A Novel Zirconia-Based Composite Presents an Aging Resistant Material for Narrow-Diameter Ceramic Implants
Abstract
:1. Introduction
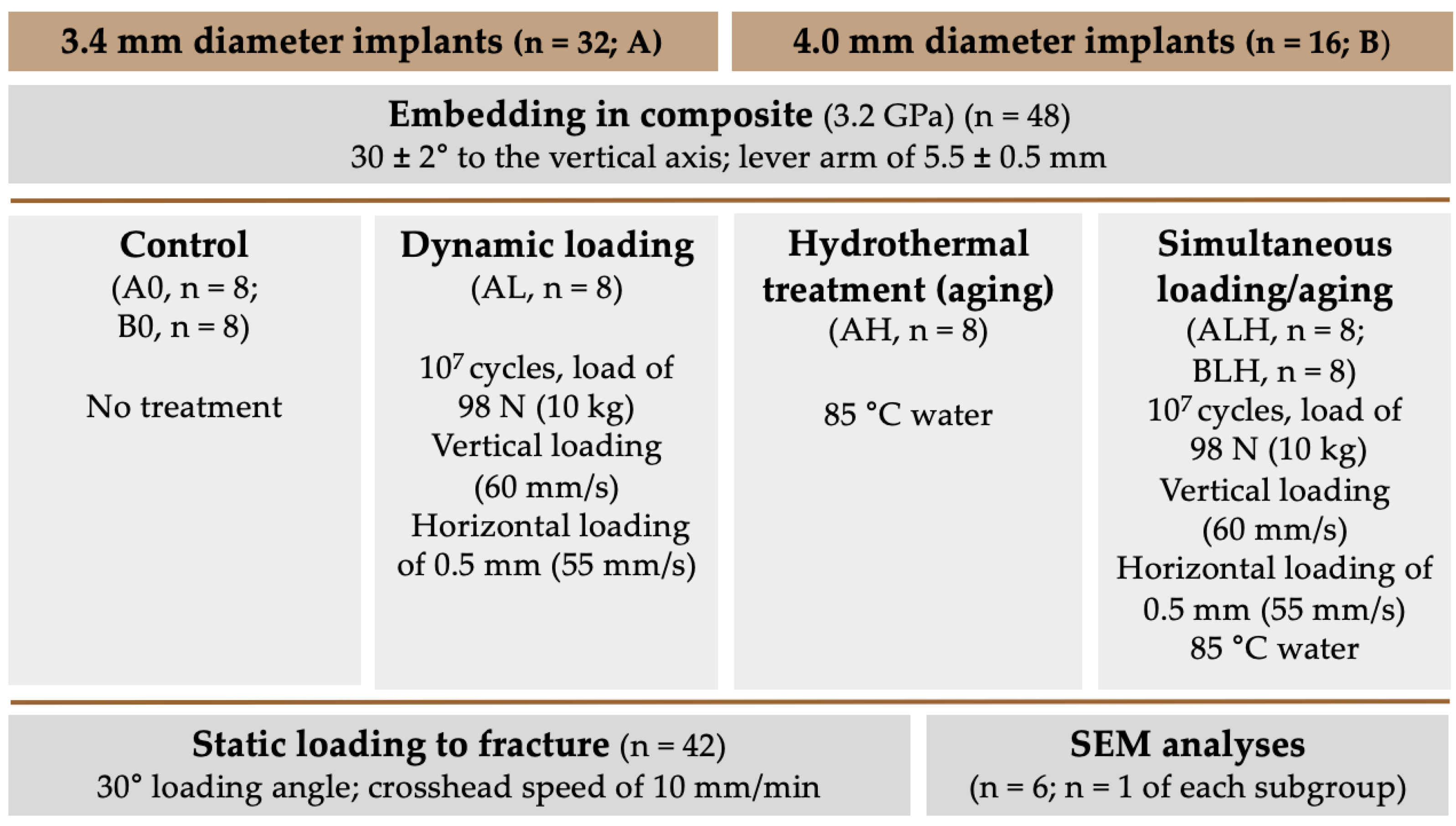
2. Materials and Methods
2.1. Experimental Setup
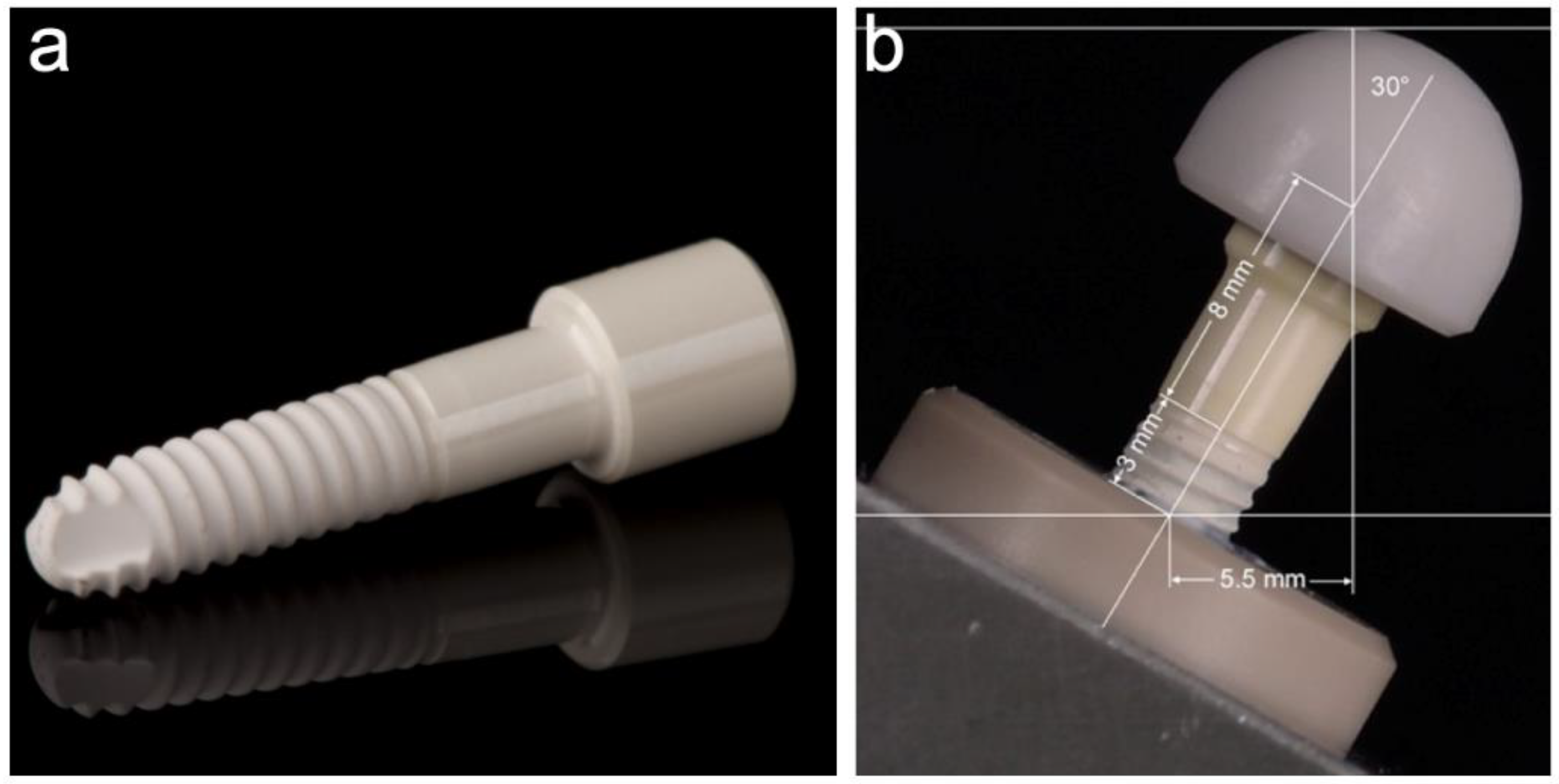
2.2. Investigated Implants
2.3. Embedding of the Samples
2.4. Dynamic Loading/Hydrothermal Treatment (-Aging)
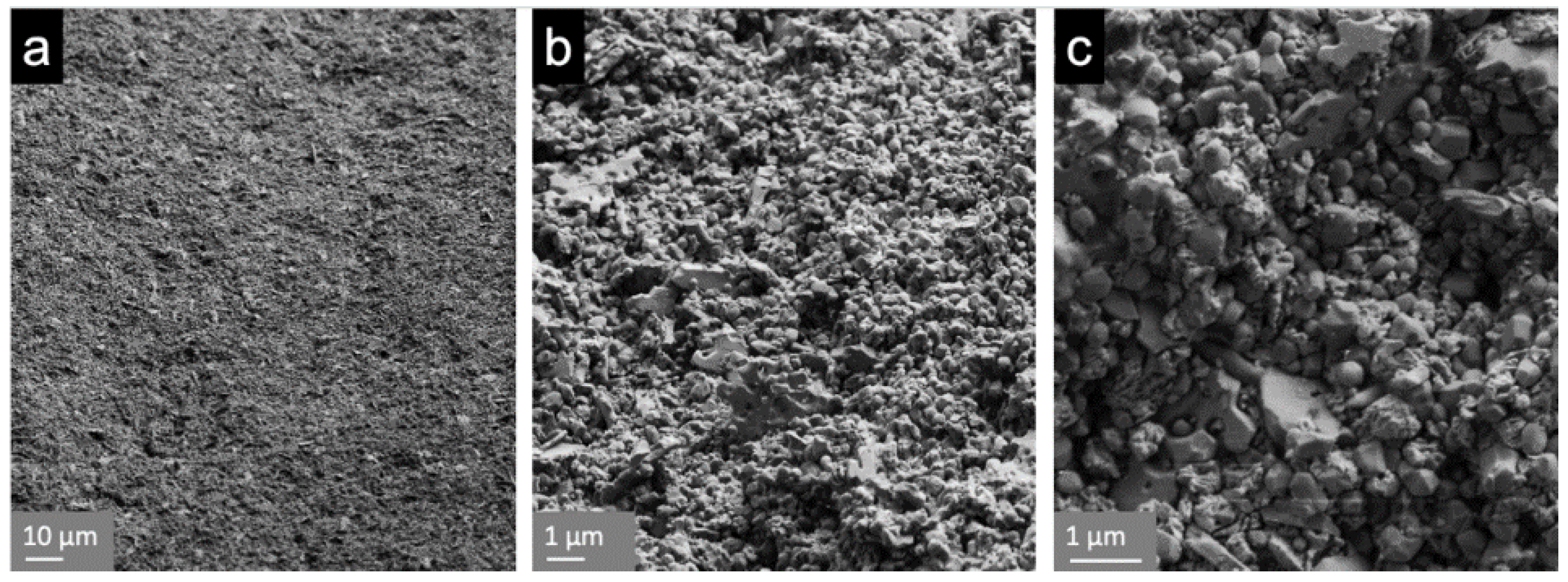
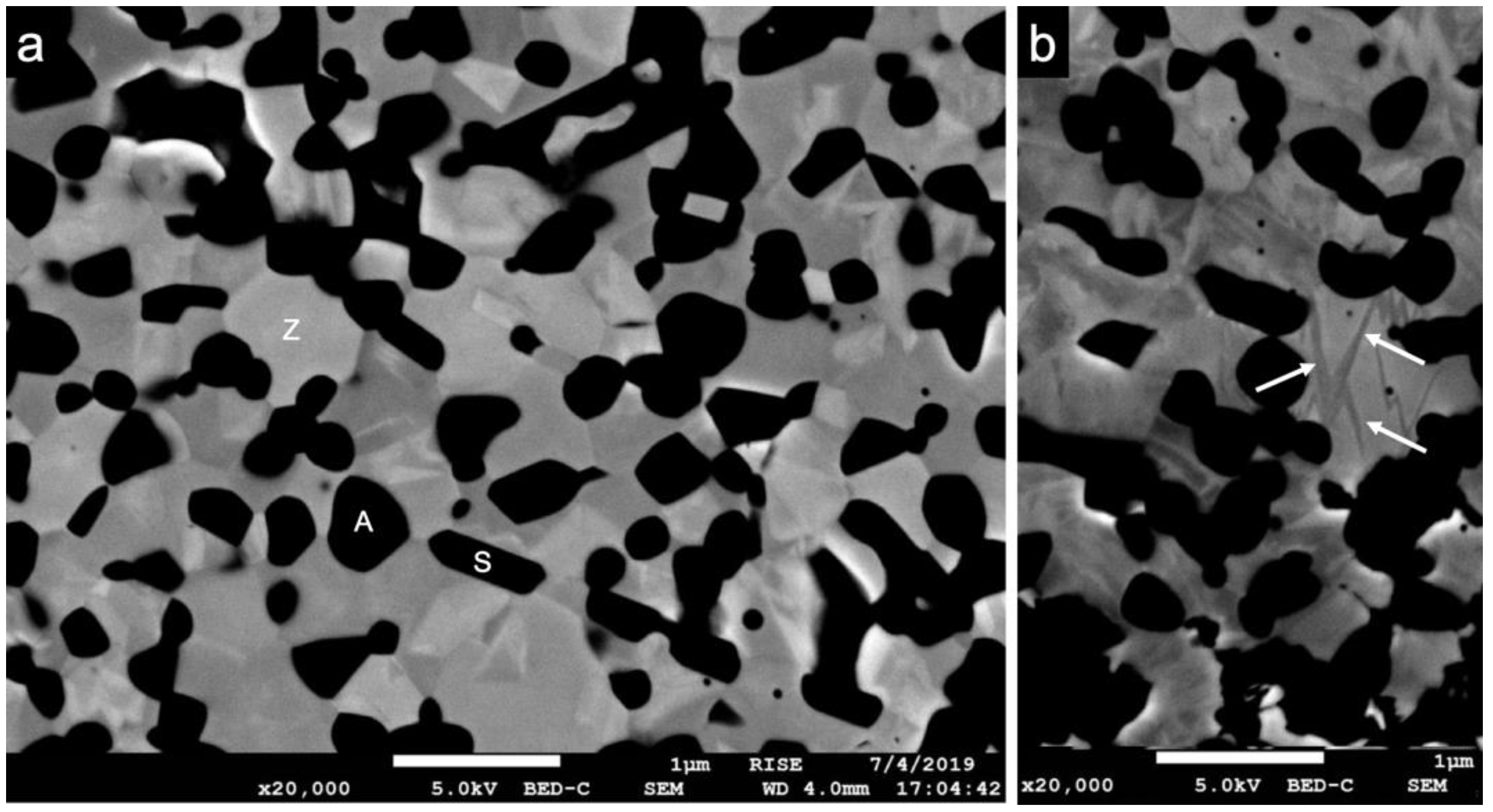
2.5. Cross-Sectioning, Scanning Electron Microscopy (SEM)
2.6. Static Loading Test
2.7. Fracture Analyses
2.8. Statistical Analyses
3. Results
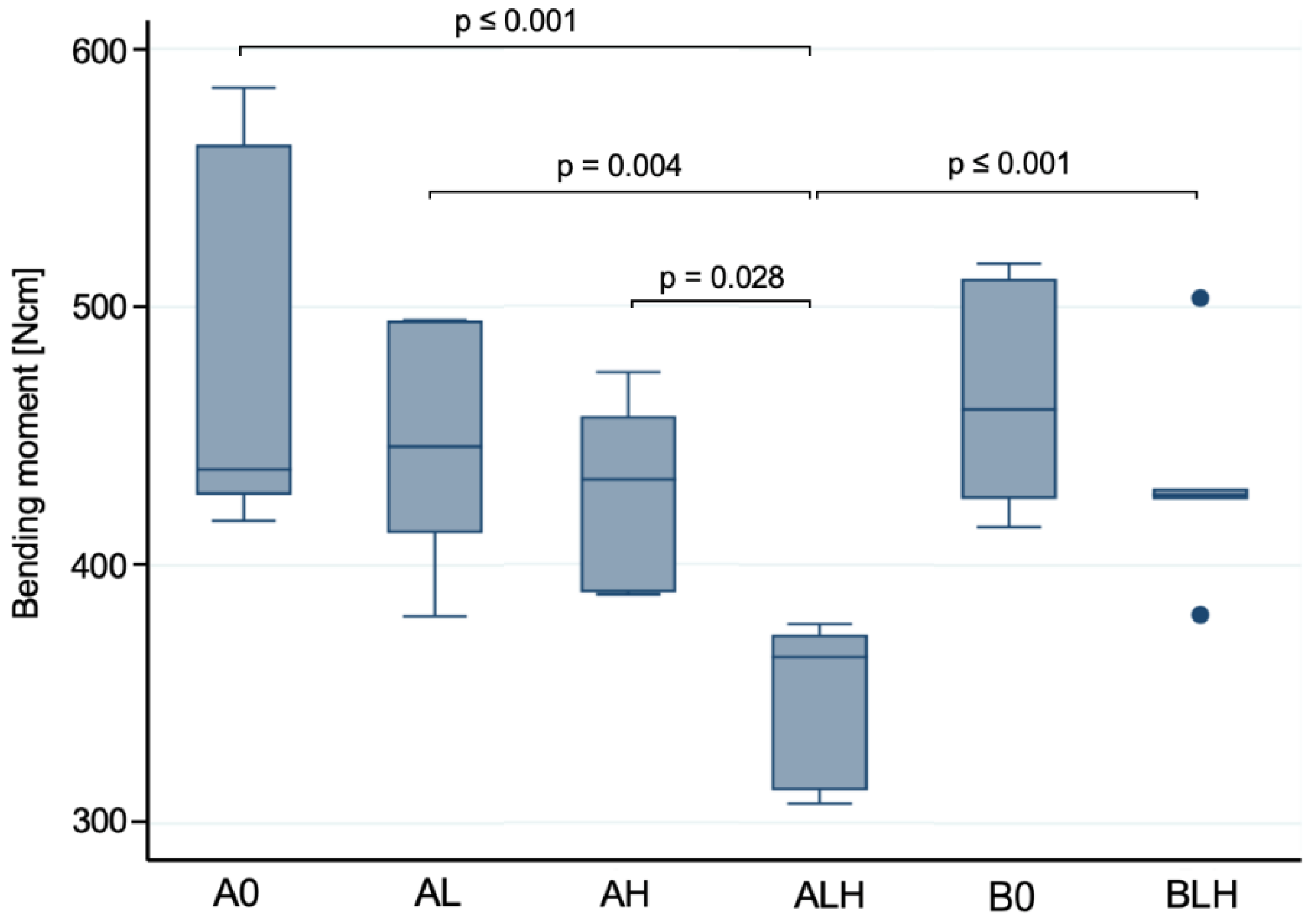
3.1. Dynamic Loading Test
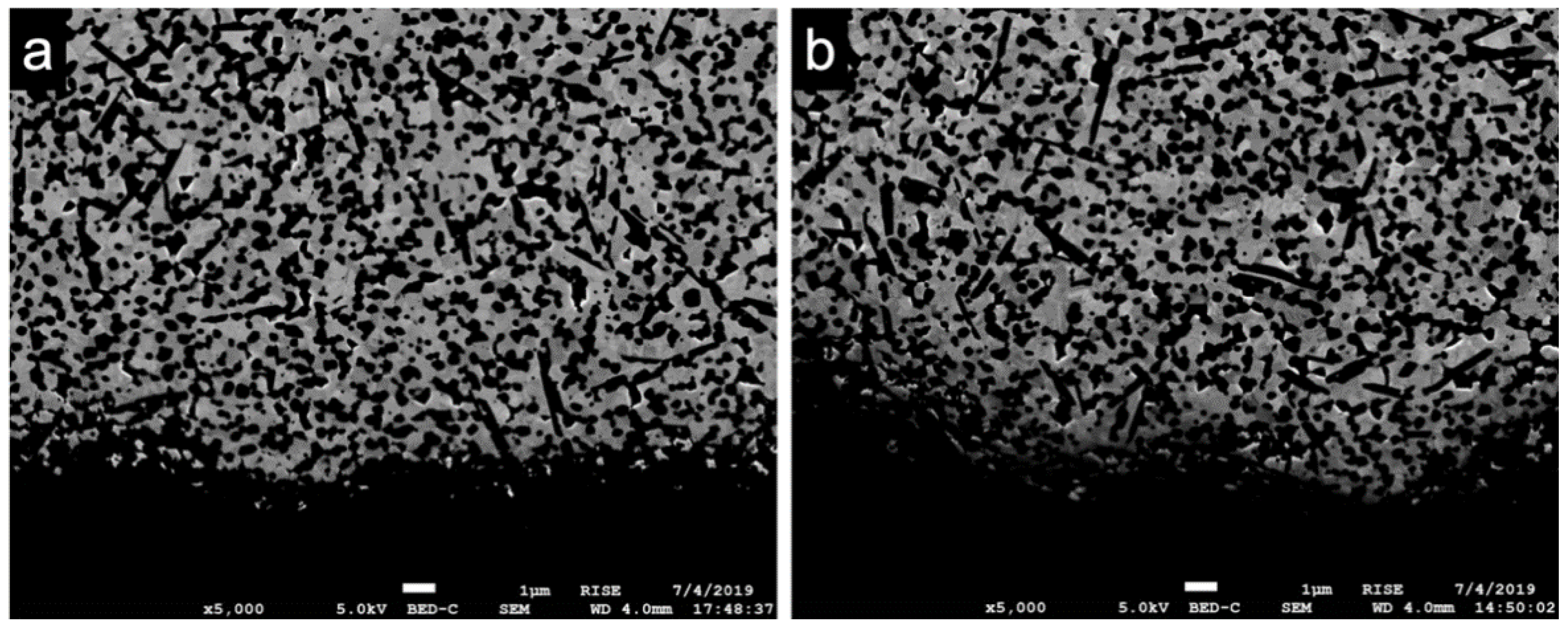
3.2. Scanning Electron Microscopy (SEM)
3.3. Static Loading Test
3.4. Fracture Analysis
4. Discussion
4.1. Dynamic Loading and Hydrothermal Aging
4.2. Scanning Electron Microscopy
4.3. Static Load to Fracture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brånemark, P.-I.; Breine, U.; Adell, R.; Hansson, B.O.; Lindström, J.; Ohlsson, Å. Intra-osseous anchorage of dental prostheses: I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Sandhaus, S. Tecnica e strumentario dell’impianto C.B.S. (Crystalline Bone Screw). Inf. Odontostomatol. 1968, 4, 19–24. (In Italian) [Google Scholar] [PubMed]
