An Evaluation of the Impact of the Amount of Potassium Hydroxide on the Porous Structure Development of Activated Carbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon Samples
2.2. Adsorption Models Considered in This Study
2.3. Textural Characterization of Activated Carbon Samples
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babich, F.; Demanega, I.; Avella, F.; Belleri, A. Low polluting building materials and ventilation for good air quality in residential buildings: A cost–benefit study. Atmosphere 2020, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Vunain, E.; Houndedjihou, D.; Monjerezi, M.; Muleja, A.A.; Kodom, B.T. Adsorption, kinetics and equilibrium studies on removal of catechol and resorcinol from aqueous solution using low-cost activated carbon prepared from sunflower (helianthus annuus) seed hull residues. Water Air Soil Pollut. 2018, 229, 366. [Google Scholar] [CrossRef]
- Wu, H.; Chen, R.; Du, H.; Zhang, J.; Shi, L.; Qin, Y.; Yue, L.; Wang, J. Synthesis of activated carbon from peanut shell as dye adsorbents for wastewater treatment. Ads. Sci. Technol. 2018, 37, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Song, Y.; Ji, X.; Ji, L.; Cai, L.; Wang, Y.; Zhang, H.; Song, W. Preparation and characterization of nanoporous activated carbon derived from prawn shell and its application for removal of heavy metal ions. Materials 2019, 12, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baby, R.; Hussein, M.Z. Ecofriendly approach for treatment of heavy-metal-contaminated water using activated carbon of kernel shell of oil palm. Materials 2020, 13, 2627. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Mulana, F.; Juniar, L.; Fathira, D.; Safitri, R.; Muchtar, S.; Bilad, M.R.; Shariff, A.H.M.; Huda, N. Development of biosorbent derived from the endocarp waste of Gayo coffee for lead removal in liquid wastewater—effects of chemical activators. Sustainability 2021, 13, 3050. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, M.; Dai, B. Cobalt-nitrogen-activated carbon as catalyst in acetylene hydrochlorination. Cat. Commun. 2017, 98, 22–25. [Google Scholar] [CrossRef]
- Bermejo, B.; Fraga, A.; Sousa-Aguiar, E. The role of sulfonated activated carbons as catalysts for the hydrolysis of cellobiose. Braz. J. Chem. Eng. 2019, 36, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Mostazo-López, M.J.; Salinas-Torres, D.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Nitrogen-doped superporous activated carbons as electrocatalysts for the oxygen reduction reaction. Materials 2019, 12, 1346. [Google Scholar] [CrossRef] [Green Version]
