Exploration of D022-Type Al3TM(TM = Sc, Ti, V, Zr, Nb, Hf, Ta): Elastic Anisotropy, Electronic Structures, Work Function and Experimental Design
Abstract
1. Introduction
2. Methods and Details
3. Results and Discussion
3.1. Structural Properties and Stability
3.2. Mechanical Stability, Elastic Properties and Moduli
(C11 − C12) > 0, (C11 + C33 − 2C13) > 0,
[2(C11 + C12) + C33 + 4C13] > 0.
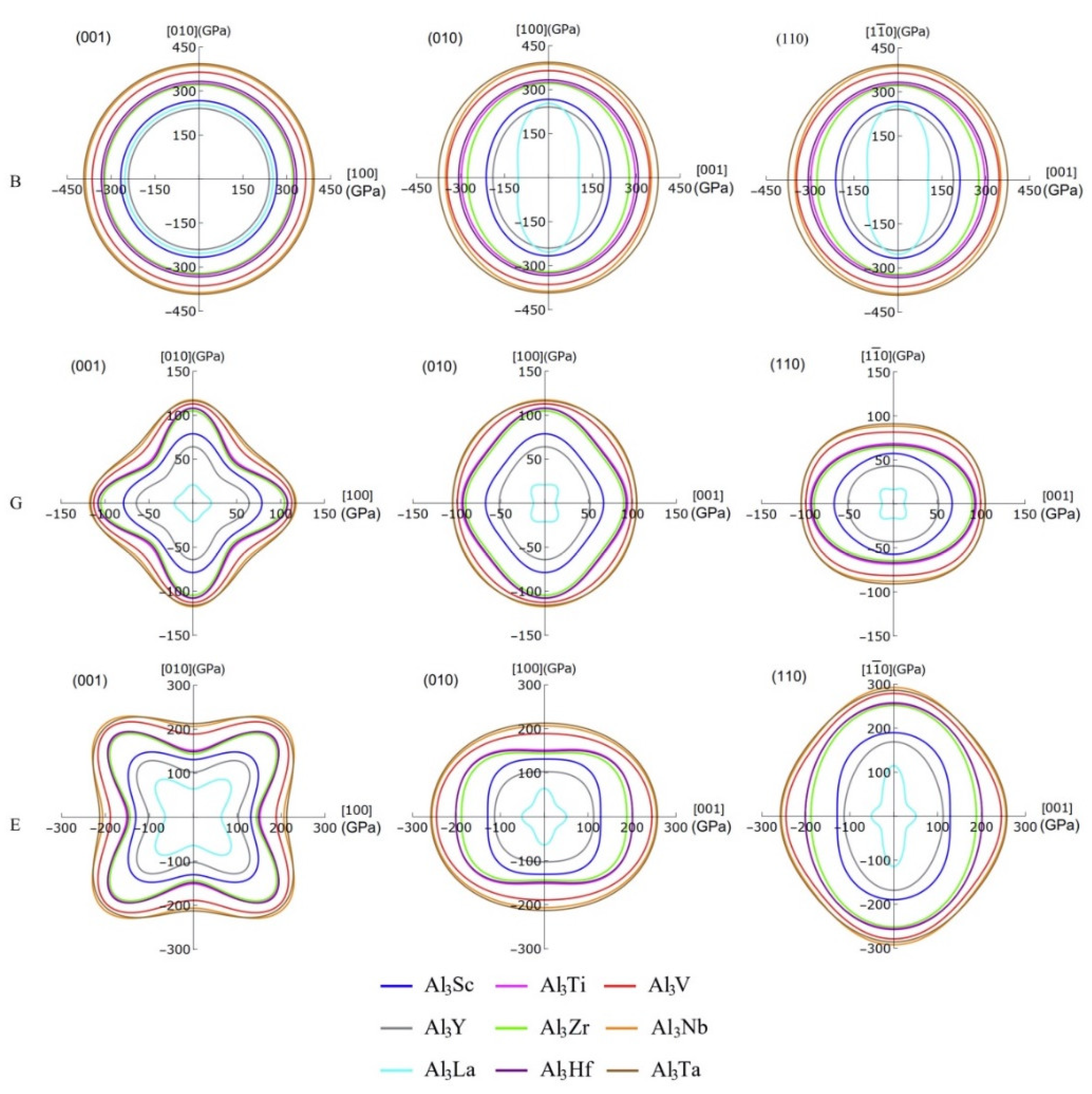
3.3. Mechanical Anisotropy
3.4. Electronic Structures
3.5. Work Function
3.6. Experimental Design
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schön, C.G.; Duong, T.; Wang, Y.; Arróyave, R. A proof of concept: Thermodynamics of aluminum–transition metal highly concentrated alloys. J. Alloy. Compd. 2019, 781, 595–605. [Google Scholar] [CrossRef]
- Engelbert, S.; Janka, O. RE4TAl (RE = Y, Sm, Gd–Tm, Lu; T = Pd, Pt)—Synthesis and magnetism of new aluminum representatives with the Gd 4 RhIn type structure. Intermetallics 2018, 96, 84–89. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, T.; Ma, H.; Li, D.; Ying, C.; Liu, C.; Wang, F. A first principles investigation on the influence of transition-metal elements on the structural, mechanical, and anisotropic properties of CaM2Al20 intermetallics. J. Mol. Graph. Model. 2020, 96, 107509. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, P.; Li, Q.; Xu, B. Vacancy-induced brittle to ductile transition of W-M co-doped Al3Ti (M = Si, Ge, Sn and Pb). Sci. Rep. 2017, 7, 13964. [Google Scholar] [CrossRef] [PubMed]
