Metallographic Testing of 19th Century Steel in an Operating Water Tower
Abstract
1. Introduction
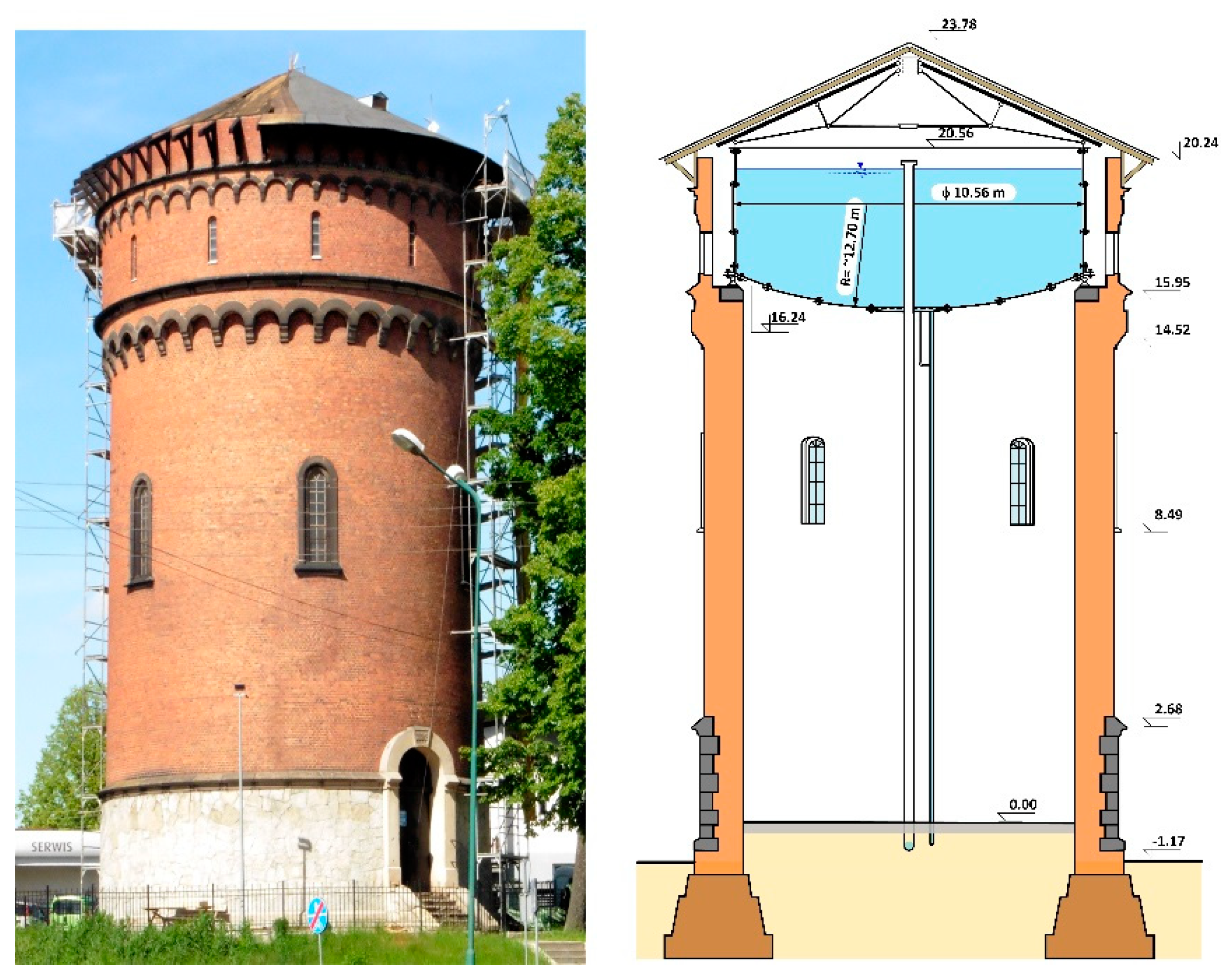
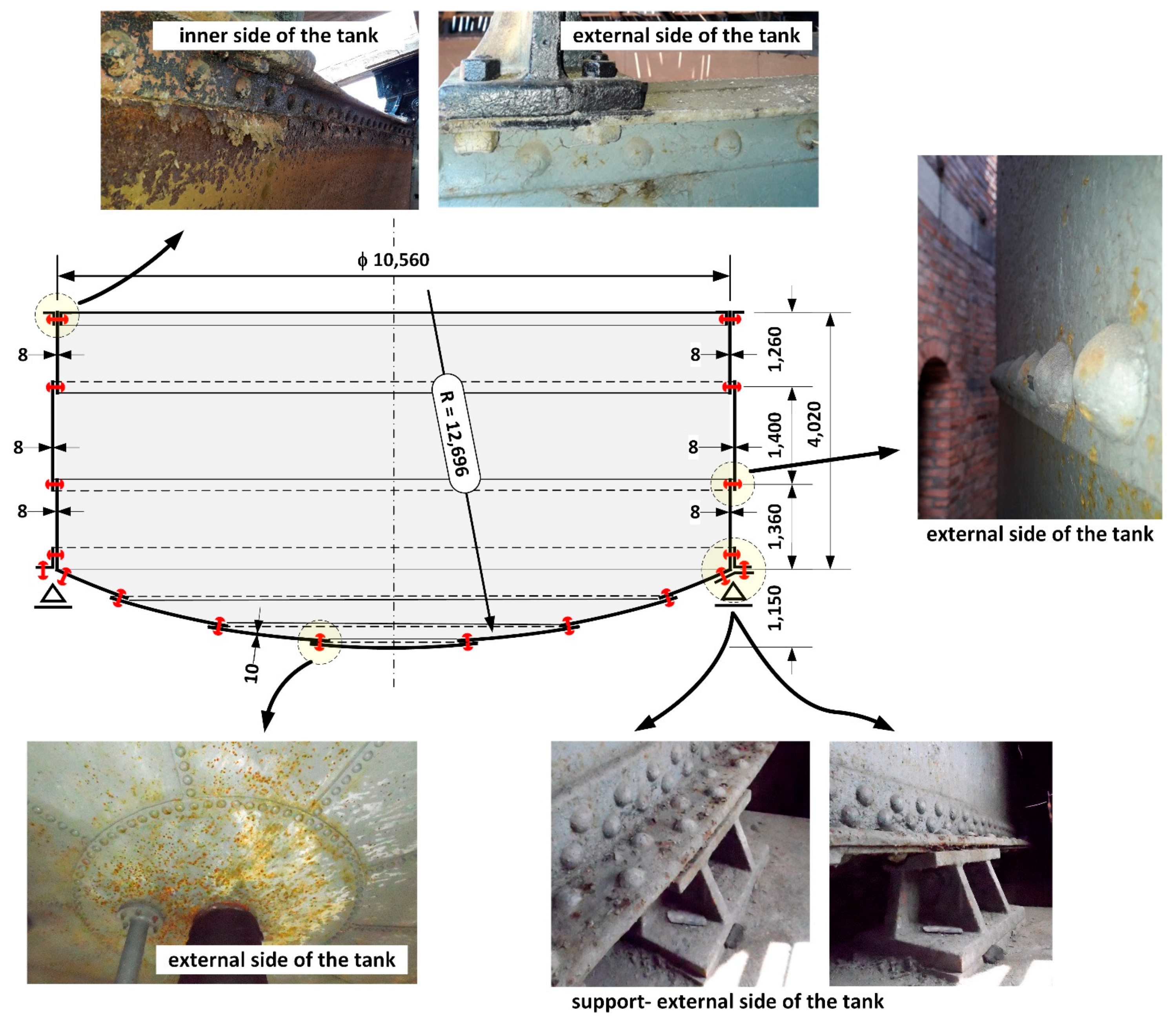
2. General Description of the Tank Structure and Its Technical Condition
