Bioactivity of PEEK GRF30 and Ti6Al4V SLM in Simulated Body Fluid and Hank’s Balanced Salt Solution
Abstract
1. Introduction
2. Materials and Methods
3. Results
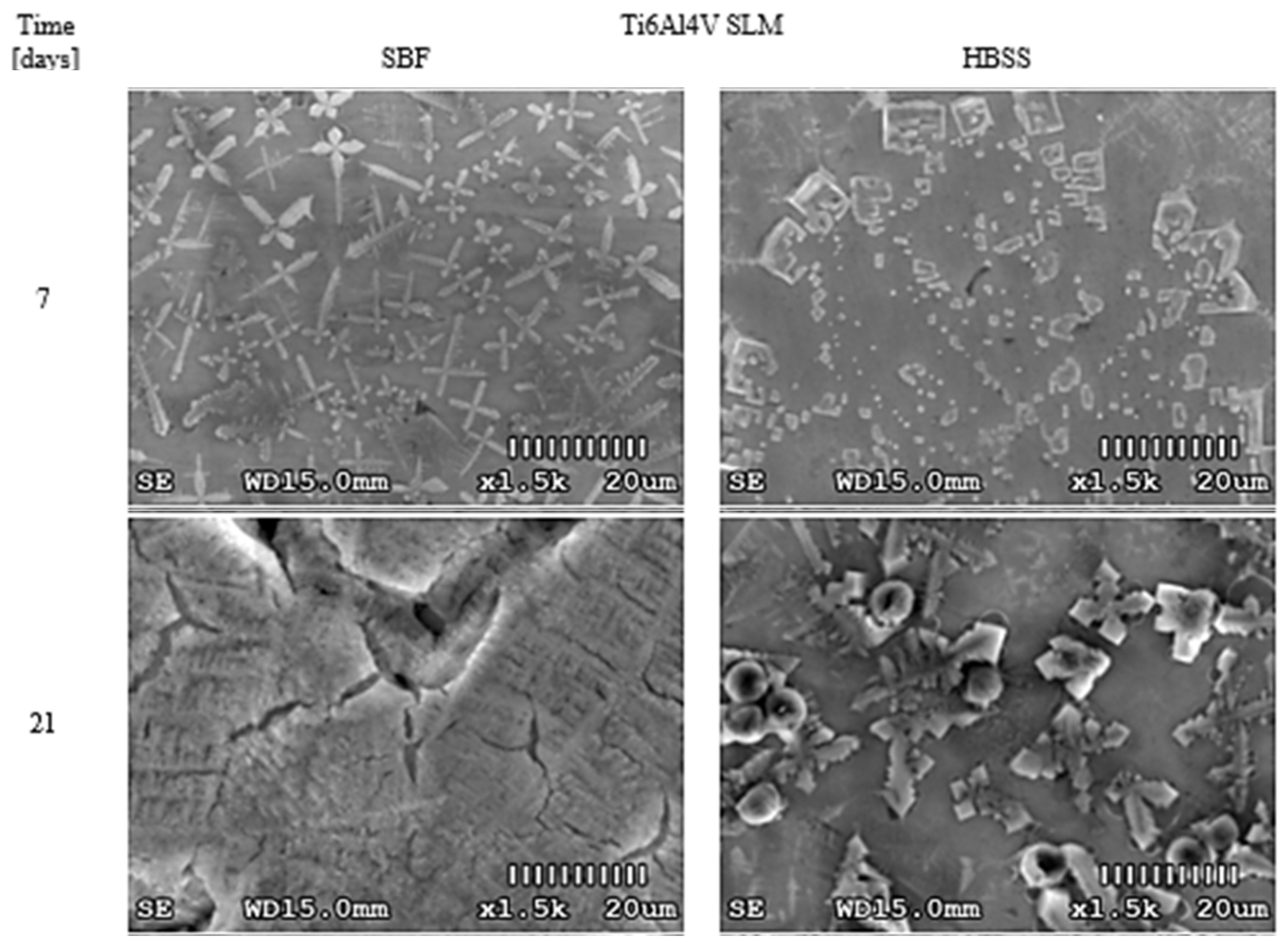
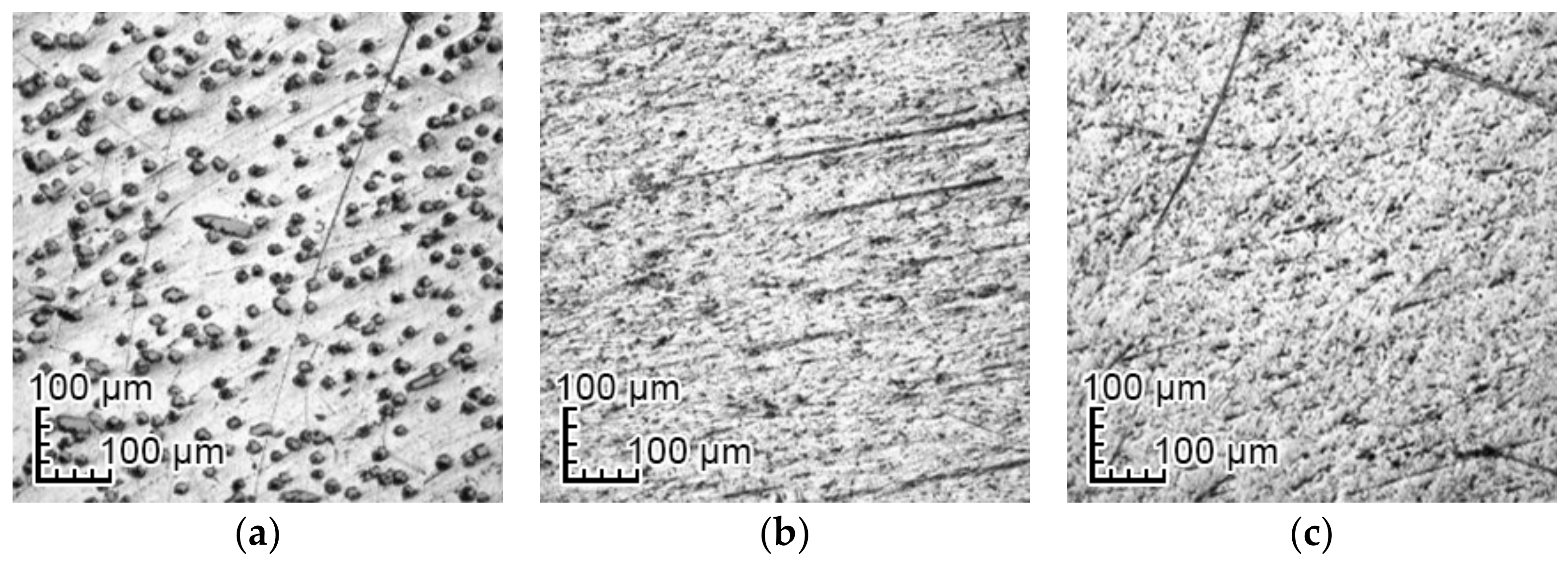
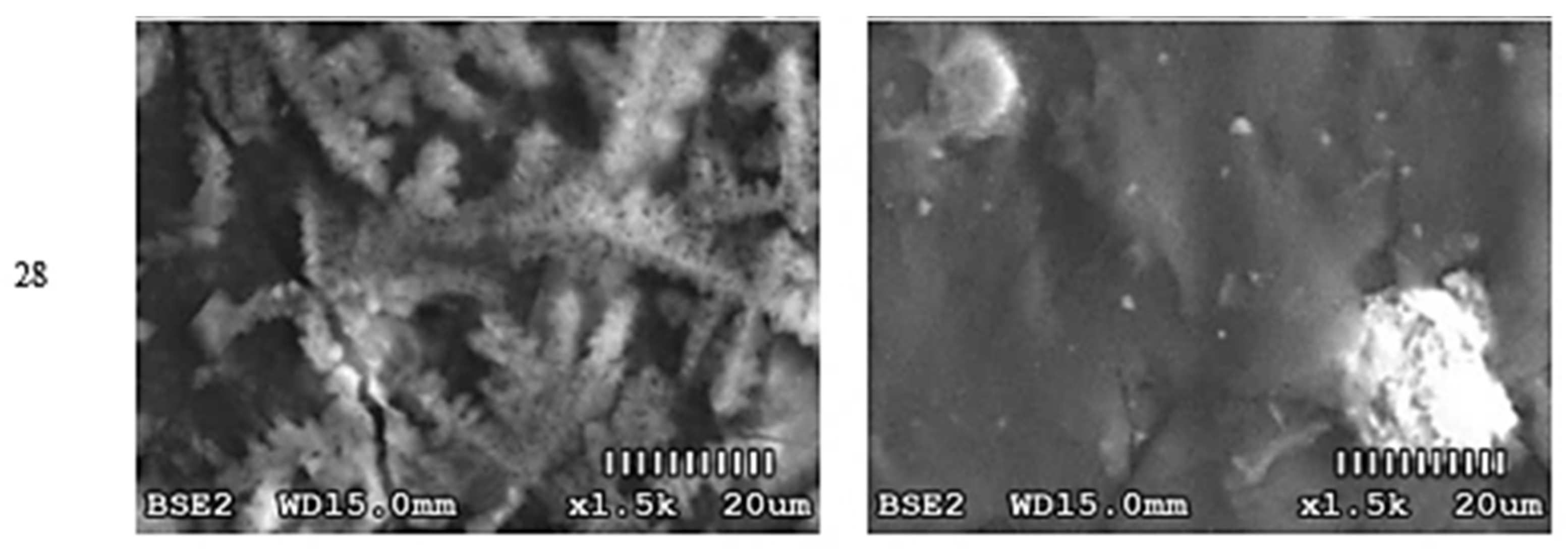
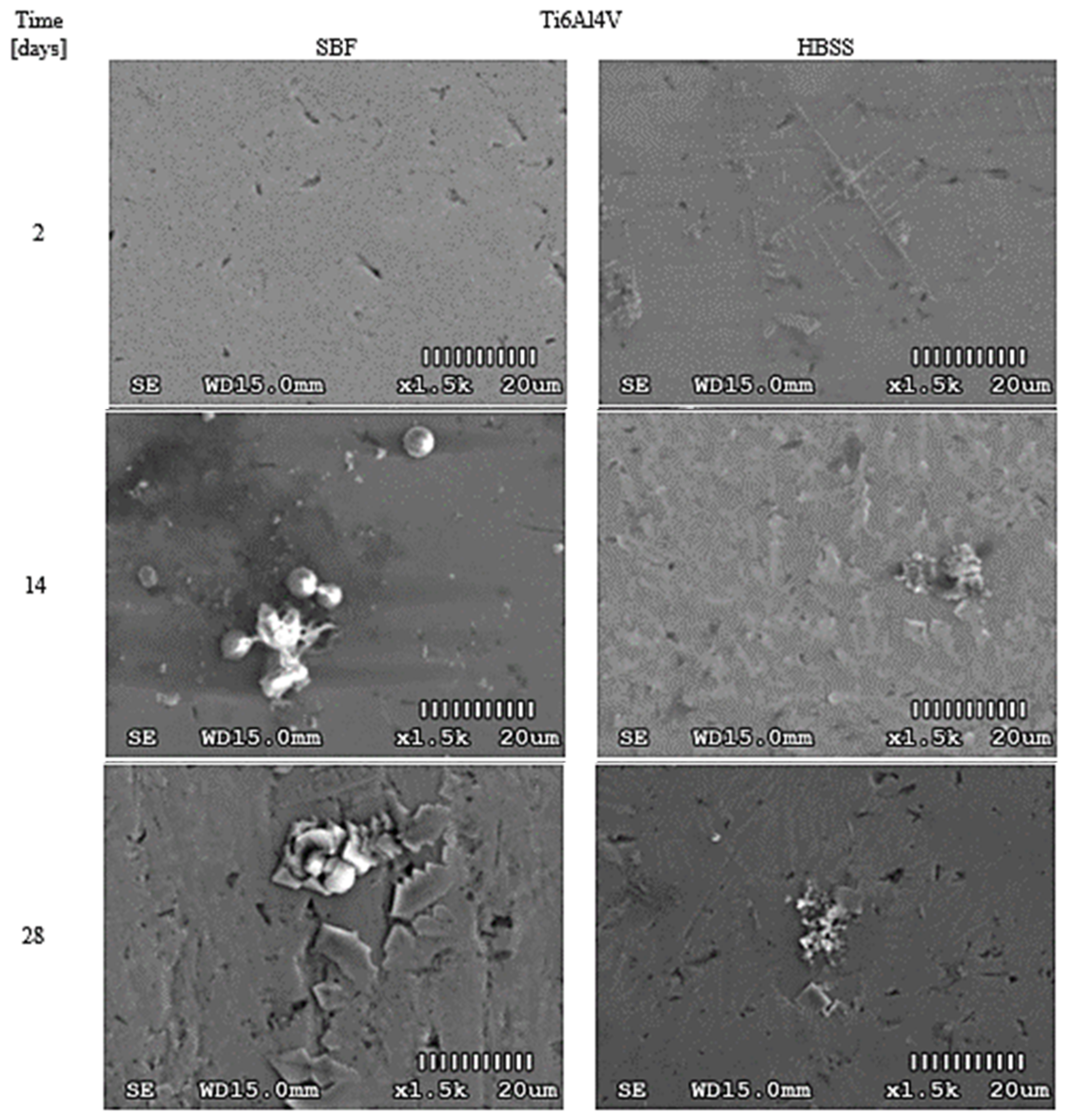
3.1. Optical Changes in the Morphology of Layers Formed on the Surface
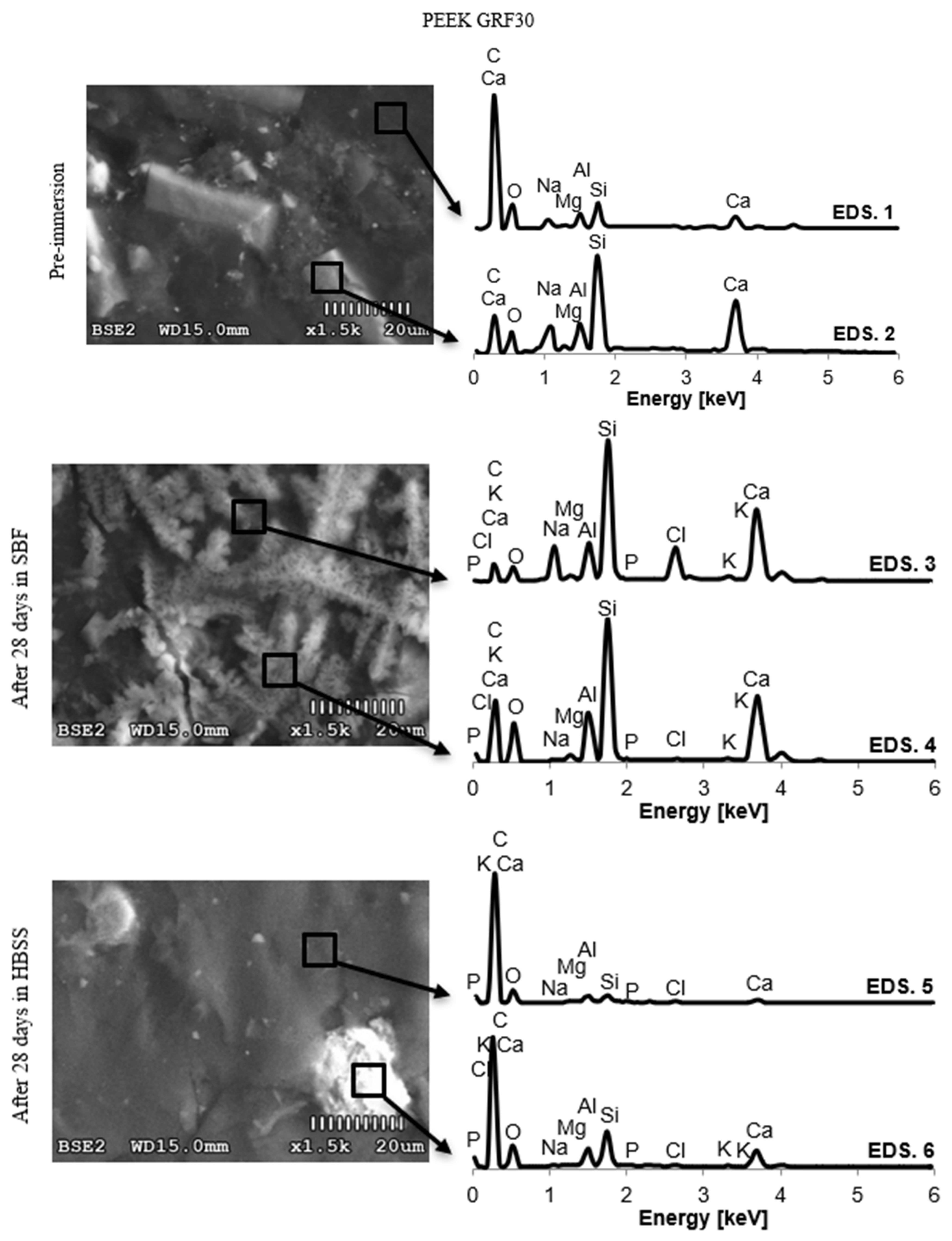
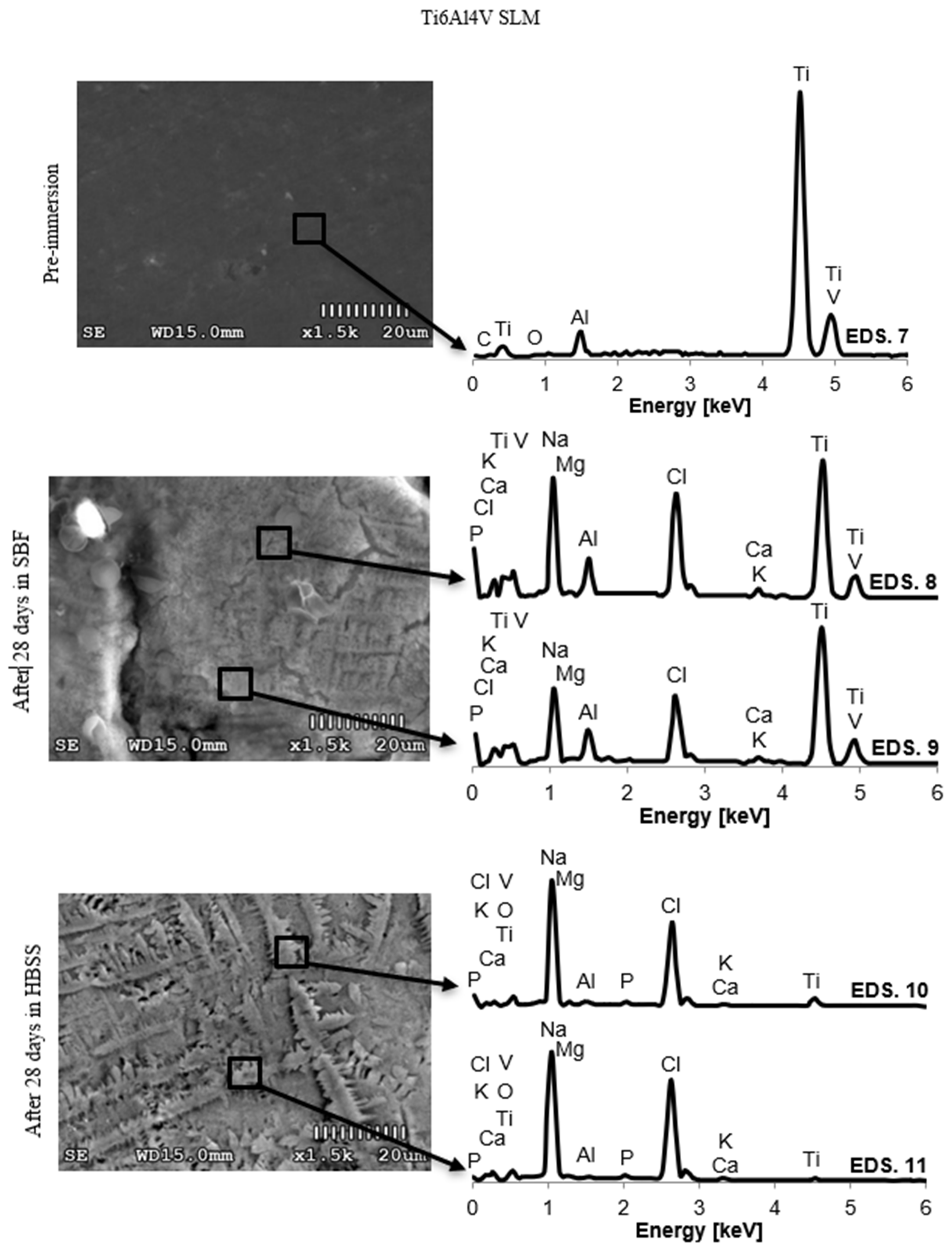
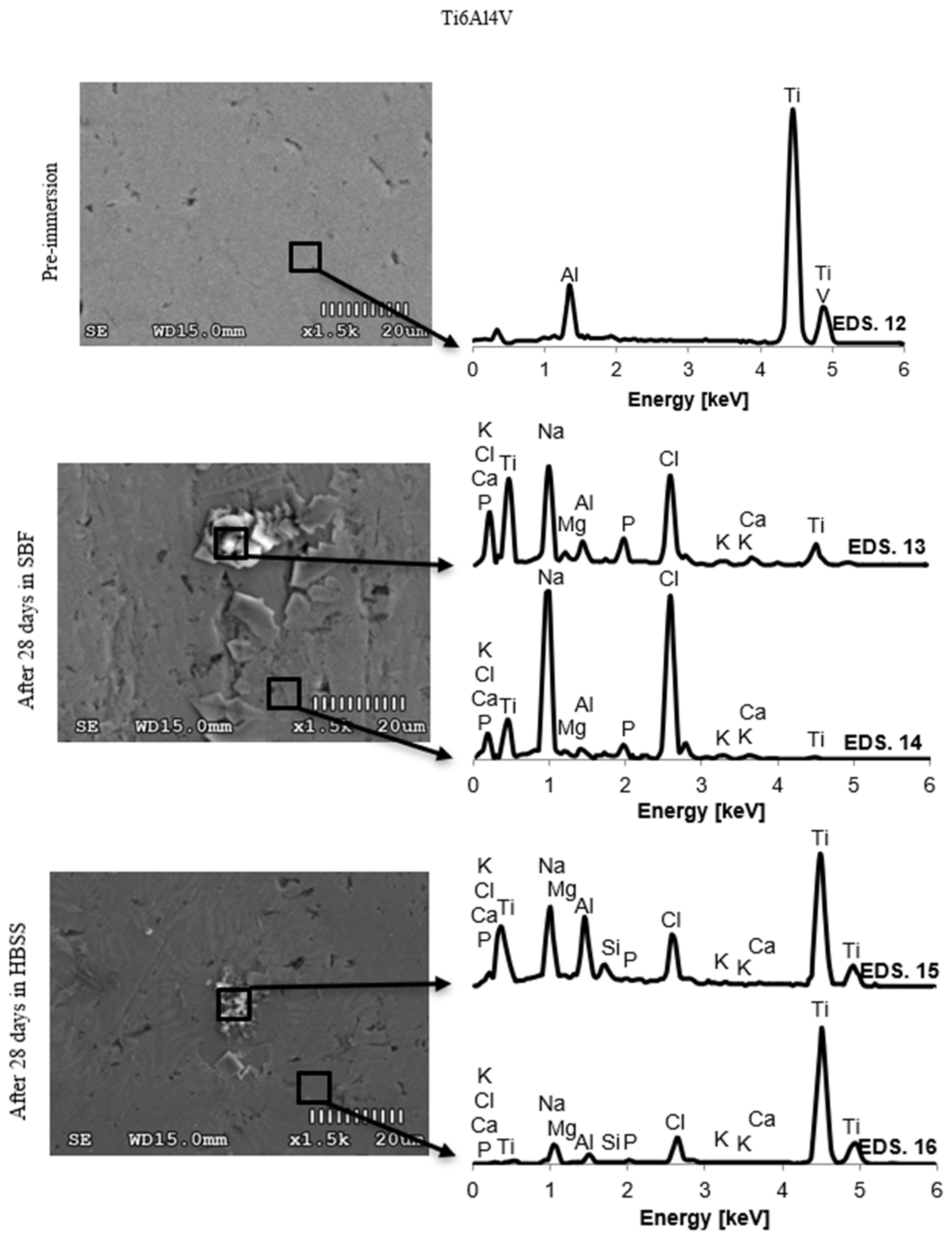
3.2. Changes in the Chemical Composition of the Surface
4. Discussion
4.1. Optical Analysis of Changes on the Surface
4.2. Changes in the Chemical Composition of the Surface