- Khenioui, Y.; Boulechfar, R.; Maazi, N.; Ghemid, S. FP-LAPW investigation of Al3(Sc1−xTix) alloys properties in L12 and D022 structures. Int. J. Mod. Phys. B 2018, 32, 1850167. [Google Scholar] [CrossRef]
- Colinet, C.; Pasturel, A. Ab initio calculation of the formation energies of L12, D022, D023 and one dimensional long period structures in TiAl3 compound. Intermetallics 2002, 10, 751–764. [Google Scholar] [CrossRef]
- Colinet, C.; Pasturel, A. Phase stability and electronic structure of the HfAl3 compound. Phys. Rev. B 2001, 64, 205102. [Google Scholar] [CrossRef]
- Kwon, O.J.; Kim, Y.C.; Kim, K.B.; Lee, Y.K.; Fleury, E. Formation of amorphous phase in the binary Cu−Zr alloy system. Met. Mater. Int. 2006, 12, 207–212. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, Y.; Peng, M.; Zhou, S. Stability, elastic properties and electronic structures of L12-ZrAl3 and D022-ZrAl3 up to 40GPa. J. Phys. Chem. Solids 2014, 75, 535–542. [Google Scholar] [CrossRef]
- Fuller, C.B.; Seidman, D.N. Temporal evolution of the nanostructure of Al (Sc, Zr) alloys: Part II-coarsening of Al3(Sc1−xZrx) precipitates. Acta Mater. 2005, 53, 5415–5428. [Google Scholar] [CrossRef]
- Moon, K.I.; Lee, S.H.; Kim, S.J. The effect of Cu and Zn on the phase stability of L12 Al3Hf intermetallic compound synthesized by mechanical alloying. Intermetallics 2002, 10, 793–800. [Google Scholar] [CrossRef]