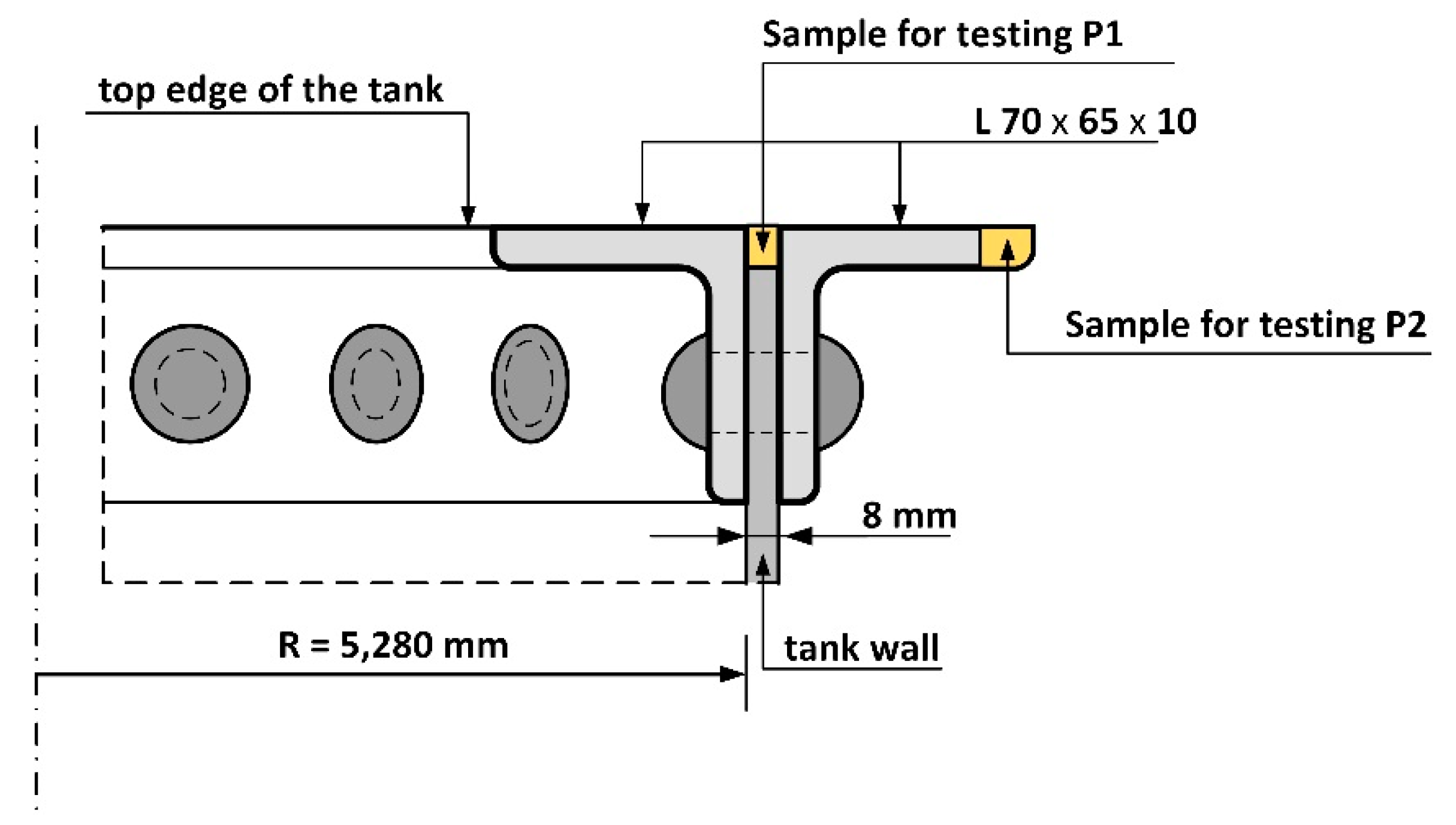
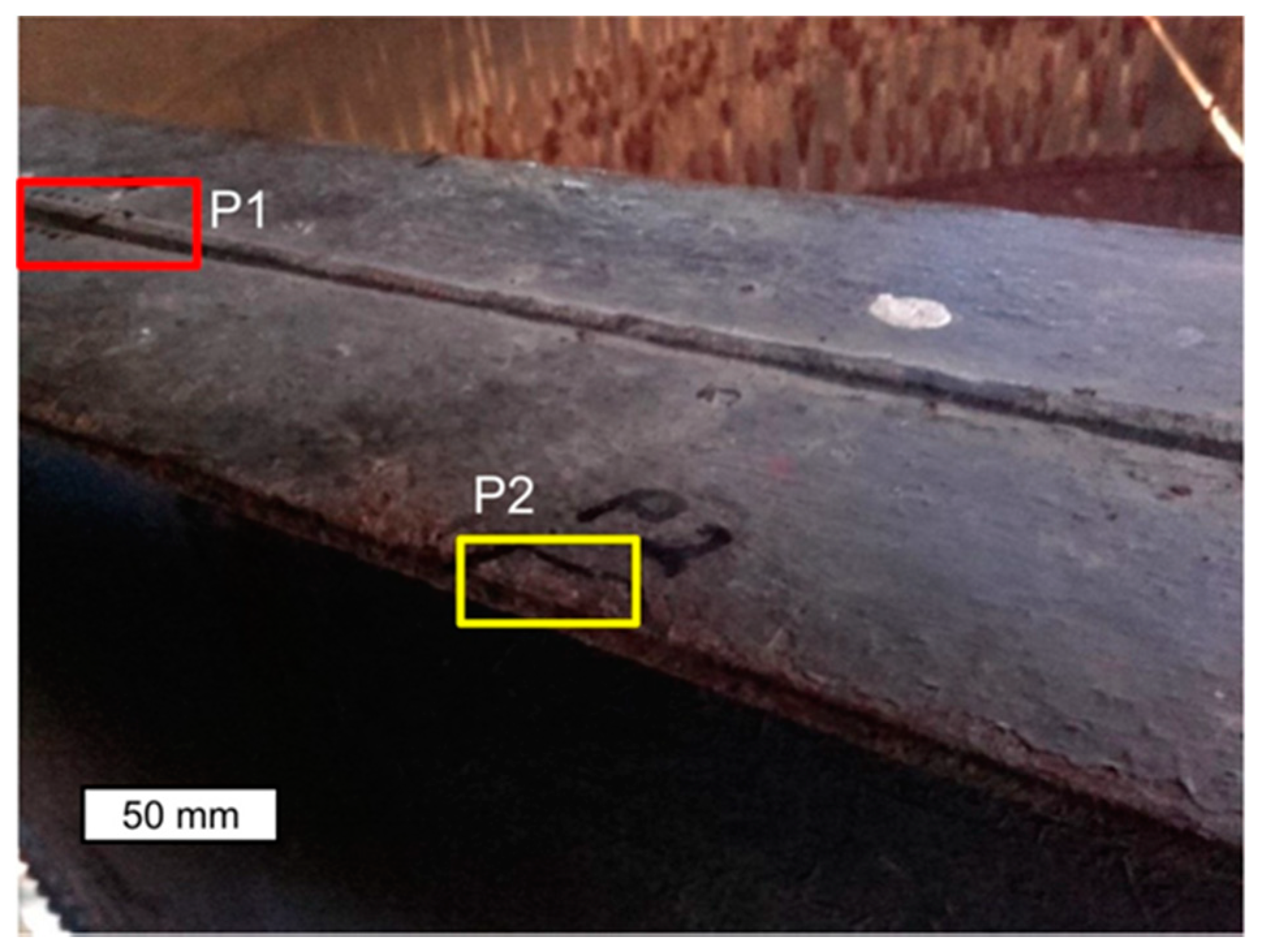
3. Research Methodology
- (1)
- The chemical composition was determined by means of the spectral method using the LECO GDS-500A (Leco Corporation, St. Joseph, MI, USA) analyser with glow discharge (GDOES).