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Siek, D.; Czechowska, J.; Mróz, W.; Zima, A.; Burdyńska, S.; Załęczny, R.; Ślósarczyk, A. Bioactivity of cement type bone substitutes. Bull. Pol. Acad. Tech. 2013, 61, 433–439. [Google Scholar] [CrossRef]
- Albrektsson, T.; Albrektsson, B. Osseointegration of bone implants. A review of an alternative mode of fixation. Acta Orthop. Scand. 1987, 58, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M.; McKee, M.D. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr. Opin. Nephrol. Hypertens. 2010, 19, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Spanos, N.; Misirlis, D.Y.; Kanellopoulou, D.G.; Koutsoukos, P.G. Seeded growth of hydroxyapatite in simulated body fluid. J. Mater. Sci. 2006, 41, 1805–1812. [Google Scholar] [CrossRef]
- Hämmerle, C.H.; Olah, A.J.; Schmid, J.; Flückiger, L.; Gogolewski, S.; Winkler, J.R.; Lang, N.P. The biological effect of natural bone mineral on bone neoformation on the rabbit skull. Clin. Oral. Implant. Res. 1997, 8, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.N.; Bahir, M.M.; Mene, R.; Mahabole, M.P.; Khairnar, R. Study of nanobiomaterial hydroxyapatite in simulated body fluid: Formation and growth of apatite. Mater. Sci. Eng. B 2010, 168, 224–230. [Google Scholar] [CrossRef]
- Maszybrocka, J.; Stwora, A.; Gapiński, B.; Skrabalak, G.; Karolus, M. Morphology and surface topography of Ti6Al4V lattice structure fabricated by selective laser sintering. Bull. Pol. Acad. Tech. 2007, 65, 85–92. [Google Scholar] [CrossRef]
- Sovak, G.; Weiss, A.; Gotman, I. Osseointegration of Ti6Al4V alloy implants coated with titanium nitride by a new method. J. Bone Jt. Surg. Br. Vol. 2000, 82, 