Polysiloxane Bonded Silica Aerogel with Enhanced Thermal Insulation and Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
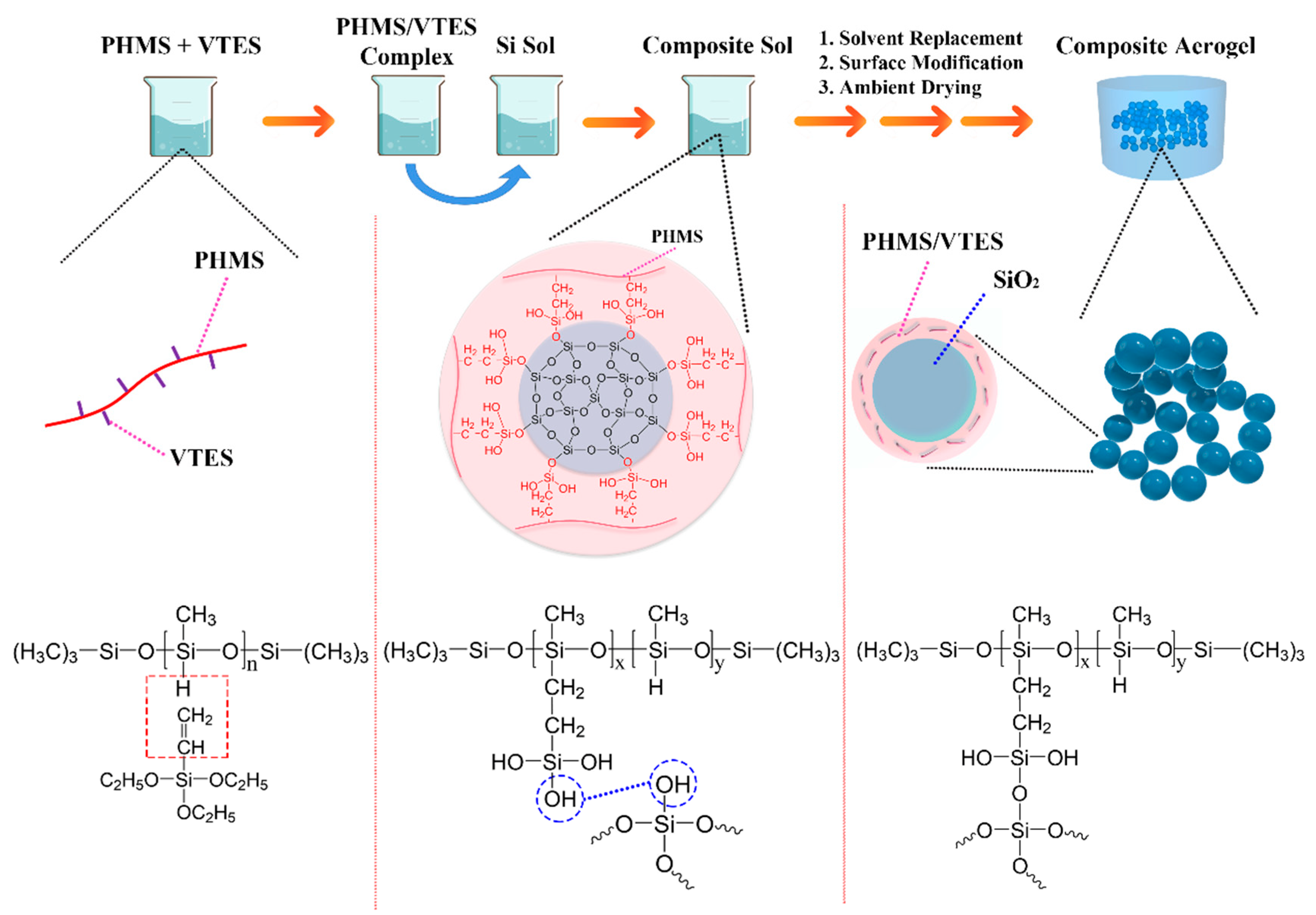
2.2.1. Preparation of the PHMS/VTES Complex
2.2.2. Preparation of the Wet Gels
2.2.3. Post-Processing of the Composite Aerogels
2.3. Characterizations
3. Results
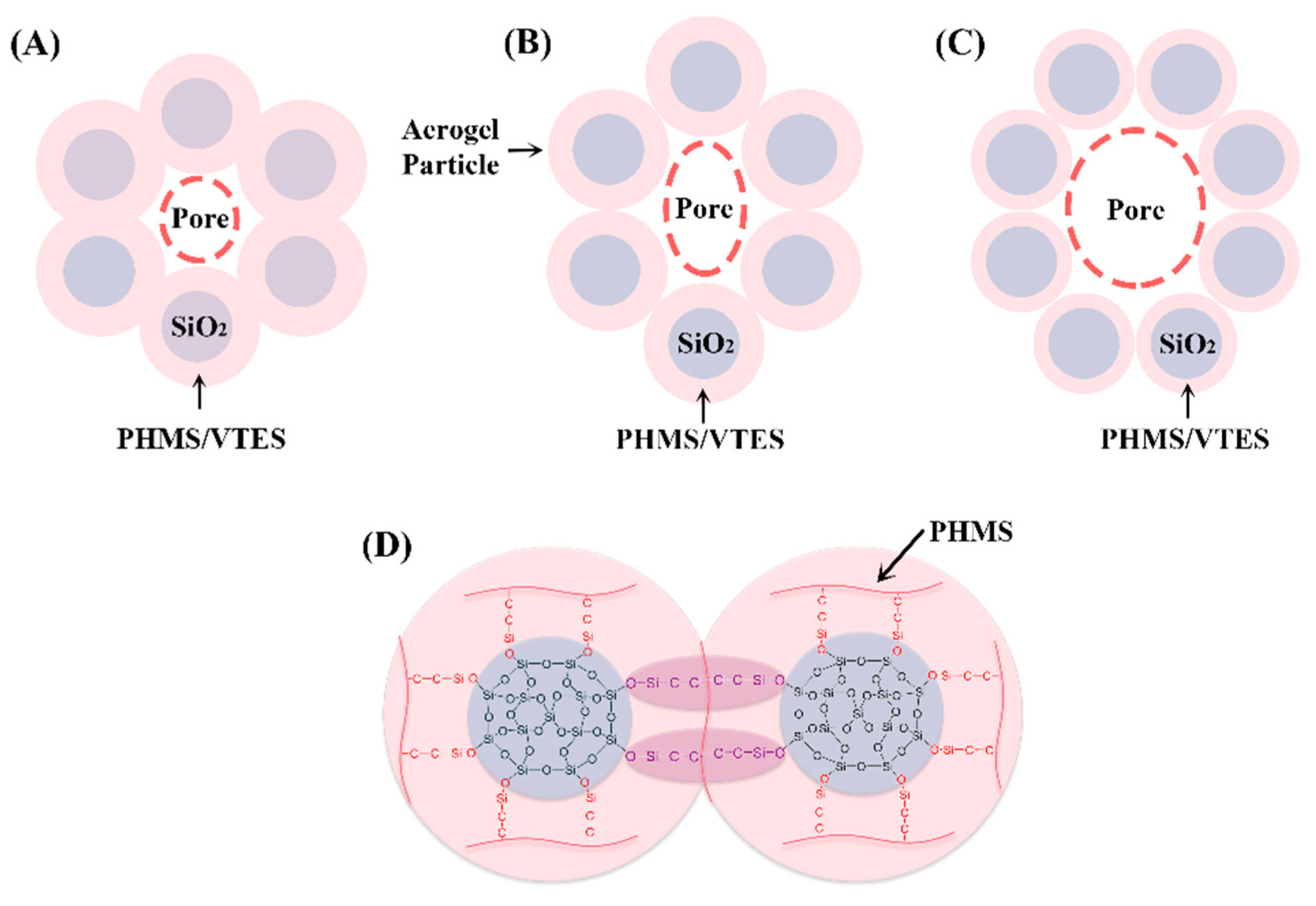
3.1. Microscopic Morphology and Structural Characteristics of the Composite Aerogels
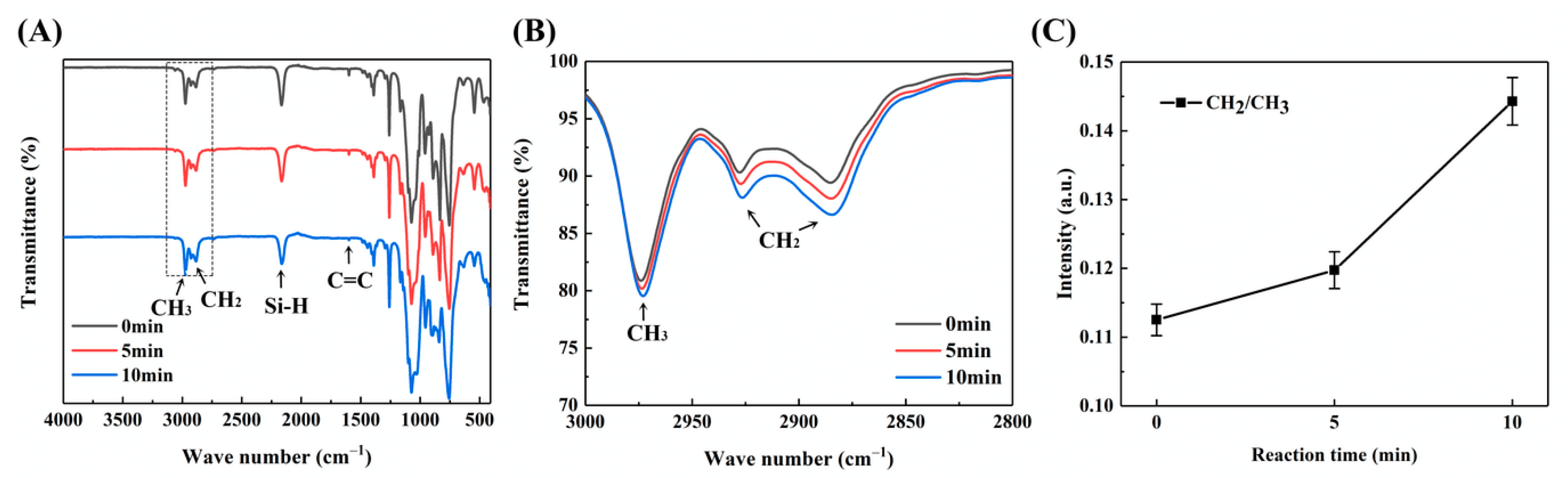
3.1.1. Chemical Composition and Reaction Mechanism
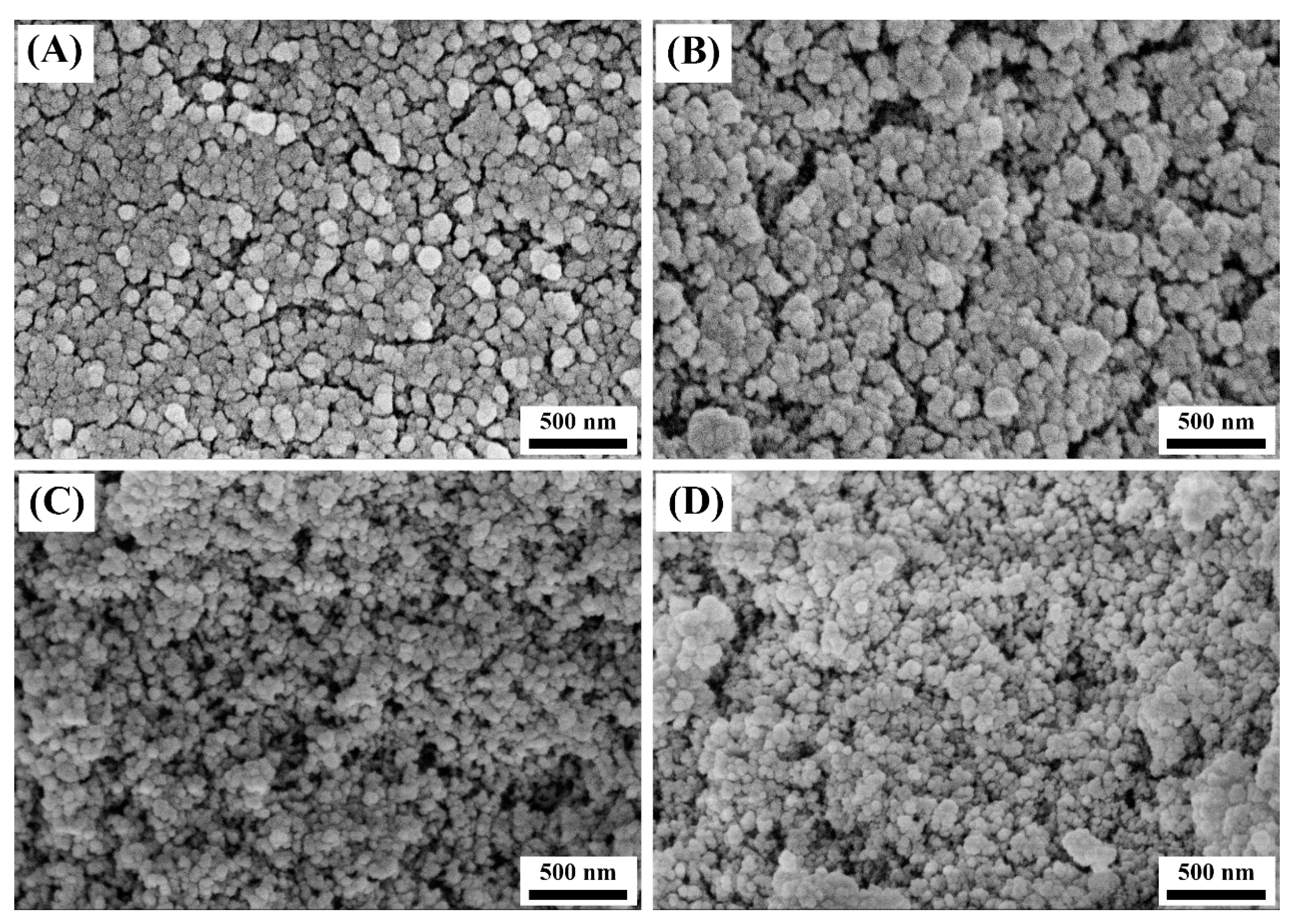
3.1.2. Microscopic Morphology
3.1.3. Pore Structure
3.2. Properties of the Composite Aerogels
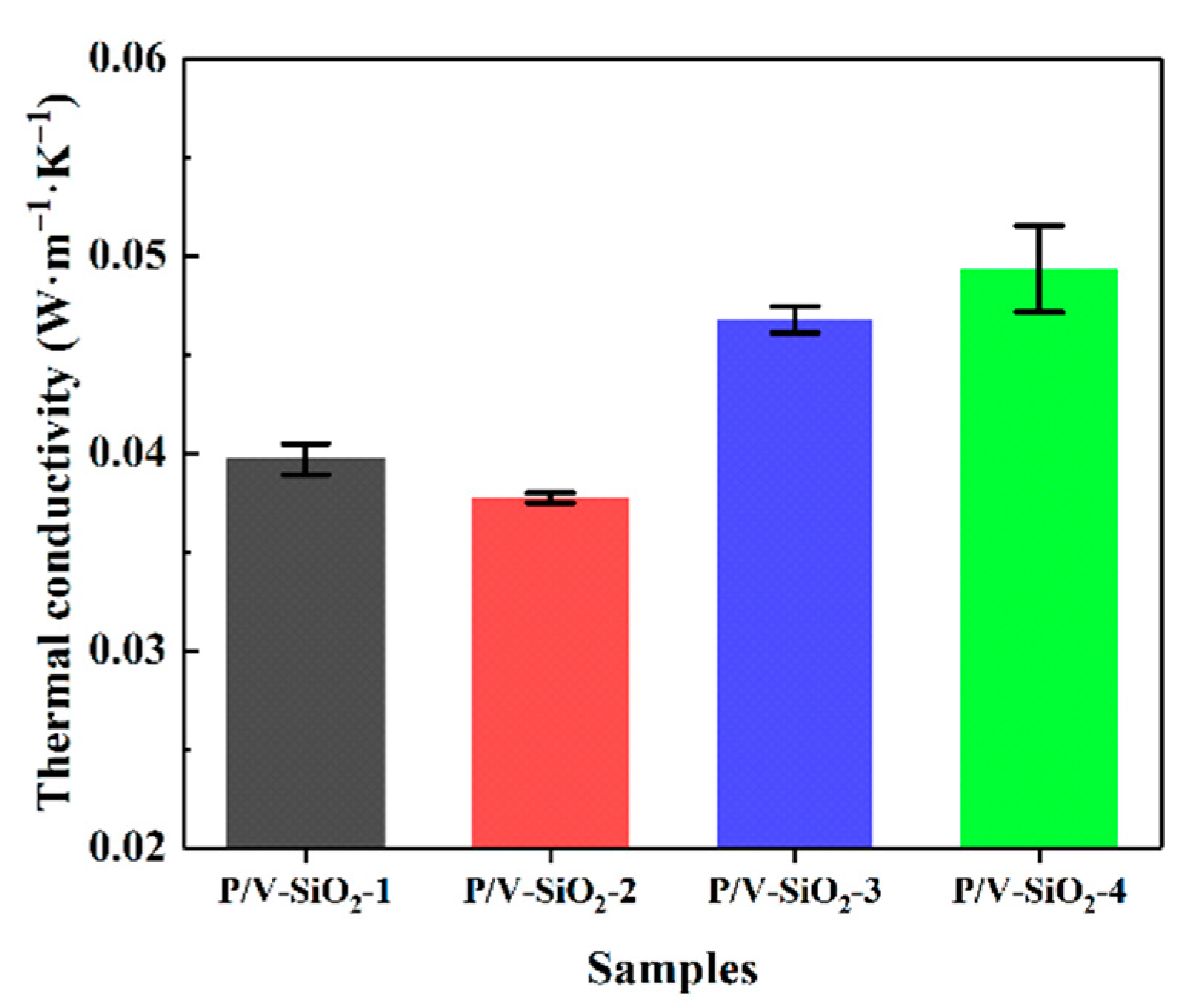
3.2.1. Thermal Properties
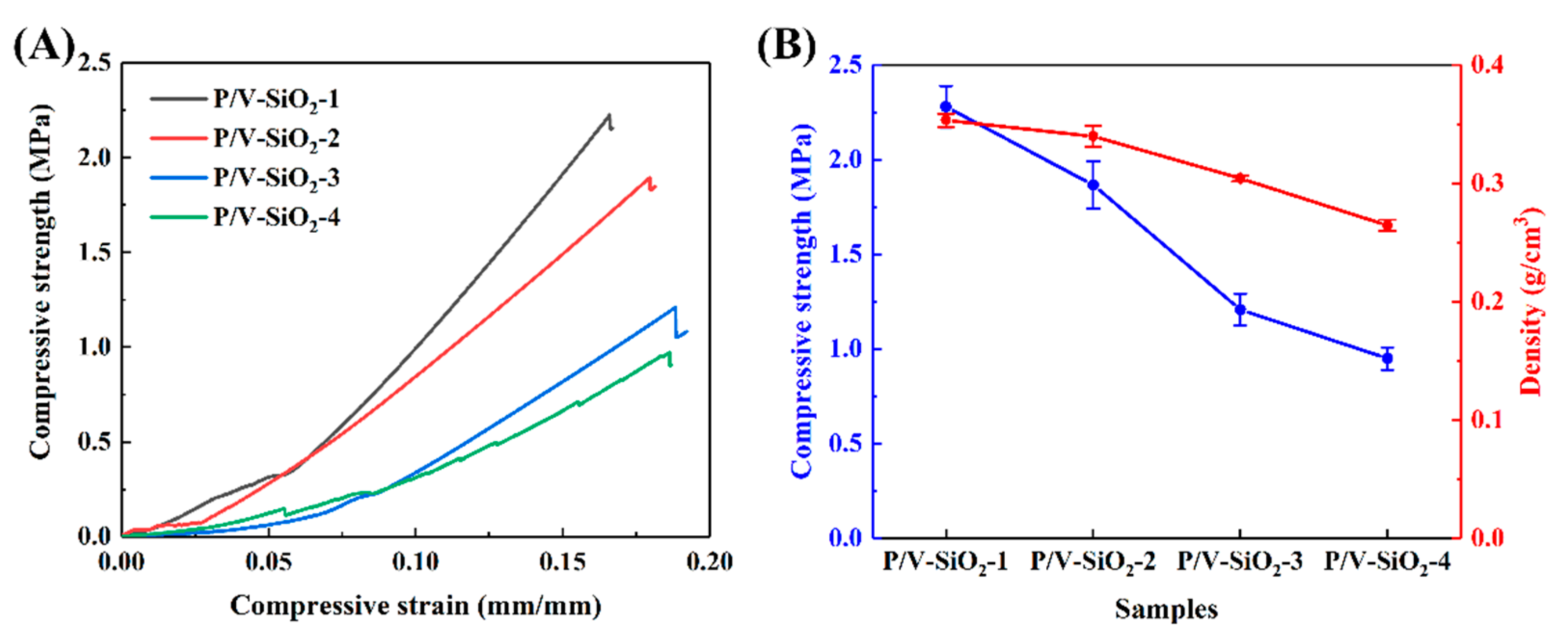
3.2.2. Mechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, D.M.; Maskara, A.; Boes, U. Aerogel-based thermal insulation. J. Non-Cryst. Solids 1998, 225, 254–259. [Google Scholar] [CrossRef]
- Fricke, J.; Emmerling, A. Aerogels—recent progress in production techniques and novel applications. J. Sol-Gel Sci. Technol. 1998, 13, 299–303. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V. Modifying the surface energy and hydrophobicity of the low-density silica aerogels through the use of combinations of surface-modification agents. J. Mater. Sci. 2010, 45, 51–63. [Google Scholar]