- (2)
- The microscopic tests were performed using the Leica M205 C stereoscopic microscope (Leica Microsystems, Wetzlar, Hesse, Germany), Leica DM6000 M metallographic microscope (Leica Microsystems, Wetzlar, Hesse, Germany), as well as the Phenom World ProX scanning electron microscope (Thermo Fisher Scientific, Waltham, MA, USA), equipped with the EDS detector on the conventionally prepared metallographic specimens. The tests were carried out on samples in a non-etched and etched with 2% Nital solution state.
- (3)
- The HV1 hardness measurements were performed using the Vickers method according to the PN-EN ISO 6507-1: 2007 standard with the application of a 1 kg load. The test was carried out with the use of a LECO LM-248AT (Leco Corporation, St. Joseph, MI, USA) hardness tester.
4. Results of Tests of the Chemical Composition of Tank Steel
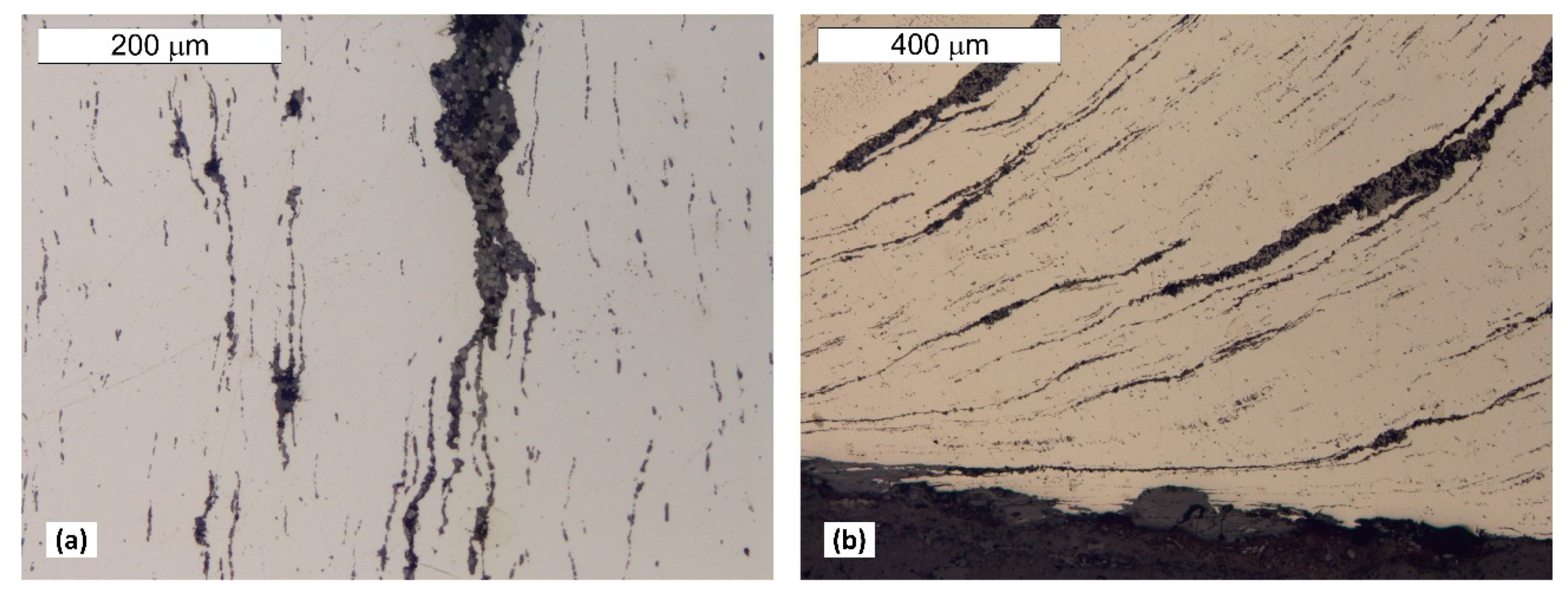
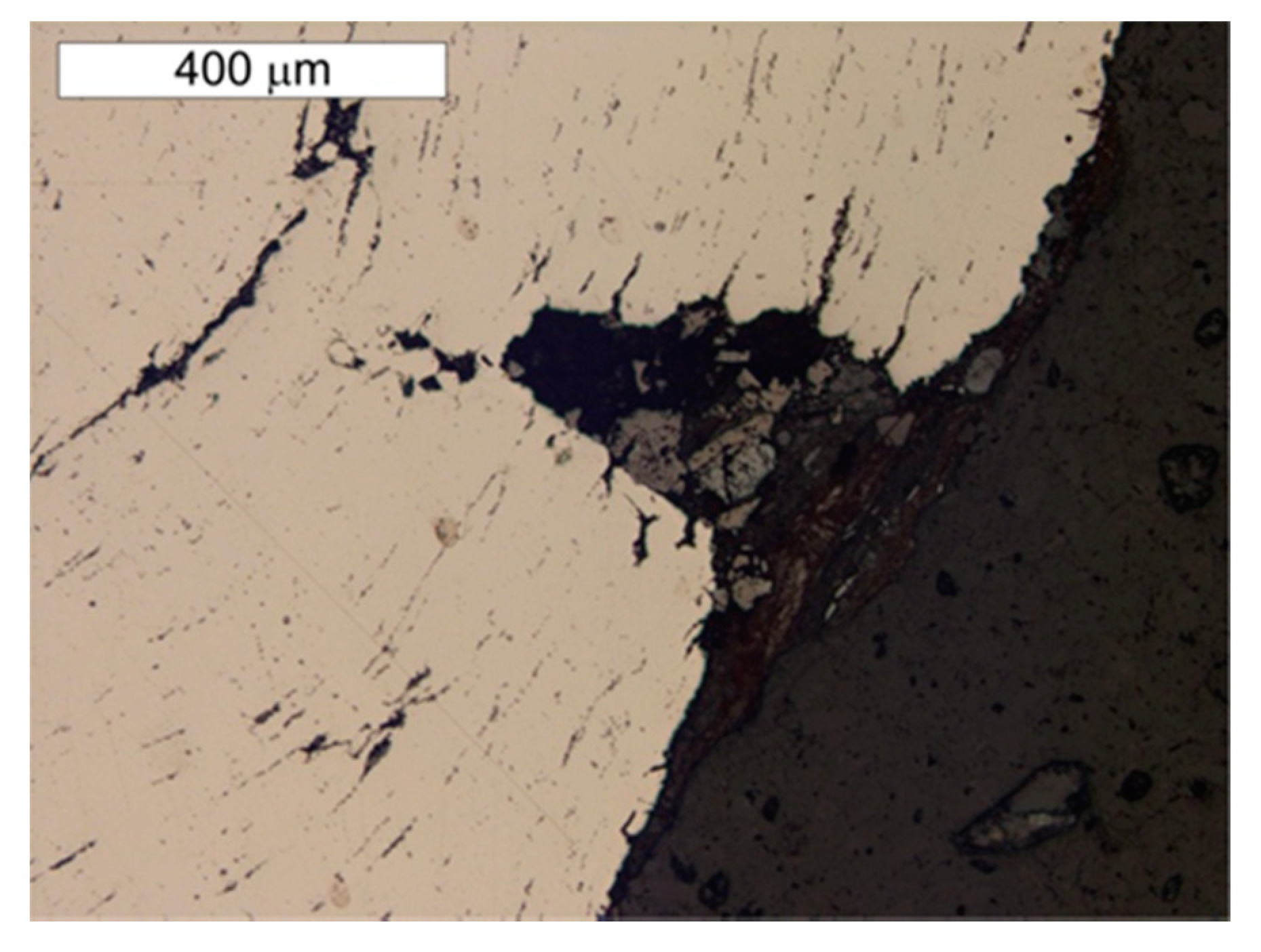
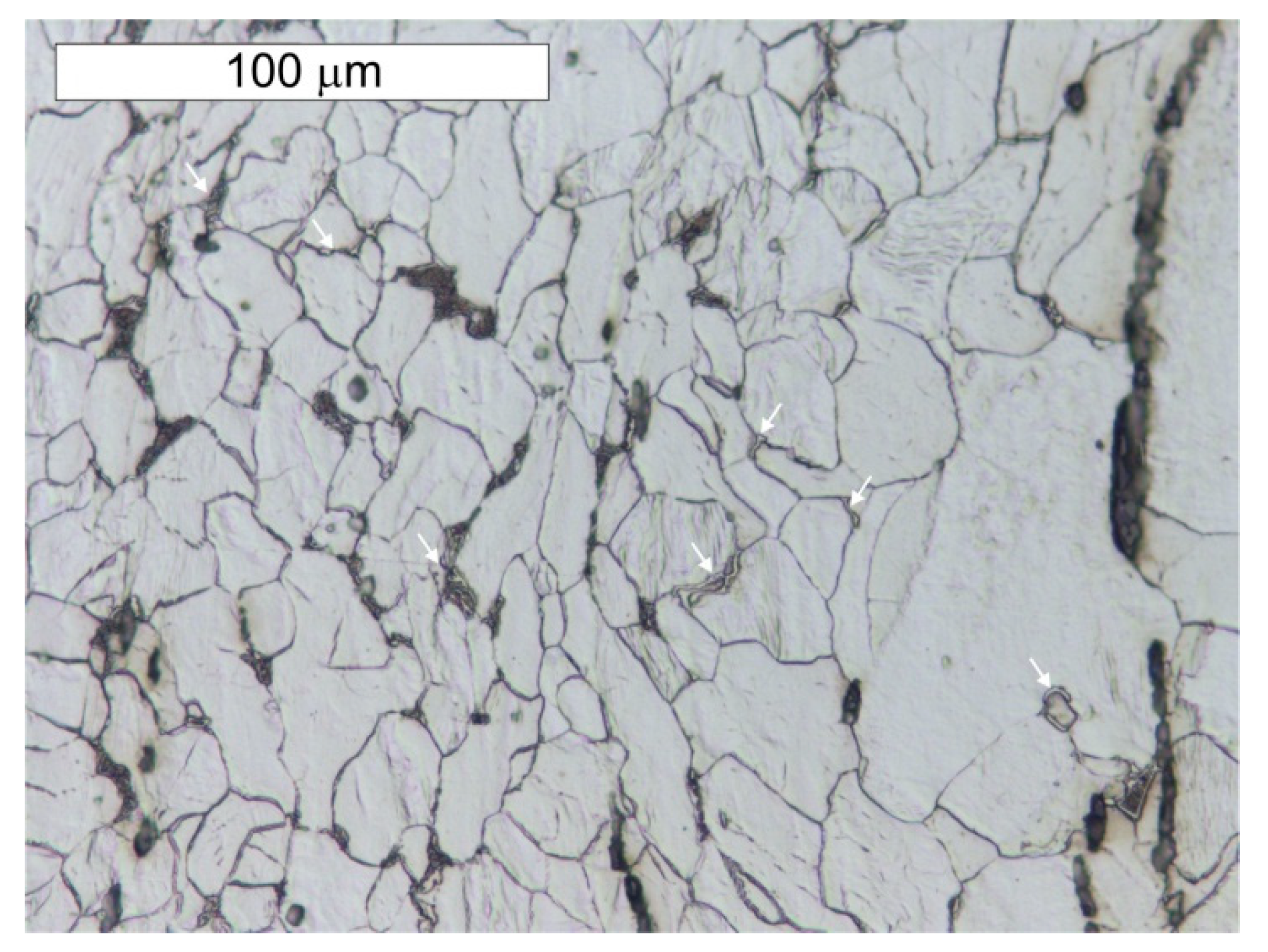