- Reshak, A.H.; Charifi, Z.; Baaziz, H. Ab-initio calculation of structural, electronic, and optical characterizations of the intermetallic trialuminides ScAl3 compound. J. Solid State Chem. 2010, 183, 1290–1296. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Luo, X. Theoretical investigations on phase stability, elastic constants and electronic structures of D022- and L12-Al3Ti under high pressure. J. Alloy. Compd. 2013, 556, 214–220. [Google Scholar] [CrossRef]
- Jahnátek, M.; Krajčí, M.; Hafner, J. Response of fcc metals and L12 and D022 type trialuminides to uniaxial loading along [100] and [001]: ab initio DFT calculations. Philos. Mag. 2011, 91, 491–516. [Google Scholar] [CrossRef]
- Zhou, S.; Peng, B.; Cao, Y.; Xu, Y.; Quan, G.; Ma, S.; Jiao, Z.; Luo, K. First-principles investigations on stability, elastic properties and electronic structures of L12-TiAl3 and D022-TiAl3 under pressure. Phys. B Condens. Matter 2019, 571, 118–129. [Google Scholar] [CrossRef]
- Jahnátek, M.; Krajčí, M.; Hafner, J. Interatomic bonds and the tensile anisotropy of trialuminides in the elastic limit: A density functional study for Al3(Sc, Ti, V, Cr). Philos. Mag. 2007, 87, 1769–1794. [Google Scholar] [CrossRef][Green Version]
- Boulechfar, R.; Meradji, H.; Ghemid, S.; Drablia, S.; Bouhafs, B. First principle calculations of structural, electronic and thermodynamic properties of Al3(TixV1−x) alloy in D022 and L12 structures. Solid State Sci. 2013, 16, 1–5. [Google Scholar] [CrossRef]
- Krajcí, M.; Hafner, J. Covalent bonding and bandgap formation in intermetallic compounds: A case study for Al3V. J. Phys. Conden. Matter. 2002, 14, 1865–1879. [Google Scholar] [CrossRef]