- Nguyen, B.N.; Meador, M.A.B.; Tousley, M.E.; Shonkwiler, B.; Mccorkle, L.; Scheiman, D.A. Tailoring elastic properties of silica aerogels cross-linked with polystyrene. ACS Appl. Mater. Interfaces 2009, 1, 621–630. [Google Scholar] [CrossRef]
- Meyer, E.; Milow, B.; Ratke, L. Development of aerogel additives for the foundry industry. J. Supercrit. Fluids 2015, 106, 62–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Q.; Li, X.; Xie, H.; Nie, C. Facile synthesis of ternary flexible silica aerogels with coarsened skeleton for oil–water separation. RSC Adv. 2020, 10, 42297–42304. [Google Scholar] [CrossRef]
- Boday, D.J.; Keng, P.Y.; Muriithi, B.; Pyun, J.; Loy, D.A. Mechanically reinforced silica aerogel nanocomposites via surface initiated atom transfer radical polymerizations. J. Mater. Chem. 2010, 20, 6863–6865. [Google Scholar] [CrossRef]
- Si, Y.; Yu, J.; Tang, X.; Ge, J.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5, 5802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez, M.; Taboada, E.; Idriss, H.; Molins, E.; Llorca, J. Fast and efficient hydrogen generation catalyzed by cobalt talc nanolayers dispersed in silica aerogel. J. Mater. Chem. 2010, 20, 4875–4883. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, Z.; Cao, B.; Chen, X.; Song, H. Enhancements of thermal insulation and mechanical property of silica aerogel monoliths by mixing graphene oxide. Mater. Chem. Phys. 2017, 187, 183–190. [Google Scholar] [CrossRef]
- Yue, S.; Li, X.; Yu, H.; Tong, Z.; Liu, Z. Preparation of high-strength silica aerogels by two-step surface modification via ambient pressure drying. J. Porous Mater. 2021, 1–9. [Google Scholar] [CrossRef]
- Jiang, L.; Kato, K.; Mayumi, K.; Yokoyama, H.; Ito, K. One-pot synthesis and characterization of polyrotaxane–silica hybrid aerogel. ACS Macro Lett. 2017, 6, 281–286. [Google Scholar] [CrossRef]
- He, S.; Chen, X. Flexible silica aerogel based on methyltrimethoxysilane with improved mechanical property. J. Non-Cryst. Solids 2017, 463, 6–11. [Google Scholar] [CrossRef]
- Kanamori, K.; Aizawa, M.; Nakanishi, K.; Hanada, T. Elastic organic–inorganic hybrid aerogels and xerogels. J. Sol-Gel Sci. Technol. 2008, 48, 172–181. [Google Scholar] [CrossRef]
- Duan, Y.; Jana, S.C.; Lama, B.; Espe, M.P. Reinforcement of silica aerogels using silane-end-capped polyurethanes. Langmuir 2013, 29, 6156–6165. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Li, H.; Ji, H.; Sun, X.; He, J. Effect of sepiolite fiber on the structure and properties of the sepiolite/silica aerogel composite. J. Sol-Gel Sci. Technol. 2013, 67, 646–653. [Google Scholar] [CrossRef]
- Hayase, G.; Kanamori, K.; Abe, K.; Yano, H.; Maeno, A.; Kaji, H.; Nakanishi, K. Polymethylsilsesquioxane–cellulose nanofiber biocomposite aerogels with high thermal insulation, bendability, and superhydrophobicity. ACS Appl. Mater. Interfaces 2014, 6, 9466–9471. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ren, H.; Zhu, J.; Bi, Y.; Xu, Y.; Zhang, L. Facile fabrication of superhydrophobic, mechanically strong multifunctional silica-based aerogels at benign temperature. J. Non-Cryst. Solids 2017, 473, 59–63. [Google Scholar] [CrossRef]
- Zu, G.; Kanamori, K.; Shimizu, T.; Zhu, Y.; Maeno, A.; Kaji, H.; Nakanishi, K.; Shen, J. Versatile double-cross-linking approach to transparent, machinable, supercompressible, highly bendable aerogel thermal superinsulators. Chem. Mater. 2018, 30, 2759–2770. [Google Scholar] [CrossRef] [Green Version]