- Kurniawansyah, F.; Pertiwi, R.; Perdana, M.; Al Muttaqii, M.; Roesyadi, A. Development of bamboo—derived activated carbon as catalyst support for glucose hydrogenation. Mater. Sci. Forum 2020, 988, 108–113. [Google Scholar] [CrossRef]
- Shrestha, P.; Jha, M.K.; Ghimire, J.; Koirala, A.R.; Shrestha, R.M.; Sharma, R.K.; Pant, B.; Park, M.; Pant, H.R. Decoration of zinc oxide nanorods into the surface of activated carbon obtained from agricultural waste for effective removal of methylene blue dye. Materials 2020, 13, 5667. [Google Scholar] [CrossRef] [PubMed]
- Rash, T.; Gillespie, A.; Holbrook, B.P.; Hiltzik, L.H.; Romanos, J.; Soo, Y.C.; Sweany, S.; Pfeifer, P. Microporous carbon monolith synthesis and production for methane storage. Fuel 2017, 200, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Men’shchikov, I.E.; Shkolin, A.V.; Strizhenov, E.M.; Khozina, E.V.; Chugaev, S.S.; Shiryaev, A.A.; Fomkin, A.A.; Zherdev, A.A. Thermodynamic behaviors of adsorbed methane storage systems based on nanoporous carbon adsorbents prepared from coconut shells. Nanomaterials 2020, 10, 2243. [Google Scholar] [CrossRef] [PubMed]
- Sdanghi, G.; Nicolas, V.; Mozet, K.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. A 70 MPa hydrogen thermally driven compressor based on cyclic adsorption-desorption on activated carbon. Carbon 2020, 161, 466–478. [Google Scholar] [CrossRef]
- Romanos, J.; Abou Dargham, S.; Roukos, R.; Pfeifer, P. Local Pressure of supercritical adsorbed hydrogen in nanopores. Materials 2018, 11, 2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murillo-Acevedo, Y.; Rodríguez-Estupiñan, P.; Giraldo Gutiérrez, L.; Moreno-Piraján, J.C. Activated carbon for hydrogen storage obtained from agro-industrial waste. In Hydrogen Storage Technologies; Sankir, M., Demirci Sankir, N., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2018; pp. 177–196. [Google Scholar] [CrossRef]
- Sdanghi, G.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. Application of the modified Dubinin-Astakhov equation for a better understanding of high-pressure hydrogen storage on activated carbons. Int. J. Hydrog. Energy 2020, 45, 25912–25926. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.S.; Brites, G.; Costa, J.; Gaspar, A.; Costa, V.A.F. Review and future trends of solar adsorption refrigeration systems. Ren. Sust. Energy Rev. 2014, 39, 102–123. [Google Scholar] [CrossRef]
- Khalil, W.; Khalifa, A.H.; Abdulazeez, H. Performance study of solar adsorption refrigeration system using activated carbon—Methanol. Al-Nahrain J. Eng. Sci. 2018, 21, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Li, Y.; Feng, Y. High electrochemical performance from oxygen functional groups containing porous activated carbon electrode of supercapacitors. Materials 2018, 11, 2455. [Google Scholar] [CrossRef] [Green Version]