- Jahnátek, M.; Krajčí, M.; Hafner, J. Interatomic bonding, elastic properties, and ideal strength of transition metal aluminides: A case study for Al3(V,Ti). Phys. Rev. B 2005, 71, 024101. [Google Scholar] [CrossRef]
- Schwarz, R.; Desch, P.; Srinivasan, S.; Nash, P. Synthesis and properties of trialuminides with ultra-fine microstructures. Nanostruct. Mater. 1992, 1, 37–42. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Chen, D.; Wu, Y.; Wang, M.; Ma, N.; Wang, H. First-principles investigation of thermodynamic, elastic and electronic properties of Al3V and Al3Nb intermetallics under pressures. J. Appl. Phys. 2015, 117, 085904. [Google Scholar] [CrossRef]
- Colinet, C.; Pasturel, A. Phase stability and electronic structure in ZrAl3 compound. J. Alloy. Compd. 2001, 319, 154–161. [Google Scholar] [CrossRef]
- Li, R.-Y.; Duan, Y.-H. Electronic structures and thermodynamic properties of HfAl3 in L12, D022 and D023 structures. Trans. Nonferrous Met. Soc. China 2016, 26, 2404–2412. [Google Scholar] [CrossRef]
- Boulechfar, R.; Meradji, H.; Chouahda, Z.; Ghemid, S.; Drablia, S.; Khenata, R. FP-LAPW investigation of the structural, electronic and thermodynamic properties of Al3Ta compound. Int. J. Mod. Phys. B 2014, 29, 1450244. [Google Scholar] [CrossRef]