- Hegde, N.D.; Rao, A.V. Physical properties of methyltrimethoxysilane based elastic silica aerogels prepared by the two-stage sol–gel process. J. Mater. Sci. 2007, 42, 6965–6971. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, H.; Li, X.; Ding, H.; Ji, H. Hyperelastic and hydrophobic silica aerogels with enhanced compressive strength by using VTES/MTMS as precursors. J. Non-Cryst. Solids 2019, 525, 119677. [Google Scholar] [CrossRef]
- Hayase, G.; Kanamori, K.; Hasegawa, G.; Maeno, A.; Kaji, H.; Nakanishi, K. A superamphiphobic macroporous silicone monolith with marshmallow-like flexibility. Angew. Chem. 2013, 125, 10988–10991. [Google Scholar] [CrossRef]
- Ma, J.; Ye, F.; Lin, S.; Zhang, B.; Yang, H.; Ding, J.; Yang, C.; Liu, Q. Large size and low density SiOC aerogel monolith prepared from triethoxyvinylsilane/tetraethoxysilane. Ceram. Int. 2017, 43, 5774–5780. [Google Scholar] [CrossRef]
- Liu, H.L.; Chu, P.; Li, H.Y.; Li, J.; Han, Y.K. Preparation and properties of silica aerogels reinforced using polyurethanes via ambient pressure drying. J. Synth. Cryst. 2015, 44, 3532–3536. [Google Scholar]