290–296. [Google Scholar] [CrossRef]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In vitro studies of plasma-sprayed hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef]
- Chien, C.S.; Liao, T.Y.; Hong, T.F.; Kuo, T.Y.; Chang, C.H.; Yeh, M.L.; Lee, T.M. Surface microstructure and bioactivity of hydroxyapatite and fluorapatite coatings deposited on Ti-6Al-4V substrates using Nd-YAG laser. J. Med. Biol. Eng. 2014, 34, 109–115. [Google Scholar] [CrossRef]
- Yang, Y.; Paital, S.R.; Dahotre, N.B. Effects of SiO2 substitution on wettability of laser deposited Ca-P biocoating on Ti-6Al-4V. J. Mater. Sci. Mater. Med. 2010, 21, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Hayakawa, T. Quantitative analysis of apatite formation on titanium and zirconia in a simulated body fluid solution using the quartz crystal microbalance method. Adv. Mater. Sci. Eng. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Lu, J.; Wei, G.; Yu, Y.; Zhao, X.; Dai, Y. Enhanced corrosion resistance of TA2 titanium via anodic oxidation in mixed acid system. Int. J. Electrochem. Sci. 2017, 12, 2763–2776. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Cheng, T.C.; Fang, T.H.; Li, M.H. The fabrication and characteristics of hydroxyapatite film grown on titanium alloy Ti-6Al-4V by anodic treatment. J. Mater. Res. Technol. 2020, 9, 4817–4825. [Google Scholar] [CrossRef]
- Baumers, M.; Dickens, P.; Tuck, C.; Hague, R. The cost of additive manufacturing: Machine productivity, economies of scale and technology-push. Technol. Forecast. Soc. Chang. 2016, 102, 193–201. [Google Scholar] [CrossRef]
- Wang, H.; Xu, M.; Zhang, W.; Kwok, D.T.; Jiang, J.; Wu, Z.; Chu, P.K. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials 2010, 31, 8181–8187. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Briem, D.; Strametz, S.; Schröder, K.; Meenen, N.M.; Lehmann, W.; Linhart, W.; Ohl, A.; Rueger, J.M. Response of primary fibroblasts and osteoblasts to plasma treated polyetheretherketone (PEEK) surfaces. J. Mater. Sci. Mater. Med. 2005, 16, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Matsunami, C.; Shirosaki, Y. Bioactive carbon-PEEK composites prepared by chemical surface treatment. Mater. Sci. Eng. C 2017, 70, 71–75. [Google Scholar] [CrossRef]
- ISO Standard 23317:2014 In Vitro Evaluation for Apatite-Forming Ability of Implant Materials; ISO: Geneva, Switzerland, 2014.