- Schulte, W.; Heimke, G. Das Tübinger Sofort-Implantat. Quintessenz 1976, 27, 17–23. [Google Scholar]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R.-J. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implants Res. 2009, 20, 32–47. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Lange, F.F. Transformation toughening. J. Mater. Sci. 1982, 17, 240–246. [Google Scholar] [CrossRef]
- Gupta, T.K.; Lange, F.F.; Bechtold, J.H. Effect of stress-induced phase transformation on the properties of polycrystalline zirconia containing metastable tetragonal phase. J. Mater. Sci. 1978, 13, 1464–1470. [Google Scholar] [CrossRef]
- Özkurt, Z.; Kazazoğlu, E. Zirconia dental implants: A literature review. J. Oral Implantol. 2011, 37, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical outcomes of zirconia dental implants: A systematic review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Noumbissi, S.; Francesco, I.; Rapone, B.; Khater, A.G.A.; Scarano, A. Scientific trends in clinical research on zirconia dental implants: A bibliometric review. Materials 2020, 13, 5534. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Loh, J.; Gremillard, L.; Meille, S.; Adolfson, E. Low-temperature degradation in zirconia with a porous surface. Acta Biomater. 2011, 7, 2986–2993. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The Tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Keuper, M.; Eder, K.; Berthold, C.; Nickel, K.G. Direct evidence for continuous linear kinetics in the low-temperature degradation of Y-TZP. Acta Biomater. 2013, 9, 4826–4835. [Google Scholar] [CrossRef] [PubMed]
- Sanon, C.; Chevalier, J.; Douillard, T.; Kohal, R.J.; Coelho, P.G.; Hjerppe, J.; Silva, N.R.F.A. Low temperature degradation and reliability of one-piece ceramic oral implants with a porous surface. Dent. Mater. 2013, 29, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Palmero, P.; Fornabaio, M.; Montanaro, L.; Reveron, H.; Esnouf, C.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants. Part I: Innovative synthesis, microstructural characterization and in vitro stability. Biomaterials 2015, 50, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Tsukuma, K.; Shimada, M. Strength, fracture toughness and Vickers hardness of CeO2-stabilized tetragonal ZrO2 polycrystals (Ce-TZP). J. Mater. Sci. 1985, 20, 1178–1184. [Google Scholar] [CrossRef]