- Lin, J.; Chen, H.; Fei, T.; Liu, C.; Zhang, J. Highly transparent and thermally stable superhydrophobic coatings from the deposition of silica aerogels. Appl. Surf. Sci. 2013, 273, 776–786. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Yang, J.; Chen, J.; Song, J. Studies of the surface wettability and hydrothermal stability of methyl-modified silica films by FT-IR and Raman spectra. Vib. Spectrosc. 2009, 50, 178–184. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.K.; Kim, H.K.; Kim, H.T. High specific surface area TEOS-based aerogels with large pore volume prepared at an ambient pressure. Appl. Surf. Sci. 2007, 254, 574–579. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si (OR) 4 and R″ Si (OR′) 3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Pan, K.; Zeng, X.; Li, H.; Lai, X. Synthesis of silane oligomers containing vinyl and epoxy group for improving the adhesion of addition-cure silicone encapsulant. J. Adhes. Sci. Technol. 2016, 30, 1131–1142. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.; Jana, S.C. Mesoporous titanium dioxide nanofibers with a significantly enhanced photocatalytic activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Radi, S.; El Abiad, C.; Moura, N.M.; Faustino, M.A.; Neves, M.G.P. New hybrid adsorbent based on porphyrin functionalized silica for heavy metals removal: Synthesis, characterization, isotherms, kinetics and thermodynamics studies. J. Hazard. Mater. 2019, 370, 80–90. [Google Scholar] [CrossRef]