- Li, C.; Cheng, N.; Chen, Z.; Xie, Z.; Hui, L. Intermetallic Growth and Interfacial Properties of the Grain Refiners in Al Alloys. Materials 2018, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, Y.; Zheng, Q. Influence of Ir concentration on the structure, elastic modulus and elastic anisotropy of Nb Ir based compounds from first-principles calculations. J. Alloy. Compd. 2019, 789, 860–866. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, N.; Zhou, H.; Xue, W. Insight into silicon-carbon multilayer films as anode materials for lithium-ion batteries: A combined experimental and first principles study. Acta Mater. 2019, 178, 173–178. [Google Scholar] [CrossRef]
- Pham, T.N.; Ohno, K.; Sahara, R.; Kuwahara, R.; Bhattacharyya, S.; Thi, N.P. Clear evidence for element partitioning effects in a Ti-6Al-4V alloy by the first-principles phase field method. J. Phys. Condens. Matter. 2020, 32, 264001. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Fadila, B.; Ameri, M.; Bensaid, D.; Noureddine, M.; Ameri, I.; Mesbah, S.; Al-Douri, Y. Structural, magnetic, electronic and mechanical properties of full-Heusler alloys Co2YAl (Y = Fe, Ti): First principles calculations with different exchange-correlation potentials. J. Magn. Magn. Mater. 2018, 448, 208–220. [Google Scholar] [CrossRef]
- Lishi, M.; Yonghua, D.; Runyue, L. Structural, elastic and electronic properties of C14-type Al2M (M = Mg, Ca, Sr and Ba) Laves phases. Phys. B Condens. Matter 2017, 507, 147–155. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, G.; Ren, X.; Wang, M.; Xu, H.; Fu, Y.; Tang, Y.; Li, D. First-principles studies on phase stability, anisotropic elastic and electronic properties of Al-La binary system intermetallic compounds. Mater. Today Commun. 2020, 24, 101101. [Google Scholar] [CrossRef]