- Castro-Gutierrez, J.; Diez, N.; Sevilla, M.; Izquierdo, M.T.; Ghanbaja, J.; Celzard, A.; Fierro, V. High-rate capability of supercapacitors based on tannin-derived ordered mesoporous carbons. ACS Sustain. Chem. Eng. 2019, 7, 17627–17635. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Ariga, K.; Shrestha, L.K. Nanoporous carbon materials derived from washnut seed with enhanced supercapacitance. Materials 2020, 13, 2371. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Feng, Z.; Li, X.; Yi, R.; Sun, W.; Zhao, C.; Yang, L. Nitrogen-doped hierarchical porous activated carbon derived from paddy for high-performance supercapacitors. Materials 2021, 14, 318. [Google Scholar] [CrossRef]
- Ania, C.O.; Armstrong, P.A.; Bandosz, T.J.; Béguin, F.; Carvalho, A.P.; Celzard, A.; Frackowiak, E.; Gilarranz, M.A.; László, K.; Matos, J.; et al. Engaging nanoporous carbons in "beyond adsorption" applications: Characterization; challenges and performance. Carbon 2020, 164, 69–84. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biom. Conv. Bioref. 2019, 9, 75–802. [Google Scholar] [CrossRef] [Green Version]
- Machrouhi, A.; Alilou, H.; Farnane, M.; El Hamidi, S.; Sadiq, M.; Abdennouri, M.; Tounsadi, H.; Barka, N. Statistical optimization of activated carbon from Thapsia transtagana stems and dyes removal efficiency using central composite design. J. Sci. Adv. Mater. Dev. 2019, 4, 544–553. [Google Scholar] [CrossRef]
- Sun, S.; Qiongfen, Y.; Ming, L.; Hong, Z.; Wu, C. Preparation of coffee-shell activated carbon and its application for water vapor adsorption. Renew. Energy 2019, 142, 11–19. [Google Scholar] [CrossRef]
- Kosheleva, R.I.; Mitropoulos, A.C.; Kyzas, G.Z. Synthesis of activated carbon from food waste. Environ. Chem. Lett. 2019, 17, 429–438. [Google Scholar] [CrossRef]
- Yokoyama, J.T.C.; Cazetta, A.L.; Bedin, K.C.; Spessato, L.; Fonseca, J.M.; Carraro, P.S.; Ronix, A.; Silva, M.C.; Silva, T.L.; Almeida, V.C. Stevia residue as new precursor of CO2-activated carbon: Optimization of preparation condition and adsorption study of triclosan. Ecotox. Environ. Safe. 2019, 172, 403–410. [Google Scholar] [CrossRef]
- Yang, P.; Rao, L.; Zhu, W.; Wang, L.; Ma, R.; Chen, F.; Lin, G.; Hu, X. Porous carbons derived from sustainable biomass via a facile one-step synthesis strategy as efficient CO2 adsorbents. Ind. Eng. Chem. Res. 2020, 59, 6194–6201. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, T.; Zhi, J.; Zheng, Q.; Chen, Q.; Zhang, C.; Li, Y. Utilization of Jujube biomass to prepare biochar by pyrolysis and activation: Characterization, adsorption characteristics, and mechanisms for nitrogen. Materials 2020, 13, 5594. [Google Scholar] [CrossRef]