- Taheri, P.; Lang, J.C.; Kenvin, J.; Kroll, P. Differential hysteresis scanning of non-templated monomodal amorphous aerogels. Phys. Chem. Chem. Phys. 2021, 23, 5422–5430. [Google Scholar] [CrossRef]
- Hilonga, A.; Kim, J.K.; Sarawade, P.B.; Kim, H.T. Low-density TEOS-based silica aerogels prepared at ambient pressure using isopropanol as the preparative solvent. J. Alloys Compd. 2009, 487, 744–750. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.Y.; Chang, T.F.; Lu, S.Y.; Chang, Y.C. Preparation of monolithic silica aerogel of low thermal conductivity by ambient pressure drying. J. Am. Ceram. Soc. 2007, 90, 2003–2007. [Google Scholar] [CrossRef]
- Ma, J.; Ye, F.; Yang, C.; Ding, J.; Lin, S.; Zhang, B.; Liu, Q. Heat-resistant, strong alumina-modified silica aerogel fabricated by impregnating silicon oxycarbide aerogel with boehmite sol. Mater. Des. 2017, 131, 226–231. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Pekala, R.W. Thermal properties of organic and inorganic aerogels. J. Mater. Res. 1994, 9, 731–738. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Hunt, A.; Greif, R. Transport properties of gas in silica aerogel. J. Non-Cryst. Solids 1995, 186, 264–270. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]