- Duan, Y.-H.; Wu, Z.-Y.; Huang, B.; Chen, S. Phase stability and anisotropic elastic properties of the Hf–Al intermetallics: A DFT calculation. Comput. Mater. Sci. 2015, 110, 10–19. [Google Scholar] [CrossRef]
- Bilic, A.; Gale, J.D.; Gibson, M.A.; Wilson, N.; McGregor, K. Prediction of novel alloy phases of Al with Sc or Ta. Sci. Rep. 2015, 5, 9909. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, W.; Cheng, Z.; Dai, X.; Khenata, R. Electronic, magnetic, half-metallic and mechanical properties of a new quaternary Heusler compound ZrRhTiTl: Insights from first-principles studies. Solid State Commun. 2018, 269, 125–130. [Google Scholar] [CrossRef]
- Zhao, J.-S.; Gao, Q.; Li, L.; Xie, H.-H.; Hu, X.-R.; Xu, C.-L.; Deng, J.-B. First-principles study of the structure, electronic, magnetic and elastic properties of half-Heusler compounds LiXGe (X = Ca, Sr and Ba). Intermetallics 2017, 89, 65–73. [Google Scholar] [CrossRef]
- Chong, X.; Jiang, Y.; Feng, J. Exploring the intrinsic ductile metastable Fe-C compounds: Complex chemical bonds, anisotropic elasticity and variable thermal expansion. J. Alloy. Compd. 2018, 745, 196–211. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, G.; Liu, H.; Xu, H.; Fu, Y.; Li, D. Effect of transition-elements substitution on mechanical properties and electronic structures of B2-AlCu compounds. Results Phys. 2021, 21, 103765. [Google Scholar] [CrossRef]
