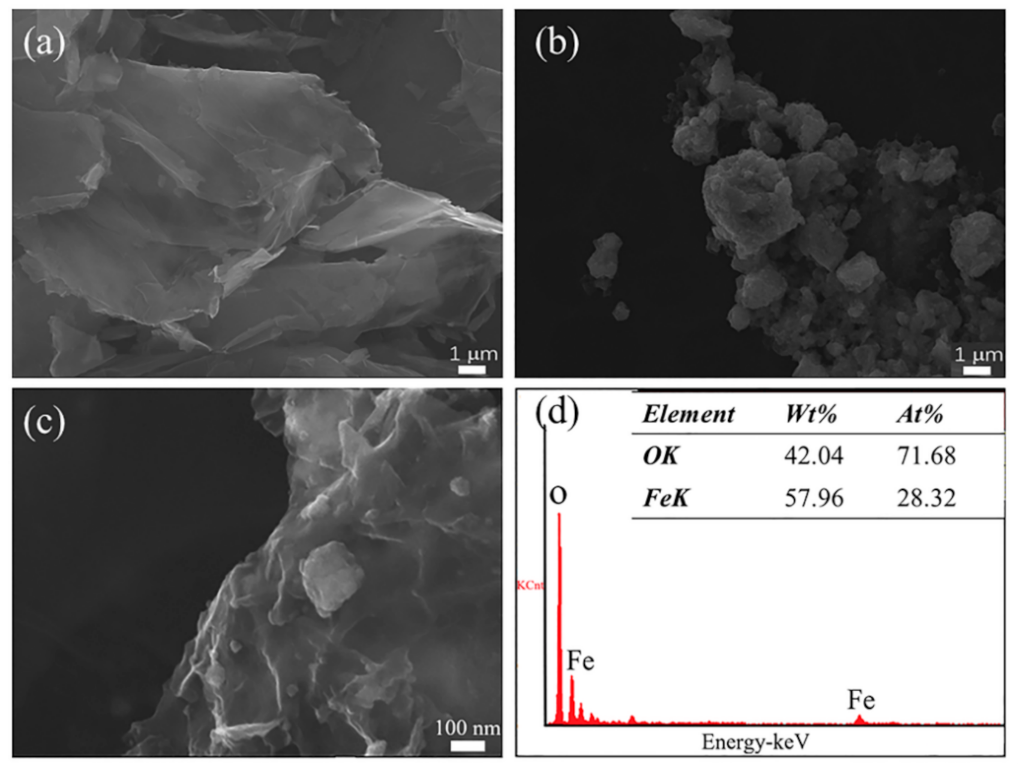
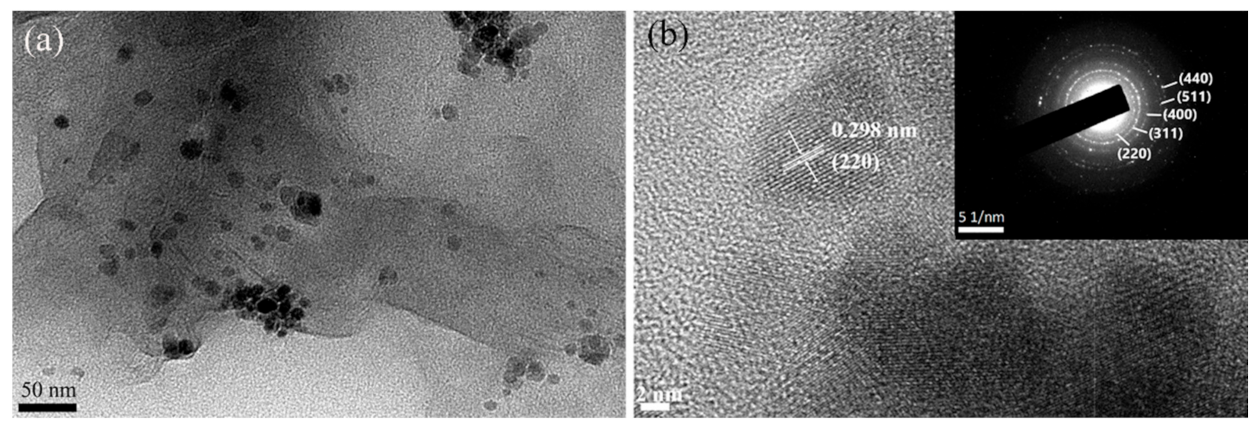
3.1. Material Characterization
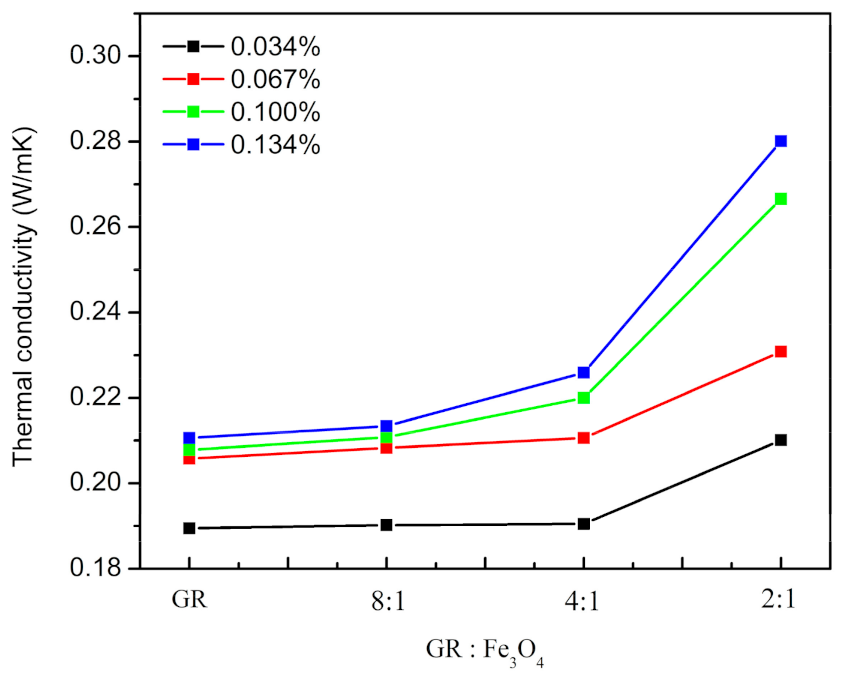
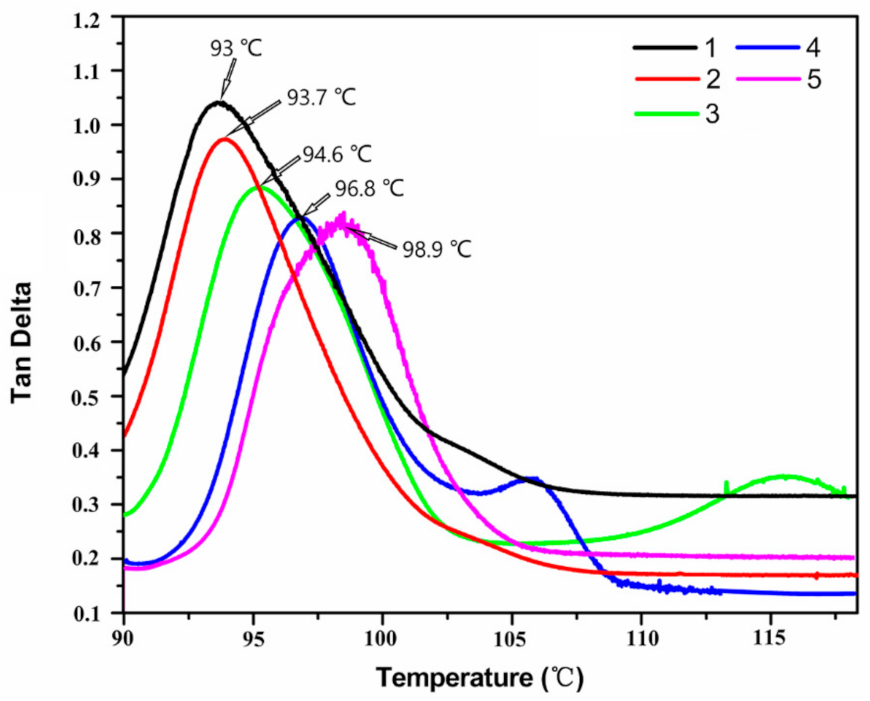
3.2. Thermal Properties and Electrical Insulation of Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, S.; Liu, H.; Li, Q.; Mu, Q.; Wen, H. Influence of magnetic alignment and layered structure of BN&Fe/EP on thermal conducting performance. J. Mater. Chem. C 2016, 4, 872–878. [Google Scholar] [CrossRef]
- Kim, J.; Ma, B.; Lee, K. Comparison of effect of epoxy and silicone adhesive on the lifetime of plastic LED package. Electron. Mater. Lett. 2013, 9, 429–432. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Mingyuan, G.U.; Liu, X. Study of the thermal conduction mechanism of nano-SiC/DGEBA/EMI-2,4 composites. Polymer 2008, 49, 4666–4672. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Hu, Z.; Jiang, P.; Li, S.; Tanaka, T. Large Dielectric Constant and High Thermal Conductivity in Poly(vinylidene fluoride)/Barium Titanate/Silicon Carbide Three-Phase Nanocomposites. ACS Appl. Mater. Interfaces 2011, 3, 4396–4403. [Google Scholar] [CrossRef]
- Zhou, W.; Qi, S.; Tu, C.; Zhao, H.; Wang, C.; Kou, J. Effect of the particle size of Al2O3 on the properties of filled heat-conductive silicone rubber. J. Appl. Polym. Sci. 2007, 104, 1312–1318. [Google Scholar] [CrossRef]
- Zhang, S.; Ke, Y.; Cao, X.; Ma, Y.; Wang, F. Effect of Al2O3 fibers on the thermal conductivity and mechanical properties of high density polyethylene with the absence and presence of compatibilizer. J. Appl. Polym. Sci. 2012, 124, 4874–4881. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J.; Kim, J. The influence of Al(OH)3-coated graphene oxide on improved thermal conductivity and maintained electrical resistivity of Al2O3/epoxy composites. J. Nanoparticle Res. 2012, 14. [Google Scholar] [CrossRef]
- Qian, R.; Yu, J.; Xie, L.; Li, Y.; Jiang, P. Efficient thermal properties enhancement to hyperbranched aromatic polyamide grafted aluminum nitride in epoxy composites. Polym. Adv. Technol. 2013, 24, 348–356. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Wang, L.; Yu, K.; Lin, Z.; He, L.; Bai, Y. Fabrication and characterization of aluminum nitride polymer matrix composites with high thermal conductivity and low dielectric constant for electronic packaging. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2012, 177, 892–896. [Google Scholar] [CrossRef]
- Yu, H.; Li, L.; Kido, T.; Xi, G.; Xu, G.; Guo, F. Thermal and insulating properties of epoxy/aluminum nitride composites used for thermal interface material. J. Appl. Polym. Sci. 2012, 124, 669–677. [Google Scholar] [CrossRef]
- Choi, S.; Im, H.; Kim, J. The thermal conductivity of embedded nano-aluminum nitride-doped multi-walled carbon nanotubes in epoxy composites containing micro-aluminum nitride particles. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Zeng, J.; Fu, R.; Shen, Y.; He, H.; Song, X. High Thermal Conductive Epoxy Molding Compound with Thermal Conductive Pathway. J. Appl. Polym. Sci. 2009, 113, 2117–2125. [Google Scholar] [CrossRef]
- Qiu, F.; Hao, Y.; Li, X.; Wang, B.; Wang, M. Functionalized graphene sheets filled isotactic polypropylene nanocomposites. Compos. Part B-Eng. 2015, 71, 175–183. [Google Scholar] [CrossRef]