| Samples | PHMS * | VTES | TEOS |
|---|---|---|---|
| P/V-SiO2-1 | 1.0 | 0.3 | 3.5 |
| P/V-SiO2-2 | 1.0 | 0.3 | 5.0 |
| P/V-SiO2-3 | 1.0 | 0.3 | 6.5 |
| P/V-SiO2-4 | 1.0 | 0.3 | 8.0 |
| Samples | BET Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| P/V-SiO2-1 | 622.8 | 1.205 | 7.288 |
| P/V-SiO2-2 | 673.1 | 1.922 | 11.43 |
| P/V-SiO2-3 | 631.3 | 2.903 | 16.12 |
| P/V-SiO2-4 | 599.7 | 2.691 | 20.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Tong, Z.; Li, R.; Su, D.; Ji, H. Polysiloxane Bonded Silica Aerogel with Enhanced Thermal Insulation and Strength. Materials 2021, 14, 2046. https://doi.org/10.3390/ma14082046
Wang W, Tong Z, Li R, Su D, Ji H. Polysiloxane Bonded Silica Aerogel with Enhanced Thermal Insulation and Strength. Materials. 2021; 14(8):2046. https://doi.org/10.3390/ma14082046
Chicago/Turabian StyleWang, Weilin, Zongwei Tong, Ran Li, Dong Su, and Huiming Ji. 2021. "Polysiloxane Bonded Silica Aerogel with Enhanced Thermal Insulation and Strength" Materials 14, no. 8: 2046. https://doi.org/10.3390/ma14082046