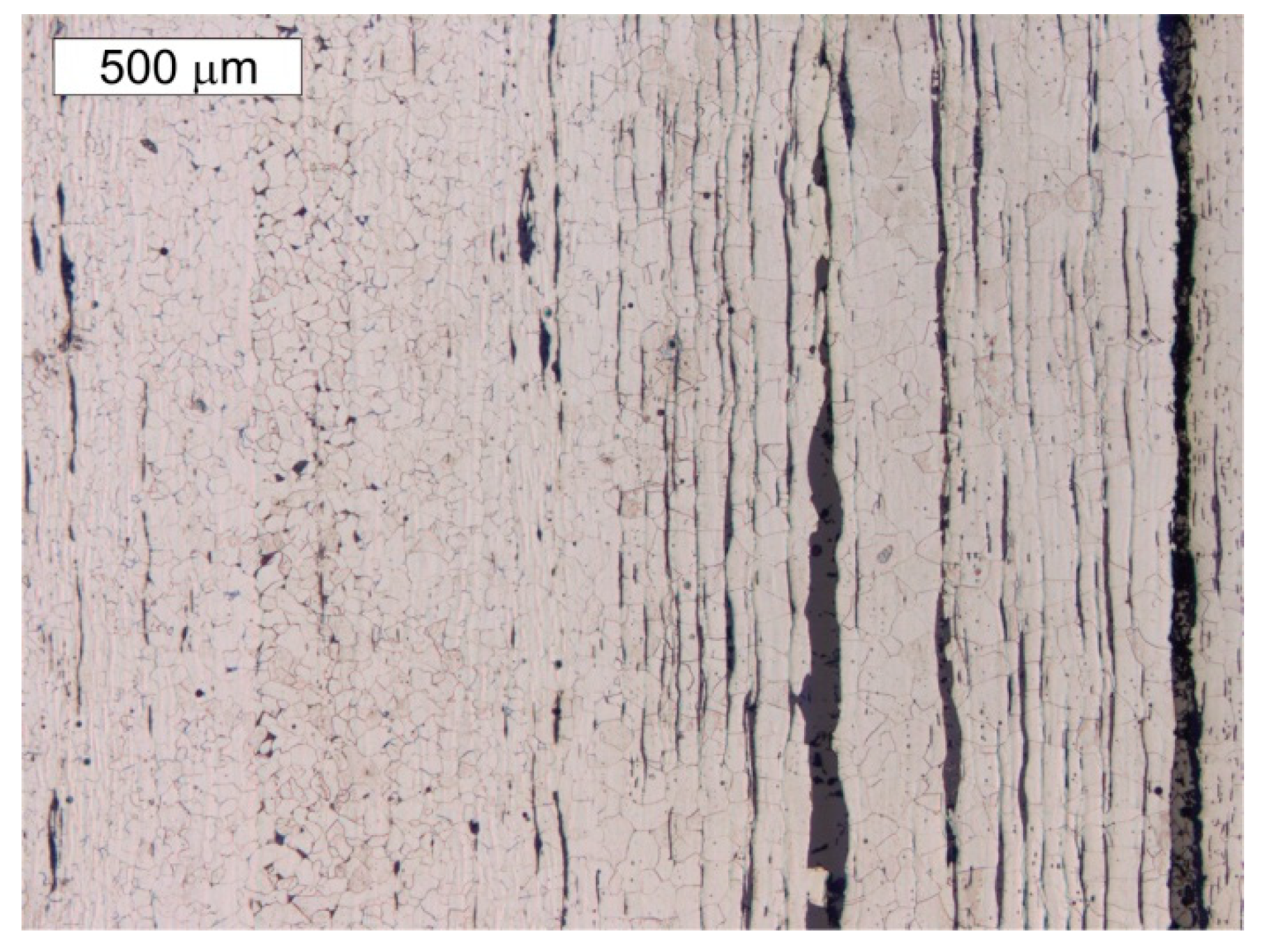
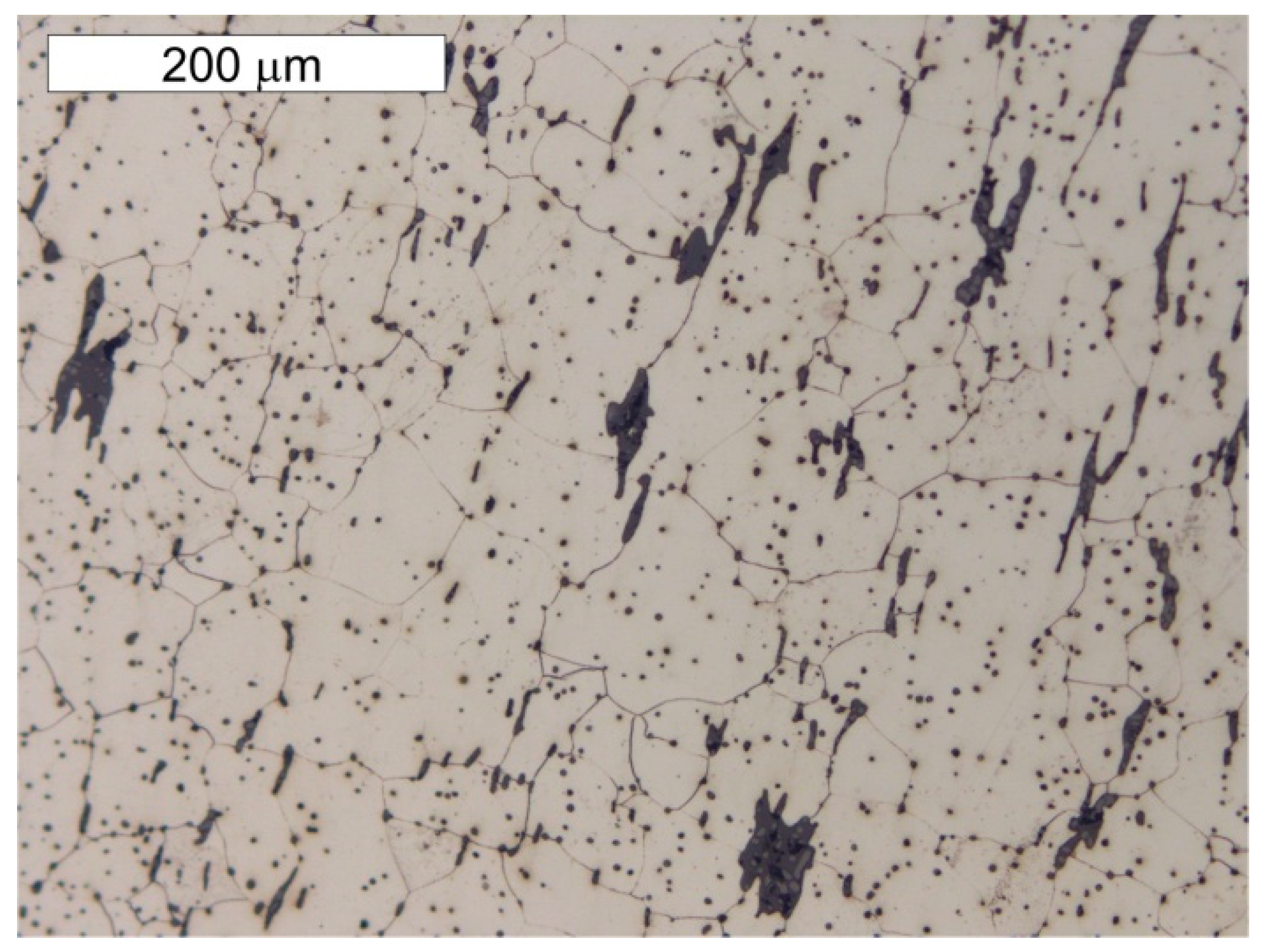
5. Results of Metallographic Tests of the Tank Steel
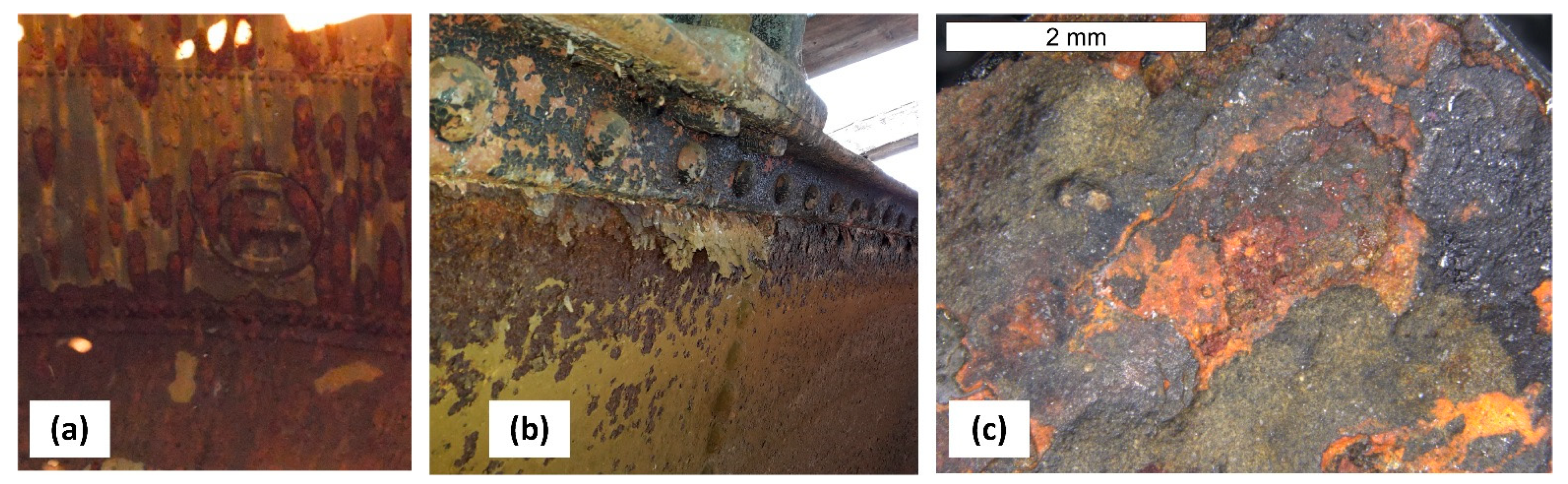
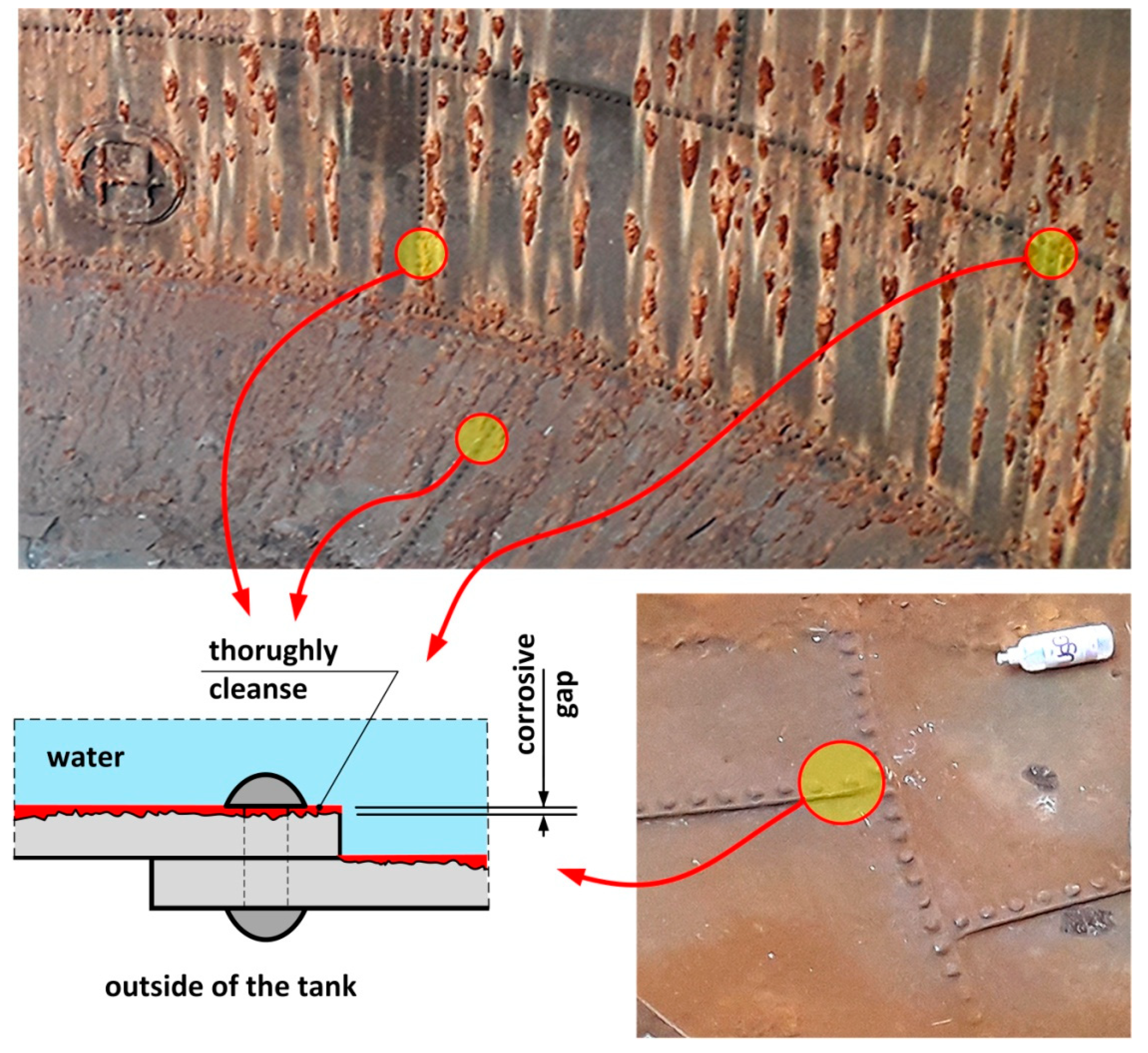
6. Assessment of Corrosion Intensity of Shell and Tank Bottom Steel
7. Conclusions
- The chemical composition of tank steel indicates that a puddled steel was used to build the pressure tower tank. Moreover, the ferritic–perlite microstructure with considerable heterogeneity and numerous non-metallic inclusions and slag is typical for this group of materials. In the microstructure, there are also numerous precipitations of tertiary cementite at the ferrite grain boundaries, the presence of which indicate advanced steel ageing. Localised degenerate perlite was also observed. This is related to the decomposition of perlite into ferrite and carbides, which is also associated with degeneration processes taking place in these types of steels.