- Zhao, W.; Kong, J.; Liu, H.; Zhuang, Q.; Gu, J.; Guo, Z. Ultra-high thermally conductive and rapid heat responsive poly(benzobisoxazole) nanocomposites with self-aligned graphene. Nanoscale 2016, 8, 19984–19993. [Google Scholar] [CrossRef]
- Lian, G.; Tuan, C.-C.; Li, L.; Jiao, S.; Wang, Q.; Moon, K.-S.; Cui, D.; Wong, C.-P. Vertically Aligned and Interconnected Graphene Networks for High Thermal Conductivity of Epoxy Composites with Ultralow Loading. Chem. Mater. 2016, 28, 6096–6104. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Han, D.; Zhao, Y.-H.; Bai, S.-L. High-performance thermal interface materials consisting of vertically aligned graphene film and polymer. Carbon 2016, 109, 552–557. [Google Scholar] [CrossRef]
- Wu, S.; Ladani, R.B.; Zhang, J.; Bafekrpour, E.; Ghorbani, K.; Mouritz, A.P.; Kinloch, A.J.; Wang, C.H. Aligning multilayer graphene flakes with an external electric field to improve multifunctional properties of epoxy nanocomposites. Carbon 2015, 94, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Mo, Z.; Zhang, C.; Wang, B.; Zhao, G.; Guo, R. Preparation of graphene-Fe3O4 nanocomposites using Fe3+ ion-containing magnetic ionic liquid. Mater. Res. Bull. 2014, 59, 223–226. [Google Scholar] [CrossRef]
- Ma, E.; Li, J.; Zhao, N.; Liu, E.; He, C.; Shi, C. Preparation of reduced graphene oxide/Fe3O4 nanocomposite and its microwave electromagnetic properties. Mater. Lett. 2013, 91, 209–212. [Google Scholar] [CrossRef]
- Guo, L.; Xu, C.; Wang, X.; Lin, Y.; Li, Y.; Ji, W. Preparation of magnetic graphene. Packag. Eng. 2017, 32–35. [Google Scholar]