- Tanaka, K.; Tamura, J.; Kawanabe, K.; Nawa, M.; Oka, M.; Uchida, M.; Kokubo, T.; Nakamura, T. Ce-TZP/Al2O3 nanocomposite as a bearing material in total joint replacement. J. Biomed. Mater. Res. 2002, 63, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Attaoui, H.E.; Saâdaoui, M.; Chevalier, J.; Fantozzi, G. Static and cyclic crack propagation in Ce-TZP ceramics with different amounts of transformation toughening. J. Eur. Ceram. Soc. 2007, 27, 483–486. [Google Scholar] [CrossRef]
- Kohorst, P.; Borchers, L.; Strempel, J.; Stiesch, M.; Hassel, T.; Bach, F.-W.; Hübsch, C. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012, 8, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.; Karygianni, L.; Al-Ahmad, A.; Butz, F.; Bächle, M.; Adolfsson, E.; Fürderer, T.; Courtois, N.; Palmero, P.; Follo, M.; et al. Assessment of novel long-lasting ceria-stabilized zirconia-based ceramics with different surface topographies as implant materials. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Reveron, H.; Fornabaio, M.; Palmero, P.; Fürderer, T.; Adolfsson, E.; Lughi, V.; Bonifacio, A.; Sergo, V.; Montanaro, L.