- Puigdollers, A.R.; Alonso, G.; Gamallo, P. First-principles study of structural, elastic and electronic properties of α-, β- and γ-graphyne. Carbon 2016, 96, 879–887. [Google Scholar] [CrossRef]
- Qin, H.; Yan, B.-L.; Zhong, M.; Jiang, C.-L.; Liu, F.-S.; Tang, B.; Liu, Q.-J. First-principles study of structural, elastic, and electronic properties of triclinic TATB under different pressures. Phys. B Condens. Matter 2019, 552, 151–158. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, F.; Ai, T.; Li, C. Structural, elastic and electronic properties of B2-type modified by ternary additions FeAl-based intermetallics: First-principles study. J. Alloy. Compd. 2017, 710, 581–588. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, J.; Fang, Q.; Feng, H. Effect of Al solute concentration on mechanical properties of AlxFeCuCrNi high-entropy alloys: A first-principles study. Phys. B Condens. Matter 2019, 566, 30–37. [Google Scholar] [CrossRef]
- Roknuzzaman, M.; Hadi, M.; Ali, M.; Hossain, M.; Jahan, N.; Uddin, M.; Alarco, J.; Ostrikov, K. First hafnium-based MAX phase in the 312 family, Hf3AlC2: A first-principles study. J. Alloy. Compd. 2017, 727, 616–626. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, W.-C.; Li, D.-J.; Li, K.; Jin, H.-L.; Xu, Y.-X.; Xu, C.-S.; Zeng, X.-Q. Mechanical, electronic and thermodynamic properties of C14-type AMg2 (A = Ca, Sr and Ba) compounds from first principles calculations. Comput. Mater. Sci. 2015, 97, 75–85. [Google Scholar] [CrossRef]