- Liu, Z.; Jiao, W.; Yan, M.; Li, J.; Ding, G.; Wang, R. A novel method for imitating nacre by utilizing magnetic graphene oxide and its magnetic field alignment in polymer nanocomposites. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Renteria, J.; Legedza, S.; Salgado, R.; Balandin, M.P.; Ramirez, S.; Saadah, M.; Kargar, F.; Balandin, A.A. Magnetically-functionalized self-aligning graphene fillers for high-efficiency thermal management applications. Mater. Des. 2015, 88, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, Y.; Bao, T.; Li, X.; Su, Y.; Duan, Y. Preparation of Ni-reduced graphene oxide nanocomposites by Pd-activated electroless deposition and their magnetic properties. Appl. Surf. Sci. 2012, 258, 8603–8608. [Google Scholar] [CrossRef]
- Mihai, A.I.; Garea, S.A.; Vasile, E.; Nistor, C.L.; Iovu, H. Functionalization of Porous Clay Heterostructures with Silane Coupling Agents. Mater. Plast. 2017, 54, 341–344. [Google Scholar] [CrossRef]
- Ozgur, M.E.; Ulu, A.; Balcioglu, S.; Ozcan, I.; Koytepe, S.; Ates, B. The Toxicity Assessment of Iron Oxide (Fe3O4) Nanoparticles on Physical and Biochemical Quality of Rainbow Trout Spermatozoon. Toxics 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Fan, M.; Hu, J.; Ruan, W.; Xiong, K.; Wei, X. Optimizing Low-Concentration Mercury Removal from Aqueous Solutions by Reduced Graphene Oxide-Supported Fe3O4 Composites with the Aid of an Artificial Neural Network and Genetic Algorithm. Materials 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.-C.; Kim, K.S. Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Jiao, W.; Shioya, M.; Wang, R.; Yang, F.; Hao, L.; Niu, Y.; Liu, W.; Zheng, L.; Yuan, F.; Wan, L.; et al. Improving the gas barrier properties of Fe3O4/graphite nanoplatelet reinforced nanocomposites by a low magnetic field induced alignment. Compos. Sci. Technol. 2014, 99, 124–130. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679. [Google Scholar] [CrossRef]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.-S.; Paik, K.-W.; Jeon, S. Enhanced Thermal Conductivity of EpoxyGraphene Composites by Using Non-Oxidized Graphene Flakes with Non-Covalent Functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Li, G.; Yao, Y.-M.; Zeng, X.-L.; Zhu, P.-L.; Sun, R. Recent advances in polymer-based electronic packaging materials. Compos. Commun. 2020, 19, 154–167. [Google Scholar] [CrossRef]
- Li, T.-L.; Hsu, S.L.-C. Enhanced Thermal Conductivity of Polyimide Films via a Hybrid of Micro- and Nano-Sized Boron Nitride. J. Phys. Chem. B 2010, 114, 6825–6829. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, G.; Hou, J.; Yu, X.; Ding, H.; Zhang, Q.; Wang, N.; Qu, X. Preparation, characterization, and thermal properties of poly (methyl methacrylate)/boron nitride composites by bulk polymerization. Polym. Compos. 2015, 36, 1675–1684. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]


| Mass Fraction of GR in EP (wt.%) | Mass Fraction of Magnetic GR in EP (wt.%) |
|---|---|
| 0.034 | 0.038 (a) |
| 0.040 (b) | |
| 0.050 (c) | |
| 0.067 | 0.075 (a) |
| 0.080 (b) | |
| 0.100 (c) | |
| 0.100 | 0.113 (a) |
| 0.125 (b) | |
| 0.150 (c) | |
| 0.134 | 0.150 (a) |
| 0.167 (b) | |
| 0.200 (c) |
| TC (W/mK) | Only GR | GR: Fe3O4/8:1 | GR: Fe3O4/4:1 | GR: Fe3O4/2:1 | |