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants: Transformation induced plasticity and its consequence on ceramic reliability. Acta Biomater. 2017, 48, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Altmann, B.; Rabel, K.; Kohal, R.J.; Proksch, S.; Tomakidi, P.; Adolfsson, E.; Bernsmann, F.; Palmero, P.; Fürderer, T.; Steinberg, T. Cellular transcriptional response to zirconia-based implant materials. Dent. Mater. 2017, 33, 241–255. [Google Scholar] [CrossRef]
- Kohal, R.J.; Adolfsson, E.; Chopard-Lallier, A.; Bruyere-Garnier, K.; Fürderer, T.; Schomer, S.; Courtois, N. Implants made from a novel zirconia material osseointegrate faster than titanium implants. An animal investigation. Clin. Oral Implants Res. 2020, 31, 146. [Google Scholar] [CrossRef]
- Benic, G.I.; Gallucci, G.O.; Mokti, M.; Hämmerle, C.H.F.; Weber, H.-P.; Jung, R.E. Titanium-zirconium narrow-diameter versus titanium regular-diameter implants for anterior and premolar single crowns: 1-Year results of a randomized controlled clinical study. J. Clin. Periodontol. 2013, 40, 1052–1061. [Google Scholar] [CrossRef] [Green Version]
- Iegami, C.M.; Uehara, P.N.; Sesma, N.; Pannuti, C.M.; Tortamano Neto, P.; Mukai, M.K. Survival rate of titanium-zirconium narrow diameter dental implants versus commercially pure titanium narrow diameter dental implants: A systematic review. Clin. Implant Dent. Relat. Res. 2017, 19, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, K.; Mushantat, A.; Esfandiari, S.; Feine, J. How successful are small-diameter implants? A literature review. Clin. Oral Implants Res. 2012, 23, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.S.; Lemos, C.A.A.; de Batista, V.E.S.; Yogui, F.C.; Oliveira, H.F.F.; Verri, F.R. Narrow-diameter implants versus regular-diameter implants for rehabilitation of the anterior region: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, S0901-5027(20)30379-9. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Voumard, B.; Zysset, P.K.; Brägger, U.; Ferrari, M. Ultimate force and stiffness of 2-piece zirconium dioxide implants with screw-retained monolithic lithium-disilicate reconstructions. J. Prosthodont. Res. 2018, 62, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Bahadirli, G.; Yilmaz, S.; Jones, T.; Sen, D. Influences of implant and framework materials on stress distribution: A three-dimensional finite element analysis study. Int. J. Oral Maxillofac. Implants 2018, 33, e117–e126. [Google Scholar] [CrossRef] [Green Version]