- The water tank should be classified as unfit for welding on account of the high phosphorus content, which is limited to 0.035 ÷ 0.05% in weldable low alloy steels, numerous slag non-metallic inclusions and the presence of tertiary cementite. This is a significant problem when riveted parts of a steel tank will need to be replaced. Riveted joints ensured a secure fit of the plates to be joined, despite their corrosion. Searching for the possibility of joining steel plates in the case of their renovation with the use of prestressed bolts requires their systematic control due to the possibility of their self-loosening. In the case of the tested tank, this problem is negligible considering the low risk of cyclical loads. However, when determining their clamping force, it is required to take into account the microstructural heterogeneity of the steel plates. Applying too much force may result in catastrophic destruction of the puddled steel.
- The presence of a considerable number of banded non-metallic inclusions in the material amid corrosive processes enables the formation of surface delamination and results in the appearance of characteristic corrosive “braids”. Such advanced corrosion means that installation of cathodic protection on the tank should be considered.
- During operations, the tank has been subjected to uniform corrosion, which was favoured by the oxygen concentration cell (differential aeration). Corrosion pits were observed locally, reaching depths of approx. 1–1.5 mm. Under the thick “braids” of corrosive products on the sheet steel of the shell, the corrosion losses were approx. 1 mm deeper. The corrosion products of the sheets inside the tank did not contain sulphides with a reaction of pH = 6.5, so Microbiologically Influenced Corrosion caused by aerobic or anaerobic bacteria was not found in this case.
- During renovation work, special attention should be paid to mechanical damage, including any physical impacts, as the material will be highly brittle due to the nature of the microstructure. Puddle joints also have much lower fatigue strength than modern steel joints. Therefore, numerical and non-destructive testing methods play an important role in their fatigue life prediction. Important recommendations in this regard are presented by Kuehn et al. [22].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lesiuk, G.; Szata, M.; Bocian, M. The mechanical properties and the microstructural degradation effect in an old low carbon steels after 100-years operating time. Arch. Civ. Mech. Eng. 2015, 15, 786–797. [Google Scholar] [CrossRef]
- Lesiuk, G.; Szata, M. Fatigue Properties and Fatigue Crack Growth in Puddled Steel with Consideration of Microstructural Degradation Processes after 100-years Operating Time. Procedia Eng. 2014, 74, 64–67. [Google Scholar] [CrossRef][Green Version]
- Lesiuk, G.; Szata, M. Aspects of structural degradation in steels of old bridges by means of fatigue crack propagation. Mater. Sci. 2011, 47, 82–88. [Google Scholar] [CrossRef]
- Tsyrulnyk, O.T.; Nykyforchyn, H.M.; Petryna, D.Y.; Hredil’, M.I.; Dz’Oba, I.M. Hydrogen degradation of steels in gas mains after long periods of operation. Mater. Sci. 2007, 43, 708–717. [Google Scholar] [CrossRef]
- Panasyuk, V.; Schüller, M.; Nykyforchyn, H.; Kutnyi, A. Corrosion-Hydrogen Degradation of the Shukhov Lattice Construction Steels. Procedia Mater. Sci. 2014, 3, 282–287. [Google Scholar] [CrossRef][Green Version]
- Zagórski, A.; Matysiak, H.; Tsyrulnyk, O.; Zvirko, O.; Nykyforchyn, H.; Kurzydłowski, K. Corrosion and stress-corrosion cracking of exploited storage tank steel. Mater. Sci. 2004, 40, 421–427. [Google Scholar] [CrossRef]
- De Jesus, A.M.; da Silva, A.L.; Correia, J.A. Fatigue of riveted and bolted joints made of puddle iron—An experimental approach. J. Constr. Steel Res. 2015, 104, 81–90. [Google Scholar] [CrossRef]
- De Jesus, A.M.P.; Figueiredo, M.A.V.; Ribeiro, A.S.; de Castro, P.M.S.T.; Fernandes, A.A. Residual Lifetime Assessment of an Ancient Riveted Steel Road Bridge. Strain 2011, 47, e402–e415. [Google Scholar] [CrossRef]
- De Jesus, A.M.; da Silva, A.L.; Figueiredo, M.V.; Correia, J.A.; Ribeiro, A.S.; Fernandes, A.A. Strain-life and crack propagation fatigue data from several Portuguese old metallic riveted bridges. Eng. Fail. Anal. 2011, 18, 148–163. [Google Scholar] [CrossRef]
- Lesiuk, G.; Correia, J.A.; De Jesus, A.; Kucharski, P. Fatigue crack propagation behavior of old puddle iron including crack closure effects. Procedia Struct. Integr. 2016, 2, 3218–3225. [Google Scholar] [CrossRef]
- Hotała, E. Historical structures of steel tanks and silos in Lower Silesia. Builder 2020, 273, 96–99. [Google Scholar] [CrossRef]
- Helmerich, R. Alte Stähle und Stahlkonstruktionen. Materialuntersuchungen, Ermüdungsversuche an originalen Brückenträgern und Messungen von 1990 bis 2003, 1st ed.; Verlag für neue Wissenschaft GmbH: Berlin, Germany, 2005; ISBN 3-86509-362-0. [Google Scholar]
- Pasternak, H.; Hoch, H.-U.; Füg, D. Stahltragwerke im Industriebau; Ernst and Sohn: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Brandes, K. Eigenschaften alter Eisen und Stähle und ihre adäquate Materialprüfung und Bewertung. Bautechnik 2008, 85, 394–406. [Google Scholar] [CrossRef]
- PN-EN 10025: 2019 Hot Rolled Products of Structural Steels—Part 2: Technical Delivery Conditions for Non-alloy Structural Steel; Polish Committee for Standardization: Warsaw, Poland, 2019; Available online: http://www/pkn.pl (accessed on 20 March 2021).