|---|---|---|---|---|---|
| The Mass Fraction of GR in EP(wt.%) | |||||
| 0.034 | 0.1895 (±0.0003) | 0.1902 (±0.0001) | 0.1905 (±0.0004) | 0.2101 (±0.0002) | |
| 0.067 | 0.2058 (±0.0004) | 0.2083 (±0.0004) | 0.2106 (±0.0002) | 0.2308 (±0.0003) | |
| 0.100 | 0.2078 (±0.0004) | 0.2108 (±0.0005) | 0.2200 (±0.0003) | 0.2666 (±0.0002) | |
| 0.134 | 0.2106 (±0.0003) | 0.2134 (±0.0002) | 0.2259 (±0.0003) | 0.2801 (±0.0004) | |
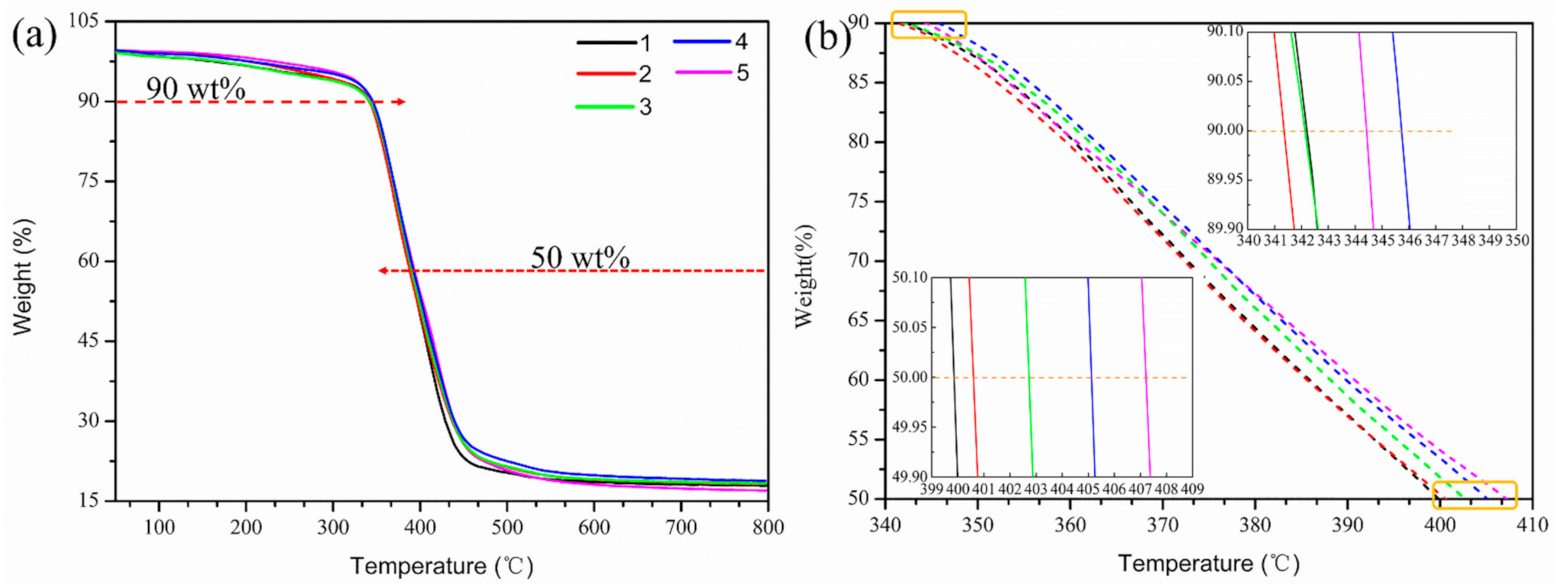
| The Mass Fraction of GR-Fe3O4 in EP (wt.%) | Tg (°C) | T10% (°C) | T50% (°C) |
|---|---|---|---|
| 0 | 93.0 | 342.4 | 399.2 |
| 0.05 | 93.7 | 341.6 | 400.8 |
| 0.10 | 94.6 | 342.0 | 402.8 |
| 0.15 | 96.8 | 345.6 | 405.6 |
| 0.20 | 98.9 | 344.4 | 407.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Chen, J.; Li, Q.; Xia, D.-H.; Deng, Y.; Zhang, Y.; Qin, Z. Preparation and Thermal Conductivity of Epoxy Resin/Graphene-Fe3O4 Composites. Materials 2021, 14, 2013. https://doi.org/10.3390/ma14082013
Wu Z, Chen J, Li Q, Xia D-H, Deng Y, Zhang Y, Qin Z. Preparation and Thermal Conductivity of Epoxy Resin/Graphene-Fe3O4 Composites. Materials. 2021; 14(8):2013. https://doi.org/10.3390/ma14082013
Chicago/Turabian StyleWu, Zhong, Jingyun Chen, Qifeng Li, Da-Hai Xia, Yida Deng, Yiwen Zhang, and Zhenbo Qin. 2021. "Preparation and Thermal Conductivity of Epoxy Resin/Graphene-Fe3O4 Composites" Materials 14, no. 8: 2013. https://doi.org/10.3390/ma14082013
APA StyleWu, Z., Chen, J., Li, Q., Xia, D.-H., Deng, Y., Zhang, Y., & Qin, Z. (2021). Preparation and Thermal Conductivity of Epoxy Resin/Graphene-Fe3O4 Composites. Materials, 14(8), 2013. https://doi.org/10.3390/ma14082013