- Bethke, A.; Pieralli, S.; Kohal, R.J.; Burkhardt, F.; von Stein-Lausnitz, M.; Vach, K.; Spies, B.C. Fracture resistance of zirconia oral implants in vitro: A systematic review and meta-analysis. Materials 2020, 13, 562. [Google Scholar] [CrossRef] [Green Version]
- ISO 14801 Dentistry-Implant-Dynamic Fatigue Test for Endosseous Dental Implants. DIN EN ISO 14801; 2008-02; ISO: Geneva, Switzerland, 2008; pp. 1–14.
- Spies, B.C.; Nold, J.; Vach, K.; Kohal, R.J. Two-piece zirconia oral implants withstand masticatory loads: An investigation in the artificial mouth. J. Mech. Behav. Biomed. Mater. 2016, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lettry, S.; Seedhom, B.B.; Berry, E.; Cuppone, M. Quality Assessment of the cortical bone of the human mandible. Bone 2003, 32, 35–44. [Google Scholar] [CrossRef]
- Mendelson, M.I. Average grain size in polycrystalline ceramics. J. Am. Ceram. Soc. 1969, 52, 443–446. [Google Scholar] [CrossRef]
- ISO 13356 Implants for Surgery-Ceramic Materials Based on Yttria-Stabilized Tetragonal Zirconia (Y-TZP), DIN EN ISO 13356; 2016-02; ISO: Geneva, Switzerland, 2016; pp. 1–18.