- Peng, S.; Feng, P.; Wu, P.; Huang, W.; Yang, Y.; Guo, W.; Gao, C.; Shuai, C. Graphene oxide as an interface phase between polyetheretherketone and hydroxyapatite for tissue engineering scaffolds. Sci. Rep. 2017, 7, 46604. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, X.; Ai, Y.; Liang, Y.; Ji, Y.; Li, J.; Wang, X. Spectroscopic and theoretical studies on the counterion effect of Cu (II) ion and graphene oxide interaction with titanium dioxide. Environ. Sci. Nano 2016, 3, 1361–1368. [Google Scholar] [CrossRef]
- Milošev, I.; Metikoš-Huković, M.; Strehblow, H.H. Passive film on orthopaedic TiAlV alloy formed in physiological solution investigatd by X-ray photoelectron spectroscopy. Biomaterials 2020, 21, 2103–2113. [Google Scholar] [CrossRef]
- Jonášová, L.; Müller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Biomimetic apatite formation on chemically treated titanium. Biomaterials 2004, 25, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, T.P.; Satheeshkumar, S.; Naveen, J. Glass fiber-reinforced polymer composites—A review. J. Reinf. Plast. Compos. 2014, 33, 1258–1275. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Cheang, P.; Khor, K.A. Mechanical properties of injection molded hydroxyapatite-polyetheretherketone biocomposites. Compos. Sci. Technol. 2003, 63, 421–425. [Google Scholar] [CrossRef]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef]
- Cazzola, M.; Ferraris, S.; Prenesti, E.; Casalegno, V.; Spriano, S. Grafting of gallic acid onto a bioactive Ti6Al4V alloy: A physico-chemical characterization. Coatings 2019, 9, 302. [Google Scholar] [CrossRef]
- Pereira, B.L.; Tummler, P.; Marino, C.E.; Soares, P.C.; Kuromoto, N.K. Titanium bioactivity surfaces obtained by chemical/electrochemical treatments. Matéria 2014, 19, 16–23. [Google Scholar] [CrossRef]
- Liang, C.Y.; Jiang, X.J.; Ji, R.L.; Li, B.E.; Zou, X.R.; Wang, H.S.; Yang, T. Preparation and surface modification of 3D printed Ti–6Al–4V porous implant. Rare Met. 2021, 40, 1164–1172. [Google Scholar] [CrossRef]
- Swaminathan, P.D.; Uddin, M.; Wooley, P.; Asmatulu, R. Fabrication and Biological Analysis of Highly Porous PEEK Bionanocomposites Incorporated with Carbon and Hydroxyapatite Nanoparticles for Biological Applications. Molecules 2020, 25, 3572. [Google Scholar] [CrossRef] [PubMed]


| HBSS pH = 7.3 | Conc. * | SBF pH = 7.3 | Conc. * |
|---|---|---|---|
| NaCl | 0.140 M | NaCl | 0.137 M |
| CaCl2 | 0.002 M | CaCl2 | 0.003 M |
| KCl | 0.005 M | KCl | 0.003 M |
| NaHCO3 | 0.004 M | NaHCO3 | 0.004 M |
| MgCl2∙6H2O | 0.002 M | MgCl2∙6H2O | 0.002 M |
| NaH2PO4 | 0.002 M | Na2SO4 | 0.001 M |
| MgSO4 | 0.002 M | K2HPO4 3H2O | 0.001 M |
| D-glucose | 0.006 M | Tris | 0.051 M |
| 1.0 M HCl | 39.000 mL | ||
| 1.0 M HCl (added until pH = 7.3) | up to 5 mL |
| EDS | C | O | Na | Mg | Al | Si | P | Cl | K | Ca | Ti | V | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEEK GR30 | Pre-immersion | 1 | 68.63 | 26.21 | 1.32 | 0.22 | 0.96 | 1.86 | 0 | 0 | 0 | 0.79 | 0 | 0 |
| ± 0.62 | ± 0.51 | ± 0.04 | ± 0.02 | ± 0.02 | ± 0.02 | ± 0.01 | ||||||||
| 2 | 53.85 | 23.59 | 0.85 | 0.33 | 1.70 | 16.38 | 0 | 0 | 0 | 3.30 | 0 | 0 | ||
| ± 0.70 | ± 0.36 | ± 0.09 | ± 0.05 | ± 0.04 | ± 0.05 | ± 0.04 | ||||||||
| SBF (28 days) | 3 | 33.88 | 25.05 | 7.22 | 0.82 | 4.37 | 15.88 | 1.42 | 3.42 | 0.36 | 7.57 | 0 | 0 | |