- Leonetti, D.; Maljaars, J.; Pasquarelli, G.; Brando, G. Rivet clamping force of as-built hot-riveted connections in steel bridges. J. Constr. Steel Res. 2020, 167, 105955. [Google Scholar] [CrossRef]
- Maljaars, J.; Leonetti, D.; Maas, C. Fatigue life prediction of hot riveted double covered butt joints. Int. J. Fatigue 2019, 124, 99–112. [Google Scholar] [CrossRef]
- Liengen, T.; Basseguy, R.; Feron, D.; Beech, I. Understanding Biocorrosion, Fundamentals and Applications, 1st ed.; Woodhead Publishing: Sawston Cambridge, UK, 2014; ISBN 9780081015476. [Google Scholar]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Maheri, M.R.; Abdollahi, A. The effects of long term uniform corrosion on the buckling of ground based steel tanks under seismic loading. Thin-Walled Struct. 2013, 62, 1–9. [Google Scholar] [CrossRef]
- Collette, Q.; Sire, S.; Vermes, W.J.; Mesler, V.J.; Wouters, I. Experimental investigations on hot-driven structural rivets in historical French and Belgian wrought-iron structures (1880s–1890s). Constr. Build. Mater. 2014, 54, 258–269. [Google Scholar] [CrossRef]
- Kühn, B.; Lukic, M.; Nussbaumer, A.; Günther, H.-P.; Helmerich, R.; Herion, S.; Kolstein, M.H.; Walbridge, S.; Androic, B.; Dijkstra, O.; et al. Assessment of Existing Steel Structures: Recommendations for Estimation of Remaining Fatigue Life, 1st ed.; Sedlacek, G., Bijlaard, F., Geradin, M., Pinto, A., Dimowa, S., Eds.; European Commision Joint Research Center: Auchen, Germany, 2008; ISSN 1018-5593. [Google Scholar]




| Element | Puddled Steel Acc. to [12] | Cast Steel to 1906 Acc. to [12] | Current Requirements According to PN EN 10025-2:2019-11 [15] | Test Results for Tank Steel (Authors) Cont. [%] ± U |
|---|---|---|---|---|
| C | 0.0032–0.15 | 0.026–0.20 | <0.17 | 0.034 ± 0.054 |
| Si | 0.003–0.42 | 0.001–0.013 | - | 0.148 ± 0.044 |
| Mn | 0.054–0.18 | 0.036–0.52 | - | 0.120 ± 0.033 |
| N | 0.0037–0.04 | 0.011–0.022 | - | - |
| P | 0.011–0.39 | 0.0009–0.136 | <0.035 | 0.115 ± 0.036 |
| S | - | 0.063–0.176 | <0.035 | 0.010 ± 0.07 |
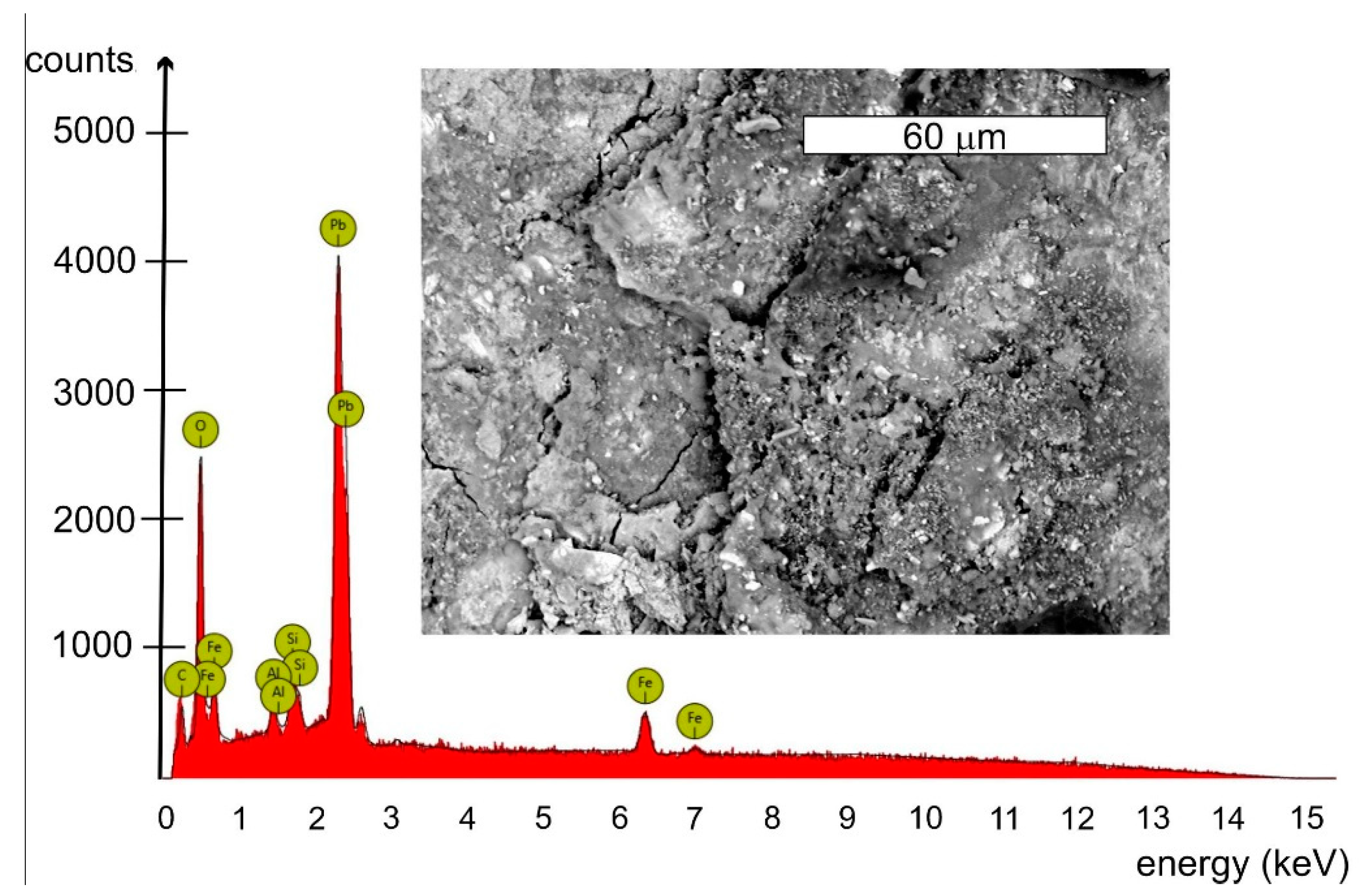
| Element Symbol | Atomic Conc. [%] | Weight Conc. [%] | Uncertainty [%] |
|---|---|---|---|
| Pb | 7.11 | 49.89 | 0.04 |
| C | 53.17 | 21.62 | 0.04 |
| O | 32.56 | 17.64 | 0.03 |
| Fe | 1.70 | 3.22 | 0.01 |
| Zn | 1.39 | 3.08 | 0.04 |
| Ca | 1.74 | 2.37 | 0.03 |
| Si | 1.81 | 1.72 | 0.03 |
| Al | 0.51 | 0.46 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotała, E.; Ignatowicz, R.; Lachowicz, M.B. Metallographic Testing of 19th Century Steel in an Operating Water Tower. Materials 2021, 14, 2204. https://doi.org/10.3390/ma14092204
Hotała E, Ignatowicz R, Lachowicz MB. Metallographic Testing of 19th Century Steel in an Operating Water Tower. Materials. 2021; 14(9):2204. https://doi.org/10.3390/ma14092204
Chicago/Turabian StyleHotała, Eugeniusz, Rajmund Ignatowicz, and Maciej B. Lachowicz. 2021. "Metallographic Testing of 19th Century Steel in an Operating Water Tower" Materials 14, no. 9: 2204. https://doi.org/10.3390/ma14092204
APA StyleHotała, E., Ignatowicz, R., & Lachowicz, M. B. (2021). Metallographic Testing of 19th Century Steel in an Operating Water Tower. Materials, 14(9), 2204. https://doi.org/10.3390/ma14092204