- Spies, B.C.; Maass, M.E.; Adolfsson, E.; Sergo, V.; Kiemle, T.; Berthold, C.; Gurian, E.; Fornasaro, S.; Vach, K.; Kohal, R.J. Long-term stability of an injection-molded zirconia bone-level implant: A testing protocol considering aging kinetics and dynamic fatigue. Dent. Mater. 2017, 33, 954–965. [Google Scholar] [CrossRef]
- Kawata, T.; Yoda, N.; Kawaguchi, T.; Kuriyagawa, T.; Sasaki, K. Behaviours of three-dimensional compressive and tensile forces exerted on a tooth during function. J. Oral Rehabil. 2007, 34, 259–266. [Google Scholar] [CrossRef]
- Schindler, H.J.; Stengel, E.; Spiess, W.E.L. Feedback control during mastication of solid food textures—a clinical-experimental study. J. Prosthet. Dent. 1998, 80, 330–336. [Google Scholar] [CrossRef]
- Zhang, F.; Meyer zur Heide, C.; Chevalier, J.; Vleugels, J.; Van Meerbeek, B.; Wesemann, C.; dos Santos, B.C.; Sergo, V.; Kohal, R.-J.; Adolfsson, E.; et al. Reliability of an injection-moulded two-piece zirconia implant with PEKK abutment after long-term thermo-mechanical loading. J. Mech. Behav. Biomed. Mater. 2020, 110, 103967. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.; Sakaguchi, R.L.; Douglas, W.H.; Pintado, M.R. The wear of dental amalgam in an artificial mouth: A clinical correlation. Dent. Mater. 1985, 1, 238–242. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Douglas, W.H.; DeLong, R.; Pintado, M.R. The wear of a posterior composite in an artificial mouth: A clinical correlation. Dent. Mater. 1986, 2, 235–240. [Google Scholar] [CrossRef]
- Rosentritt, M.; Behr, M.; Gebhard, R.; Handel, G. Influence of stress simulation parameters on the fracture strength of all-Ceramic fixed-partial dentures. Dent. Mater. 2006, 22, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Serrao, G.; Dellavia, C.; Tartaglia, G.M. Single tooth bite forces in healthy young adults. J. Oral Rehabil. 2004, 31, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Zanotti, G.; Tartaglia, G.M. Maximal bite forces in healthy young adults as predicted by surface electromyography. J. Dent. 2004, 32, 451–457. [Google Scholar] [CrossRef]
- Rauchs, G.; Fett, T.; Munz, D.; Oberacker, R. Tetragonal-to-monoclinic phase transformation in CeO2-stabilised zirconia under uniaxial loading. J. Eur. Ceram. Soc. 2001, 21, 2229–2241. [Google Scholar] [CrossRef]
- Liens, A.; Swain, M.; Reveron, H.; Cavoret, J.; Sainsot, P.; Courtois, N.; Fabrègue, D.; Chevalier, J. Development of transformation bands in ceria-stabilized-zirconia based composites during bending at room temperature. J. Eur. Ceram. Soc. 2021, 41, 691–705. [Google Scholar] [CrossRef]
- Spies, B.C.; Sauter, C.; Wolkewitz, M.; Kohal, R.J. Alumina reinforced zirconia implants: Effects of cyclic loading and abutment modification on fracture resistance. Dent. Mater. 2015, 31, 262–272. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Rolauffs, B.; Adolfsson, E.; Palmero, P.; Fürderer, T.; Altmann, B. Controlling osteoblast morphology and proliferation via surface micro-topographies of implant biomaterials. Sci. Rep. 2020, 10, 12810. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Iwasa, F.; Hotta, Y.; Matsumoto, T.; Oshima, Y.; Baba, K. Effects of surface roughness of ceria-stabilized zirconia/alumina nanocomposite on the morphology and function of human gingival fibroblasts. Dent. Mater. 2020. [CrossRef]

| Groups | A0 | AL | AH | ALH | B0 | BLH |
|---|---|---|---|---|---|---|
| Load (N) | 854 ± 116 a | 806 ± 73 a | 762 ± 62 a | 628 ± 56 b | 845 ± 70 a,c | 782 ± 60 c |
| Bending Moment (Ncm) | 477 ± 70 a | 448 ± 44 a | 427 ± 35 a | 349 ± 29 b | 466 ± 40 a,c | 432 ± 36 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkhardt, F.; Harlass, M.; Adolfsson, E.; Vach, K.; Spies, B.C.; Kohal, R.-J. A Novel Zirconia-Based Composite Presents an Aging Resistant Material for Narrow-Diameter Ceramic Implants. Materials 2021, 14, 2151. https://doi.org/10.3390/ma14092151
Burkhardt F, Harlass M, Adolfsson E, Vach K, Spies BC, Kohal R-J. A Novel Zirconia-Based Composite Presents an Aging Resistant Material for Narrow-Diameter Ceramic Implants. Materials. 2021; 14(9):2151. https://doi.org/10.3390/ma14092151
Chicago/Turabian StyleBurkhardt, Felix, Markus Harlass, Erik Adolfsson, Kirstin Vach, Benedikt Christopher Spies, and Ralf-Joachim Kohal. 2021. "A Novel Zirconia-Based Composite Presents an Aging Resistant Material for Narrow-Diameter Ceramic Implants" Materials 14, no. 9: 2151. https://doi.org/10.3390/ma14092151