| ± 0.46 | ± 0.56 | ± 0.11 | ± 0.05 | ± 0.08 | ± 0.11 | ± 0.03 | ± 0.03 | ± 0.02 | ± 0.08 | |||||
| 4 | 46.97 | 34.59 | 0.31 | 0.46 | 3.09 | 9.24 | 1.14 | 0.10 | 0.14 | 3.95 | 0 | 0 | ||
| ± 0.34 | ± 0.35 | ± 0.03 | ± 0.01 | ± 0.03 | ± 0.05 | ± 0.01 | ± 0.01 | ± 0.01 | ± 0.03 | |||||
| HBSS (28 days) | 5 | 74.96 | 22.67 | 0.07 | 0.04 | 0.80 | 0.75 | 0.28 | 0.04 | 0.01 | 0.38 | 0 | 0 | |
| ± 0.48 | ± 0.41 | ± 0.02 | ± 0.02 | ± 0.02 | ± 0.01 | ± 0.01 | ± 0.01 | ± 0.01 | ± 0.02 | |||||
| 6 | 68.02 | 25.69 | 0.14 | 0.15 | 1.34 | 2.75 | 0.50 | 0.09 | 0.04 | 1.27 | 0 | 0 | ||
| ± 0.45 | ± 0.34 | ± 0.02 | ± 0.01 | ± 0.02 | ± 0.03 | ± 0.01 | ± 0.01 | ± 0.02 | ± 0.02 | |||||
| Ti6Al4V SLM | Pre-immersion | 7 | 0.23 | 0.54 | 0 | 0 | 10.09 | 0 | 0 | 0 | 0 | 0 | 85.52 | 3.62 |
| ± 0.08 | ± 0.09 | ± 0.08 | ± 0.24 | ± 0.07 | ||||||||||
| SBF (28 days) | 8 | 0 | 0.49 | 0.38 | 0.15 | 9.00 | 0 | 6.04 | 0.68 | 0.48 | 10.28 | 69.2 | 3.29 | |
| ± 0.05 | ± 0.14 | ± 0.13 | ± 0.10 | ± 0.06 | ± 0.02 | ± 0.06 | ± 0.14 | ± 0.44 | ± 0.16 | |||||
| 9 | 0 | 0.51 | 0.46 | 0.17 | 8.79 | 0.18 | 7.60 | 0.87 | 0.45 | 12.7 | 64.95 | 3.32 | ||
| ± 0.07 | ± 0.14 | ± 0.11 | ± 0.13 | ± 0.05 | ± 0.04 | ± 0.19 | ± 0.03 | ± 0.09 | ± 0.52 | ± 0.12 | ||||
| HBSS (28 days) | 10 | 0 | 0.16 | 1.34 | 0.11 | 5.13 | 0 | 6.72 | 0.96 | 0.17 | 10.8 | 72.27 | 2.34 | |
| ± 0.11 | ± 0.02 | ± 0.04 | ± 0.09 | ± 0.14 | ± 0.07 | ± 0.05 | ± 0.11 | ± 0.32 | ± 0.07 | |||||
| 11 | 0 | 0.13 | 1.09 | 0.14 | 4.80 | 0 | 7.05 | 0.1 | 0.19 | 11.8 | 72.02 | 1.90 | ||
| ± 0.05 | ± 0.13 | ± 0.03 | ± 0.16 | ± 0.05 | ± 0.12 | ± 0.06 | ± 0.08 | ± 0.27 | ± 0.09 | |||||
| Ti6Al4V | Pre-immersion | 12 | 0 | 0 | 0 | 0 | 9.02 | 0 | 0 | 0 | 0 | 0 | 88.04 | 2.94 |
| ± 0.10 | ± 0.46 | ± 0.15 | ||||||||||||
| SBF (28 days) | 13 | 0 | 0 | 4.12 | 0.50 | 6.4 | 0 | 5.81 | 3.2 | 1.58 | 8.42 | 67.52 | 2.45 | |
| ± 0.41 | ± 0.14 | ± 0.09 | ± 0.11 | ± 0.55 | ± 0.14 | ± 0.31 | ± 0.22 | ± 0.03 | ||||||
| 14 | 0 | 0 | 2.15 | 1.52 | 6.80 | 0 | 5.67 | 2.73 | 1.64 | 8.07 | 68.29 | 3.13 | ||
| ± 0.37 | ± 0.18 | ± 0.14 | ± 0.16 | ± 0.50 | ± 0.14 | ± 0.20 | ± 0.66 | ± 0.09 | ||||||
| HBSS (28 days) | 15 | 0 | 0 | 1.2 | 0.24 | 8.11 | 0.91 | 4.72 | 0.70 | 0.76 | 7.19 | 73.22 | 2.95 | |
| ± 0.08 | ± 0.02 | ± 0.04 | ± 0.10 | ± 0.16 | ± 0.13 | ± 0.07 | ± 0.06 | ± 0.36 | ± 0.09 | |||||
| 16 | 0 | 0 | 1.0 | 0.76 | 8.14 | 0.62 | 4.57 | 0.74 | 1.24 | 7.21 | 72.57 | 3.05 | ||
| ± 0.04 | ± 0.14 | ± 0.04 | ± 0.17 | ± 0.11 | ± 0.05 | ± 0.12 | ± 0.07 | ± 0.31 | ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prochor, P.; Mierzejewska, Ż.A. Bioactivity of PEEK GRF30 and Ti6Al4V SLM in Simulated Body Fluid and Hank’s Balanced Salt Solution. Materials 2021, 14, 2059. https://doi.org/10.3390/ma14082059
Prochor P, Mierzejewska ŻA. Bioactivity of PEEK GRF30 and Ti6Al4V SLM in Simulated Body Fluid and Hank’s Balanced Salt Solution. Materials. 2021; 14(8):2059. https://doi.org/10.3390/ma14082059
Chicago/Turabian StyleProchor, Piotr, and Żaneta Anna Mierzejewska. 2021. "Bioactivity of PEEK GRF30 and Ti6Al4V SLM in Simulated Body Fluid and Hank’s Balanced Salt Solution" Materials 14, no. 8: 2059. https://doi.org/10.3390/ma14082059
APA StyleProchor, P., & Mierzejewska, Ż. A. (2021). Bioactivity of PEEK GRF30 and Ti6Al4V SLM in Simulated Body Fluid and Hank’s Balanced Salt Solution. Materials, 14(8), 2059. https://doi.org/10.3390/ma14082059