- Perdana, Y.A.; Joni, R.; Aziz, E.H. Effect of KOH activator on the performance of activated carbon from oil palm kernel shell as supercapacitor electrode material. J. Aceh Phys. Soc. 2020, 9, 13–19. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Zhang, H.; Shao, L.-M.; Lü, F.; He, P.-J. Preparation and application of hierarchical porous carbon materials from waste and biomass: A review. Waste Biomass Valor. 2021, 12, 1699–1724. [Google Scholar] [CrossRef]
- Kaewtrakulchai, N.; Faungnawakij, K.; Eiad-Ua, A. Parametric study on microwave-assisted pyrolysis combined KOH activation of oil palm male flowers derived nanoporous carbons. Materials 2020, 13, 2876. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Wang, L.; Chen, F.; Shao, J.; Hu, X. Efficient nitrogen doped porous carbonaceous CO2 adsorbents based on lotus leaf. J. Environ. Sci. 2021, 103, 268–278. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Kwiatkowski, M. Computer analysis of microporous structure by employing the LBET class models with various variants of the adsorption energy distribution in comparison to the classical equations. Langmuir 2007, 23, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, M.; Fierro, V.; Celzard, A. Numerical studies of the effects of process conditions on the development of the porous structure of adsorbents prepared by chemical activation of lignin with alkali hydroxides. J. Colloid Interface Sci. 2017, 486, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, M.; Fierro, V.; Celzard, A. Confrontation of various adsorption models for assessing the porous structure of activated carbons. Adsorption 2019, 25, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, M.; Vargas Delgadillo, D.P. Computer analysis of the effect of the type of activating agent on the formation of the porous structure of activated carbon monoliths. J. Mater. Res. Technol. 2019, 8, 4457–4463. [Google Scholar] [CrossRef]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micromesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Jagiello, J.; Kenvin, J.; Celzard, A.; Fierro, V. Enhanced resolution of ultra micropore size determination of biochars and activated carbons by dual gas analysis using N2 and CO2 with 2D-NLDFT adsorption models. Carbon 2019, 144, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Jagiello, J.; Kenvin, J.; Ania, C.; Parra, J.; Celzard, A.; Fierro, V. Exploiting the adsorption of simple gases O2 and H2 with minimal quadrupole moments for the dual gas characterization of nanoporous carbons using 2D-NLDFT models. Carbon 2020, 160, 164–175. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Caguiat, J.; Kirk, D.; Jia, C. Uncertainties in characterization of nanoporous carbons using density functional theory-based gas physisorption. Carbon 2014, 72, 47–56. [Google Scholar] [CrossRef]
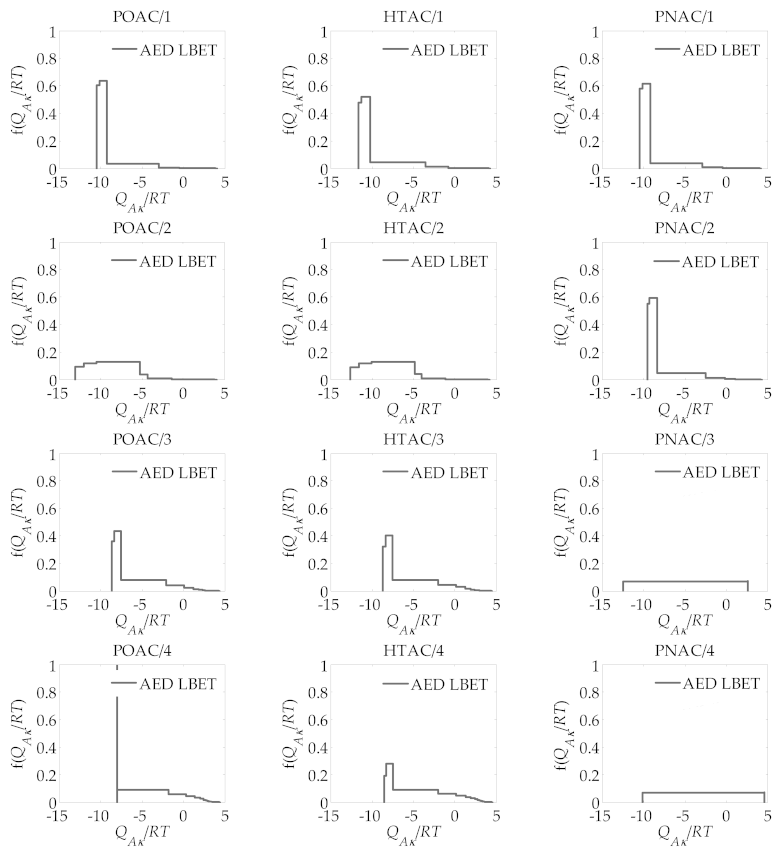
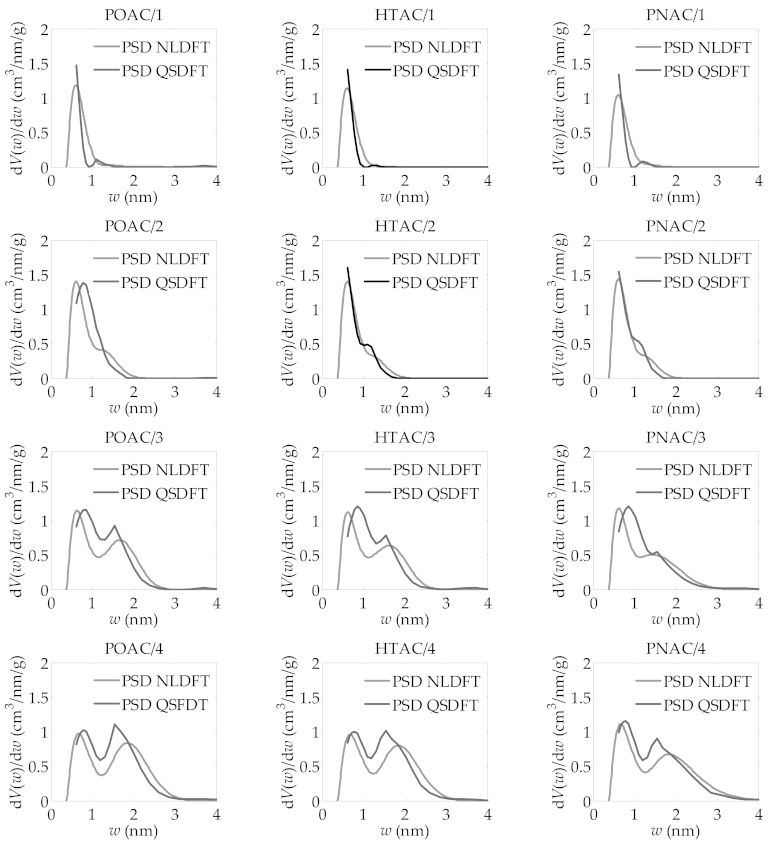
| R | ABET m2/g | VDR cm3/g | VT cm3/g | Vmeso cm3/g |
|---|---|---|---|---|
| POAC/R | ||||
| 1 | 1109 | 0.478 | 0.508 | 0.030 |
| 2 | 2004 | 0.801 | 0.893 | 0.092 |
| 3 | 2911 | 0.964 | 1.337 | 0.373 |
| 4 | 3240 | 0.990 | 1.568 | 0.578 |
| HTAC/R | ||||
| 1 | 1063 | 0.461 | 0.476 | 0.015 |
| 2 | 1804 | 0.747 | 0.810 | 0.063 |
| 3 | 2687 | 0.903 | 1.233 | 0.330 |
| 4 | 3163 | 0.984 | 1.529 | 0.545 |
| PNAC/R | ||||
| 1 | 1017 | 0.438 | 0.451 | 0.013 |
| 2 | 1845 | 0.762 | 0.827 | 0.065 |
| 3 | 2493 | 0.884 | 1.184 | 0.300 |
| 4 | 3183 | 1.048 | 1.630 | 0.582 |
| R | LBET No. | VhA cm3/g | QA/RT | BC | h | α | β |
|---|---|---|---|---|---|---|---|
| POAC/R | |||||||
| 1 | 22 | 0.426 | −10.43 | 7.98 | 5 | 0.25 | 1.00 |
| 2 | 15 | 0.731 | −13.10 | 7.51 | 9 | 0.27 | 1.00 |
| 3 | 22 | 1.329 | −8.62 | 6.92 | 5 | 0.61 | 1.00 |
| 4 | 19 | 1.434 | −8.02 | 7.23 | 3 | 0.75 | 1.00 |
| HTAC/R | |||||||
| 1 | 22 | 0.443 | −11.62 | 7.96 | 5 | 0.35 | 1.00 |
| 2 | 15 | 0.811 | −12.62 | 7.23 | 9 | 0.30 | 1.00 |
| 3 | 22 | 1.225 | −8.65 | 6.26 | 5 | 0.64 | 1.00 |
| 4 | 22 | 1.489 | −8.56 | 5.66 | 5 | 0.79 | 1.00 |
| PNAC/R | |||||||
| 1 | 22 | 0.407 | −10.40 | 7.66 | 5 | 0.28 | 1.00 |
| 2 | 22 | 0.743 | −9.54 | 7.66 | 5 | 0.35 | 1.00 |
| 3 | 2 | 1.350 | −12.41 | 6.04 | 1 | 0.00 | 1.00 |
| 4 | 2 | 2.131 | −10.12 | 7.66 | 1 | 0.33 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, M.; Broniek, E.; Fierro, V.; Celzard, A. An Evaluation of the Impact of the Amount of Potassium Hydroxide on the Porous Structure Development of Activated Carbons. Materials 2021, 14, 2045. https://doi.org/10.3390/ma14082045
Kwiatkowski M, Broniek E, Fierro V, Celzard A. An Evaluation of the Impact of the Amount of Potassium Hydroxide on the Porous Structure Development of Activated Carbons. Materials. 2021; 14(8):2045. https://doi.org/10.3390/ma14082045
Chicago/Turabian StyleKwiatkowski, Mirosław, Elżbieta Broniek, Vanessa Fierro, and Alain Celzard. 2021. "An Evaluation of the Impact of the Amount of Potassium Hydroxide on the Porous Structure Development of Activated Carbons" Materials 14, no. 8: 2045. https://doi.org/10.3390/ma14082045