- Panda, K.B.; Chandran, K.S. First principles determination of elastic constants and chemical bonding of titanium boride (TiB) on the basis of density functional theory. Acta Mater. 2006, 54, 1641–1657. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Lu, H.; Huang, X.; Li, N. Understanding the bond-energy, hardness, and adhesive force from the phase diagram via the electron work function. J. Appl. Phys. 2014, 116, 173506. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, H.; Cui, J.; Yan, D.; Yin, F.; Li, D. Mechanical characteristics of FeAl2O4 and AlFe2O4 spinel phases in coatings—A study combining experimental evaluation and first-principles calculations. Ceram. Int. 2017, 43, 16094–16100. [Google Scholar] [CrossRef]
- Lu, H.; Li, L.; Huang, X.; Li, D. An electron work function based mechanism for solid solution hardening. J. Alloy. Compd. 2018, 737, 323–329. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Li, D. The relationship between the electron work function and friction behavior of passive alloys under different conditions. Appl. Surf. Sci. 2015, 351, 316–319. [Google Scholar] [CrossRef]
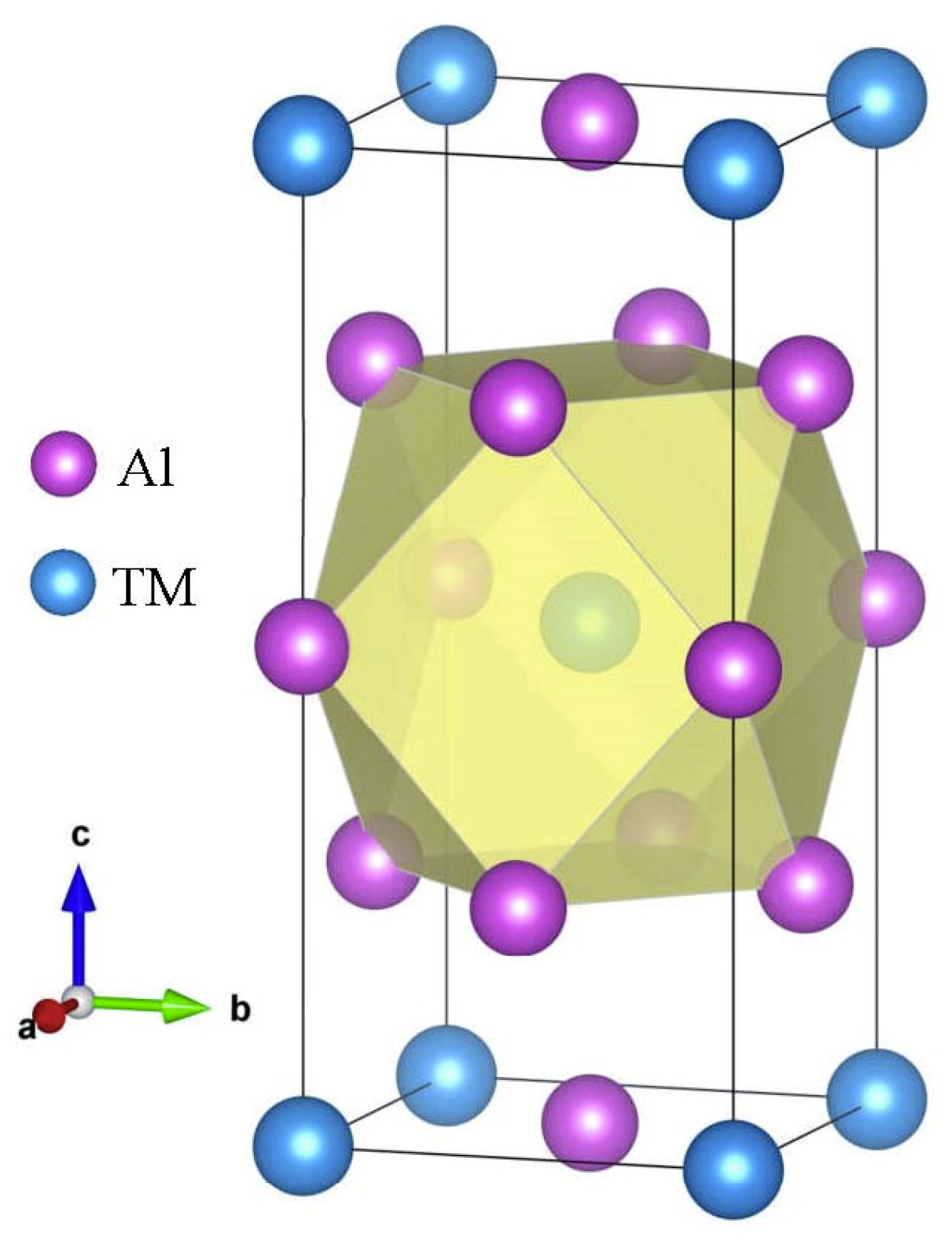
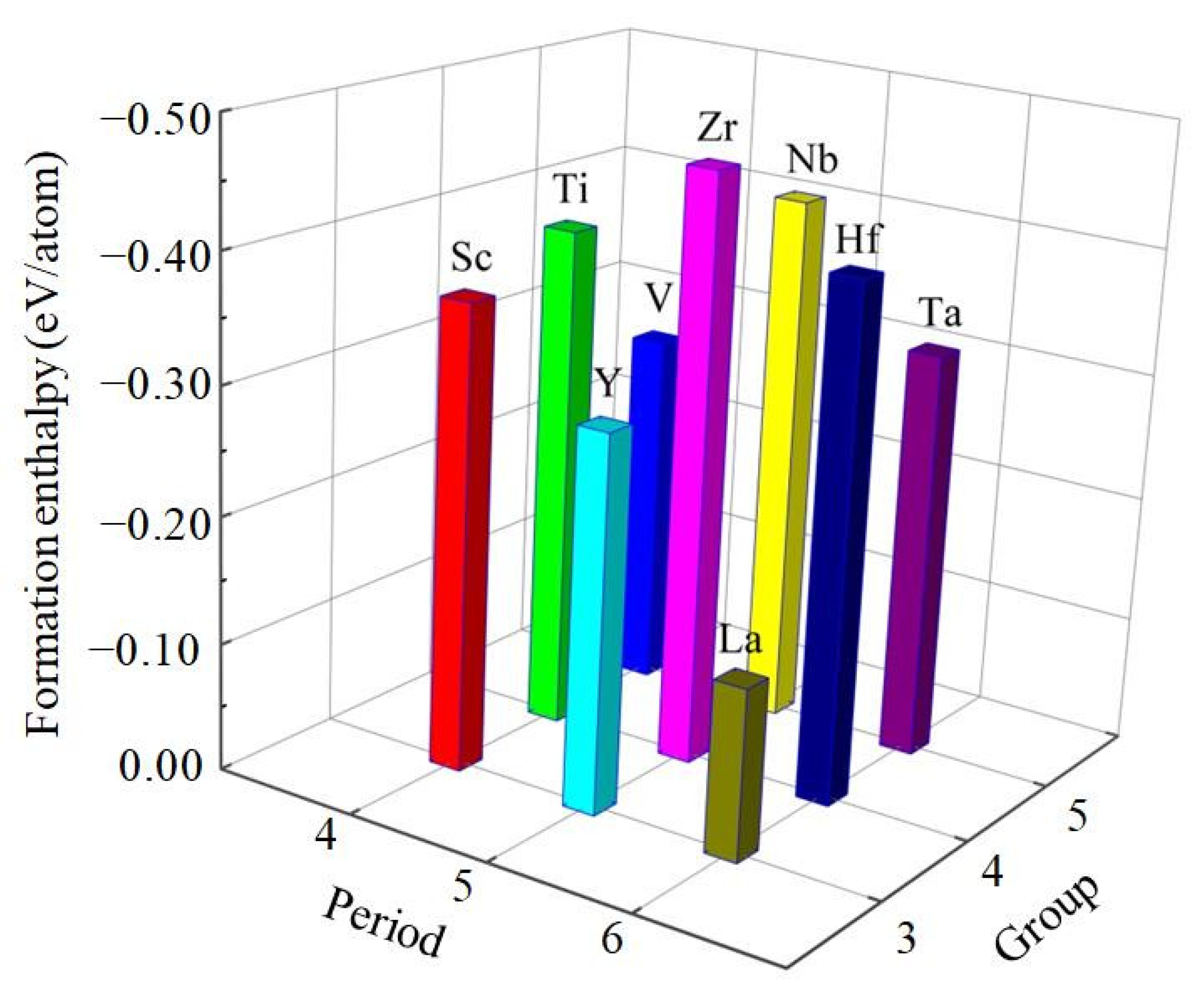
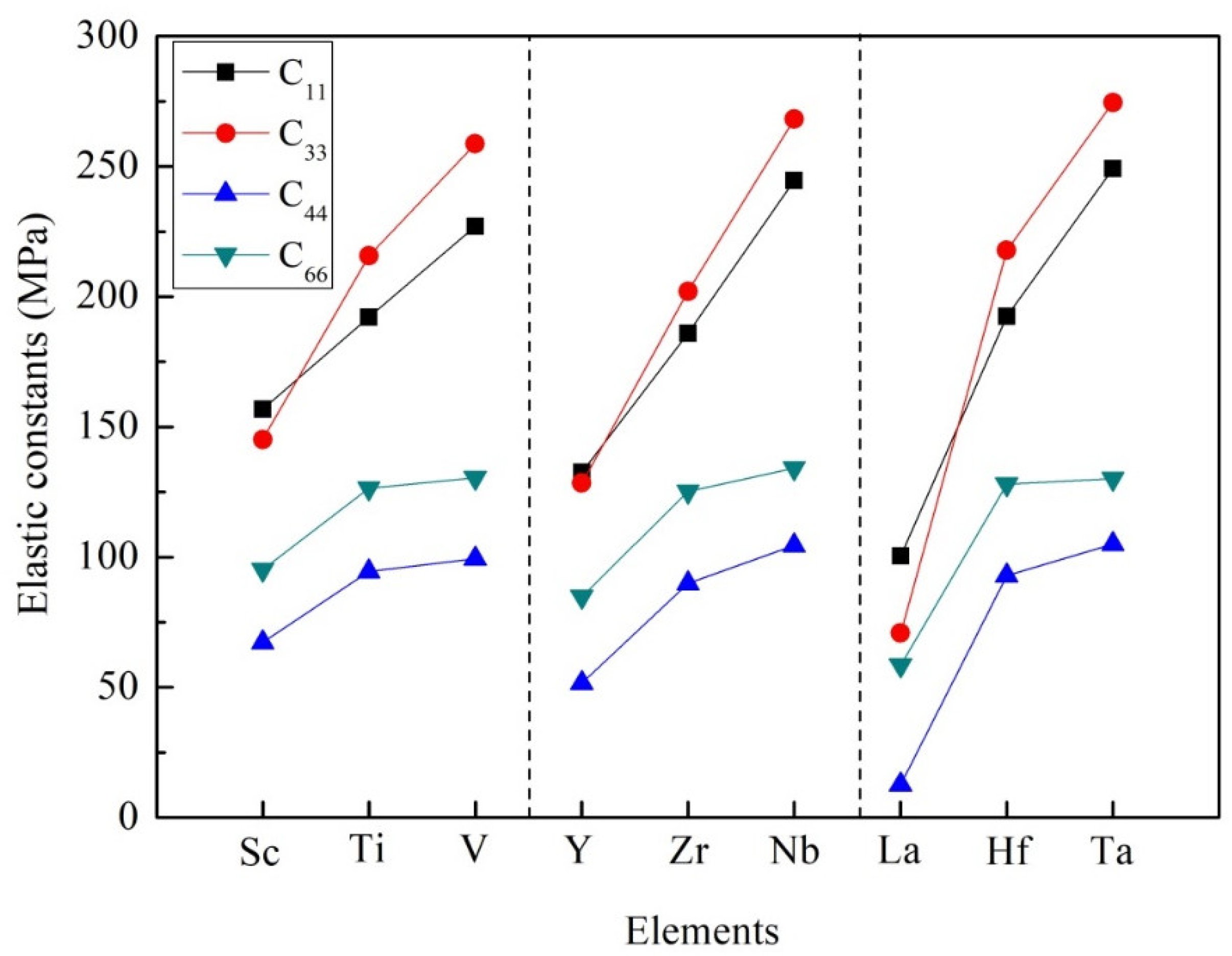
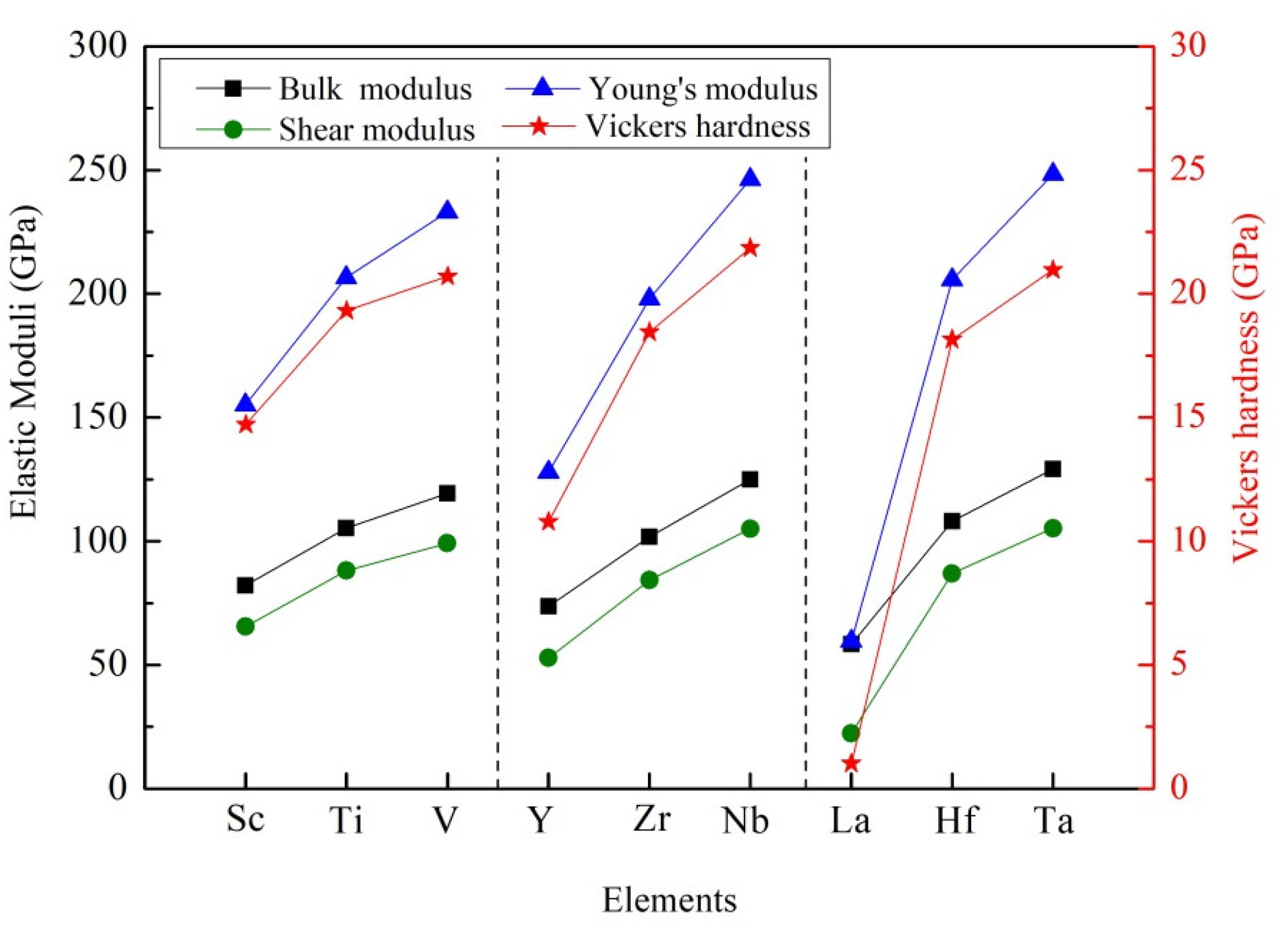
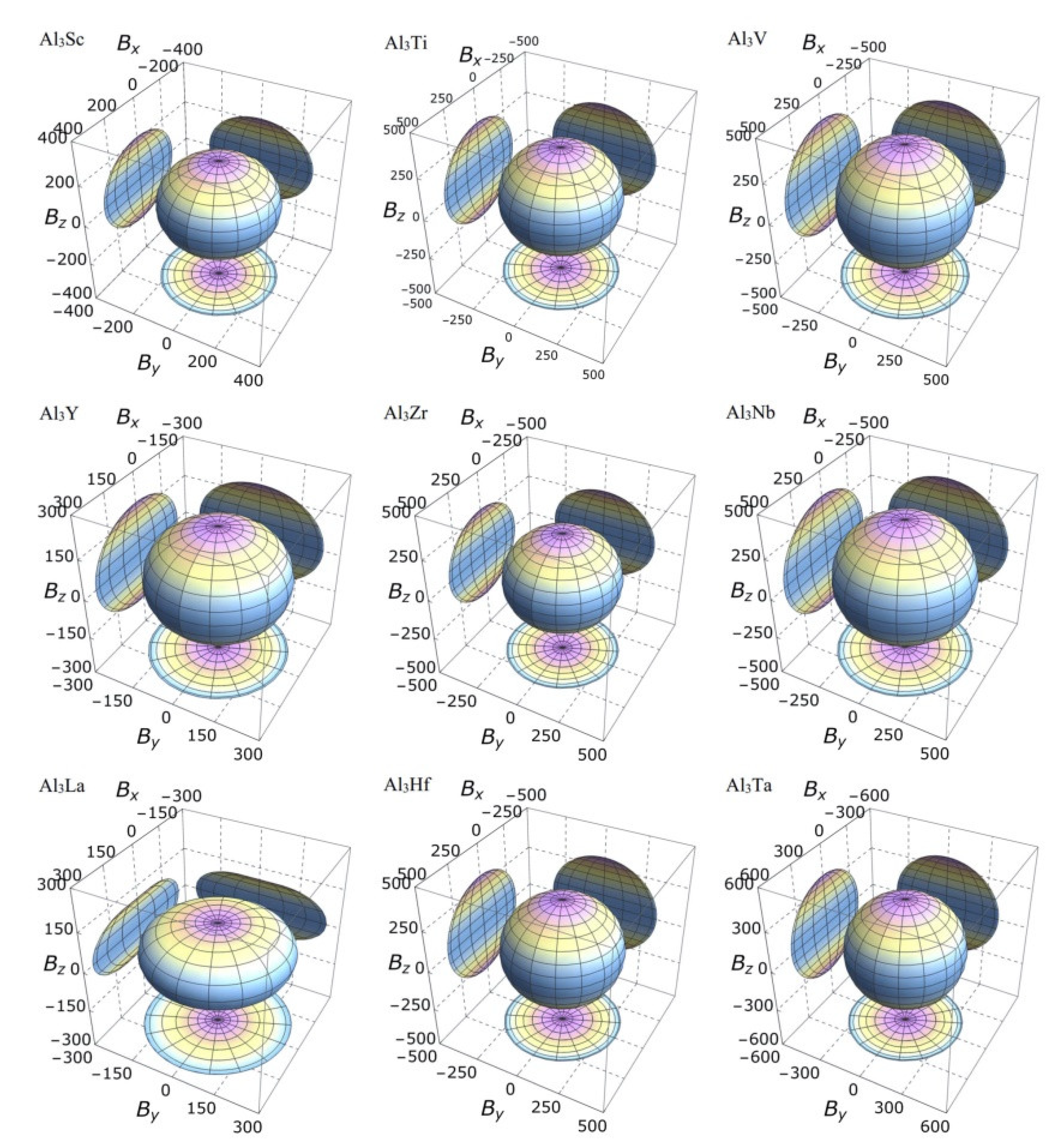
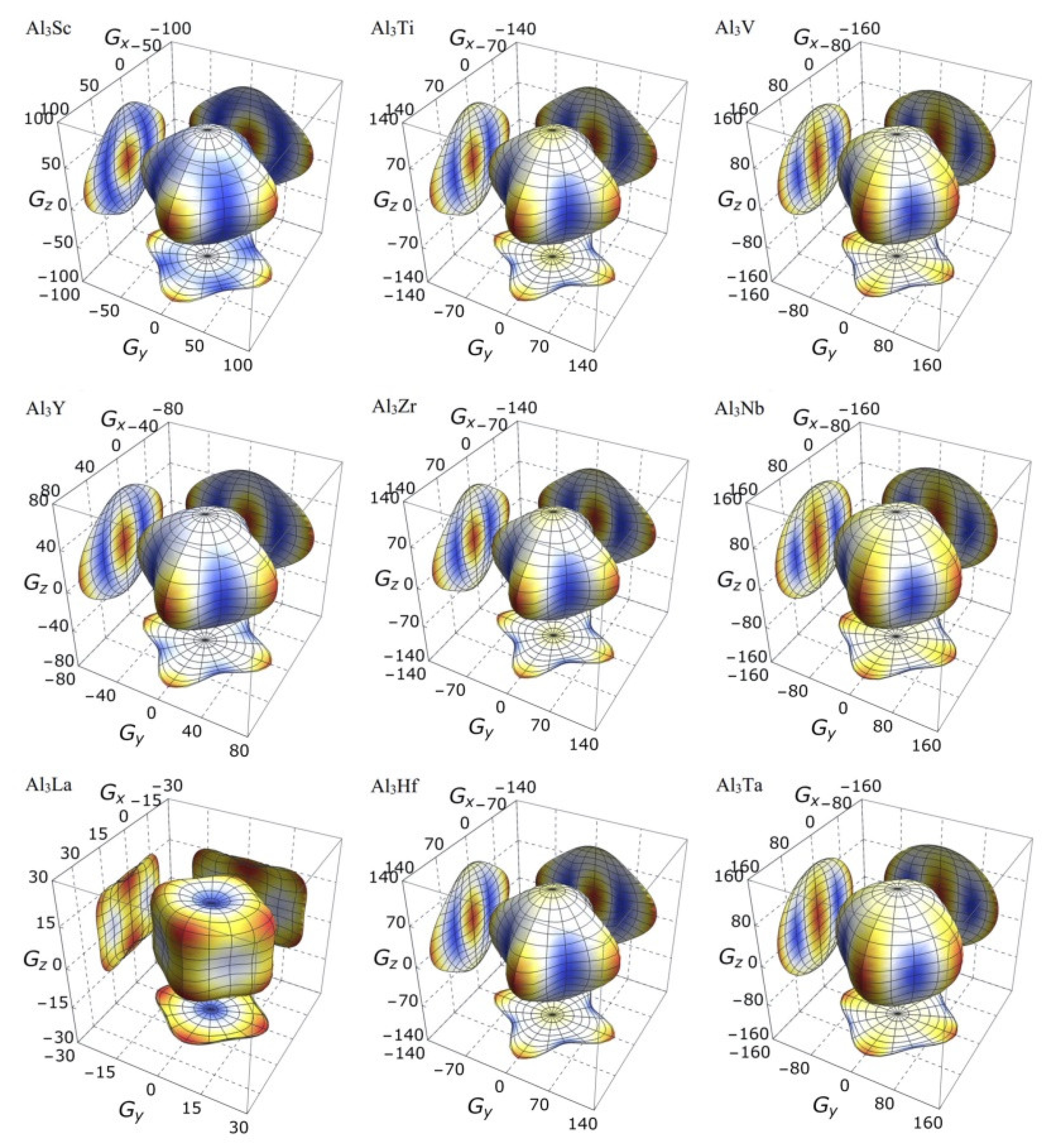
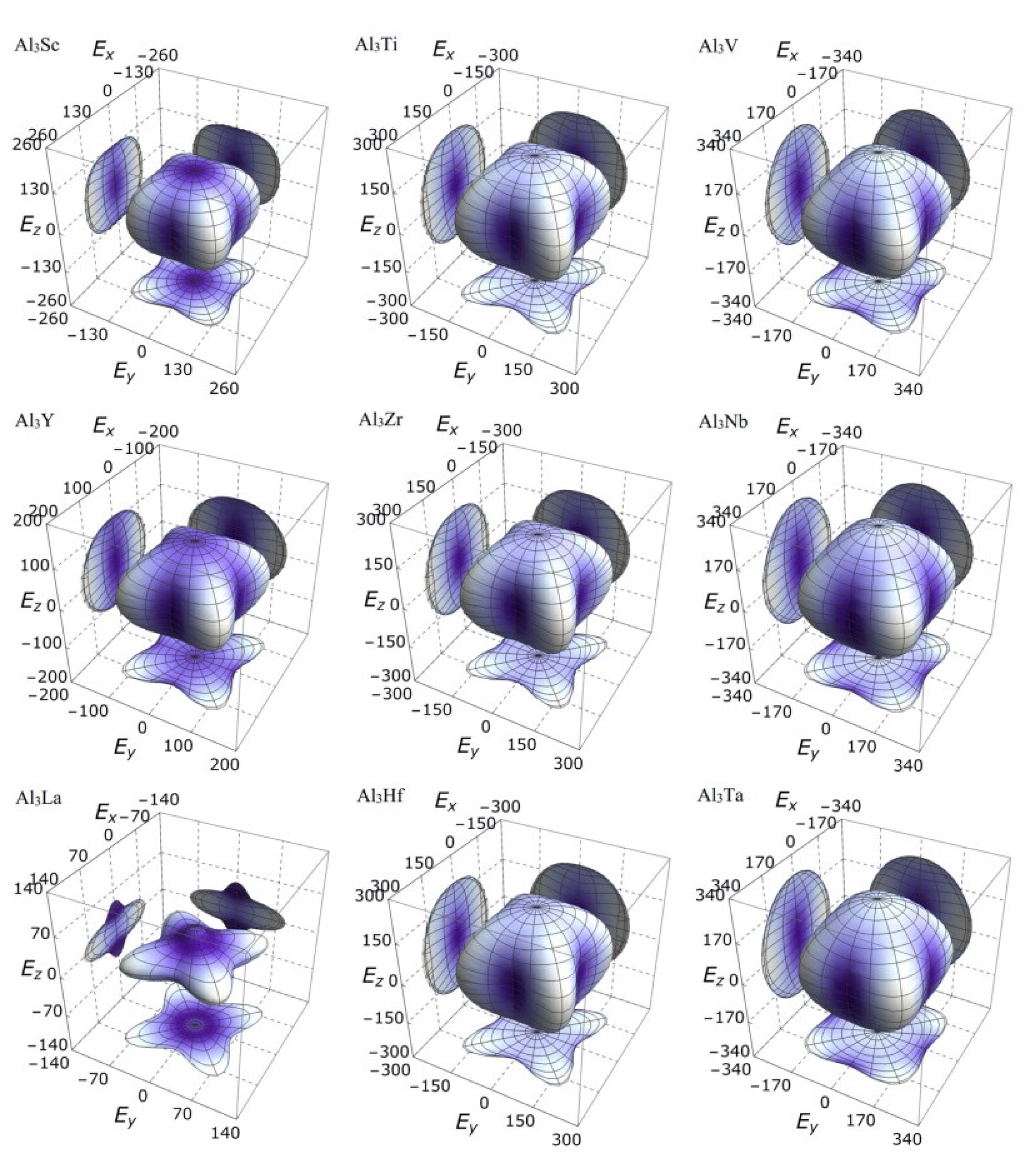
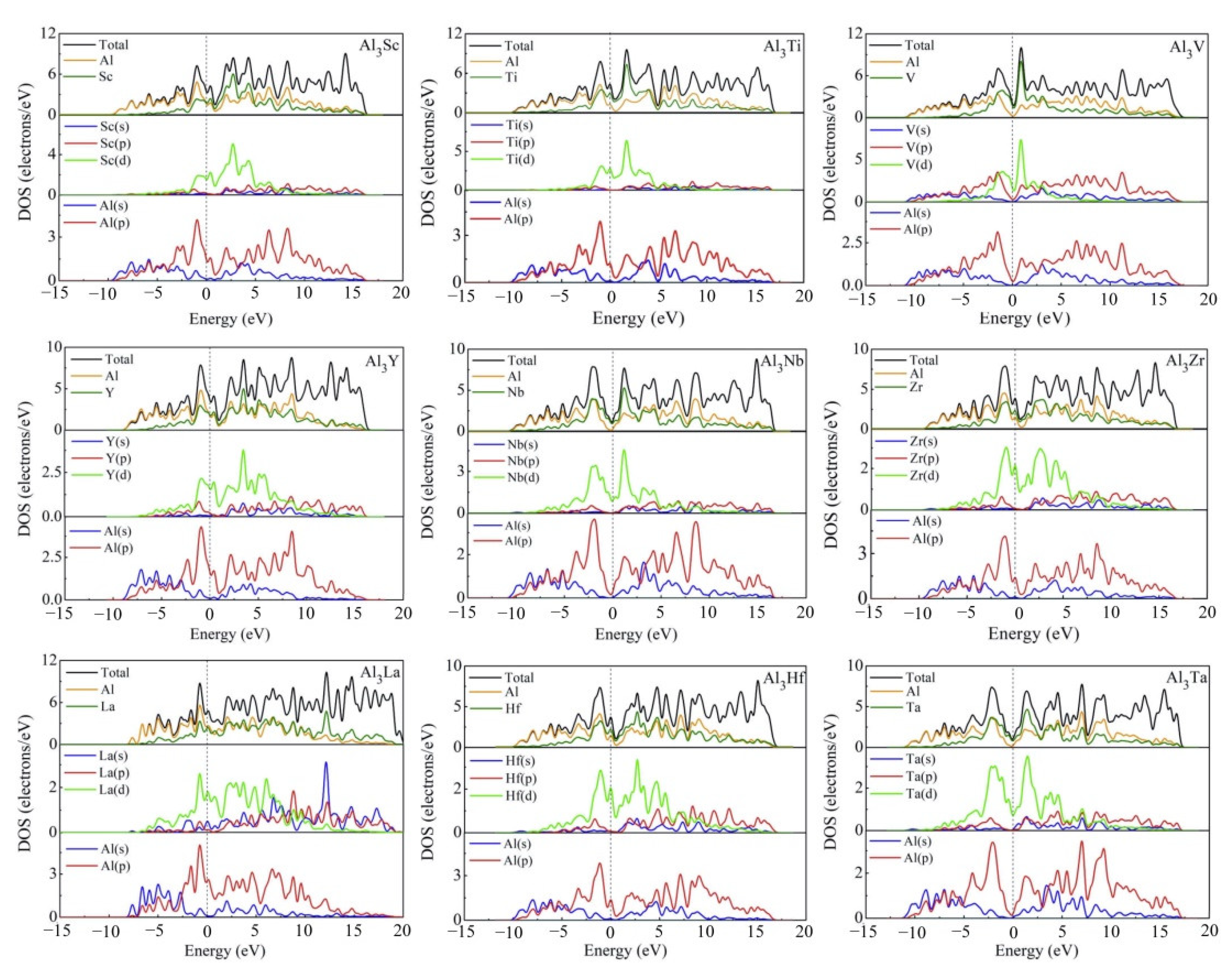
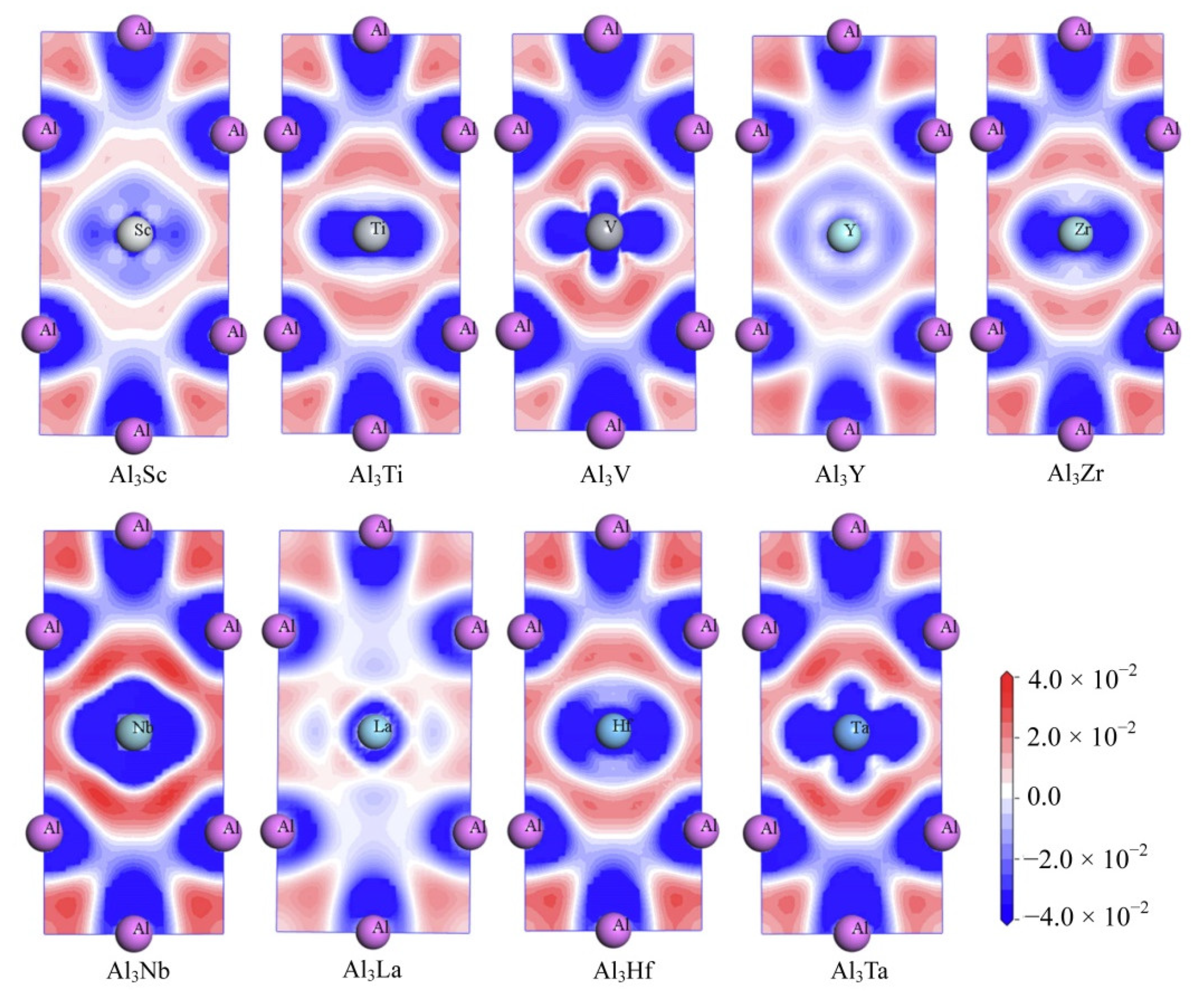
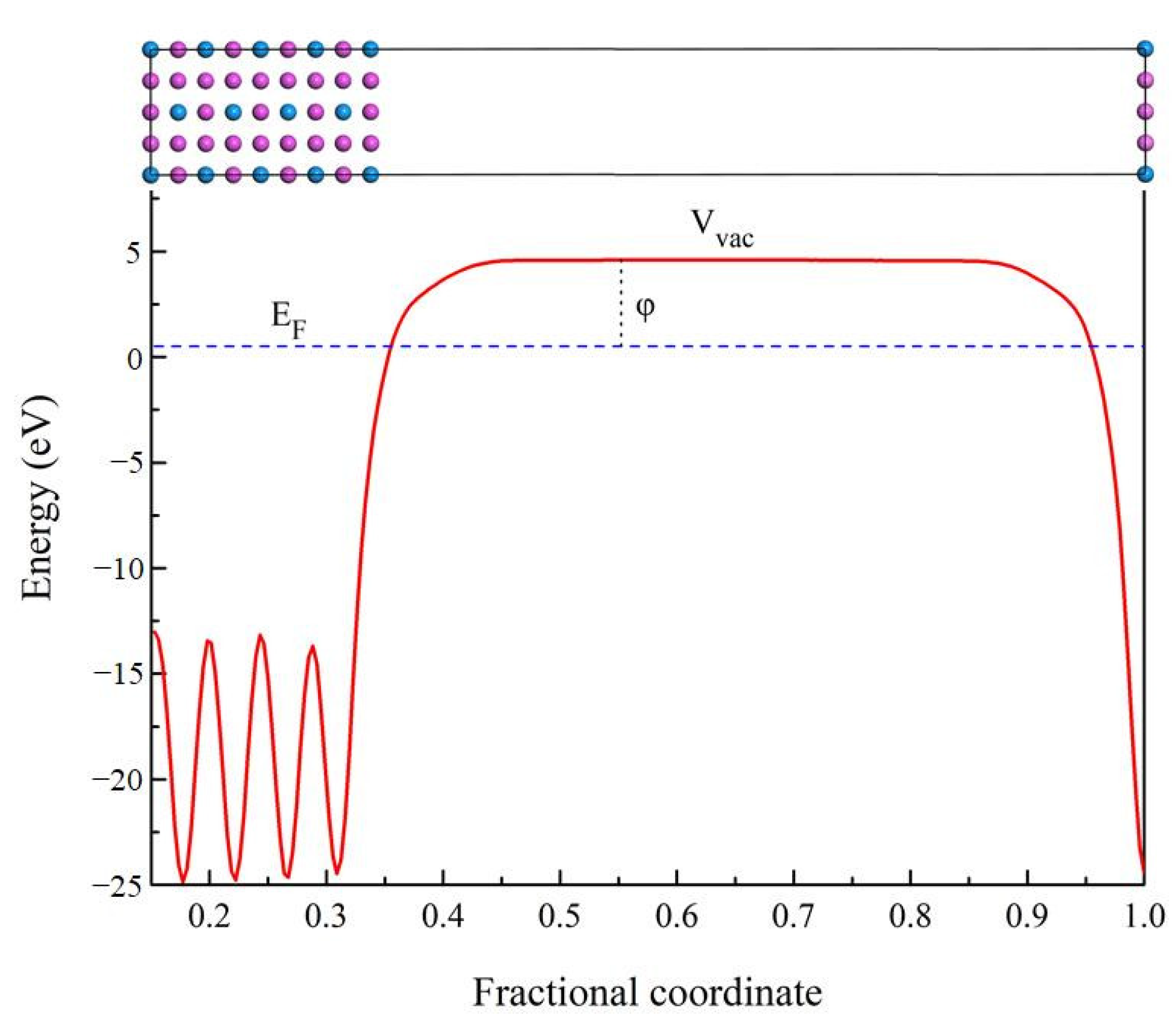
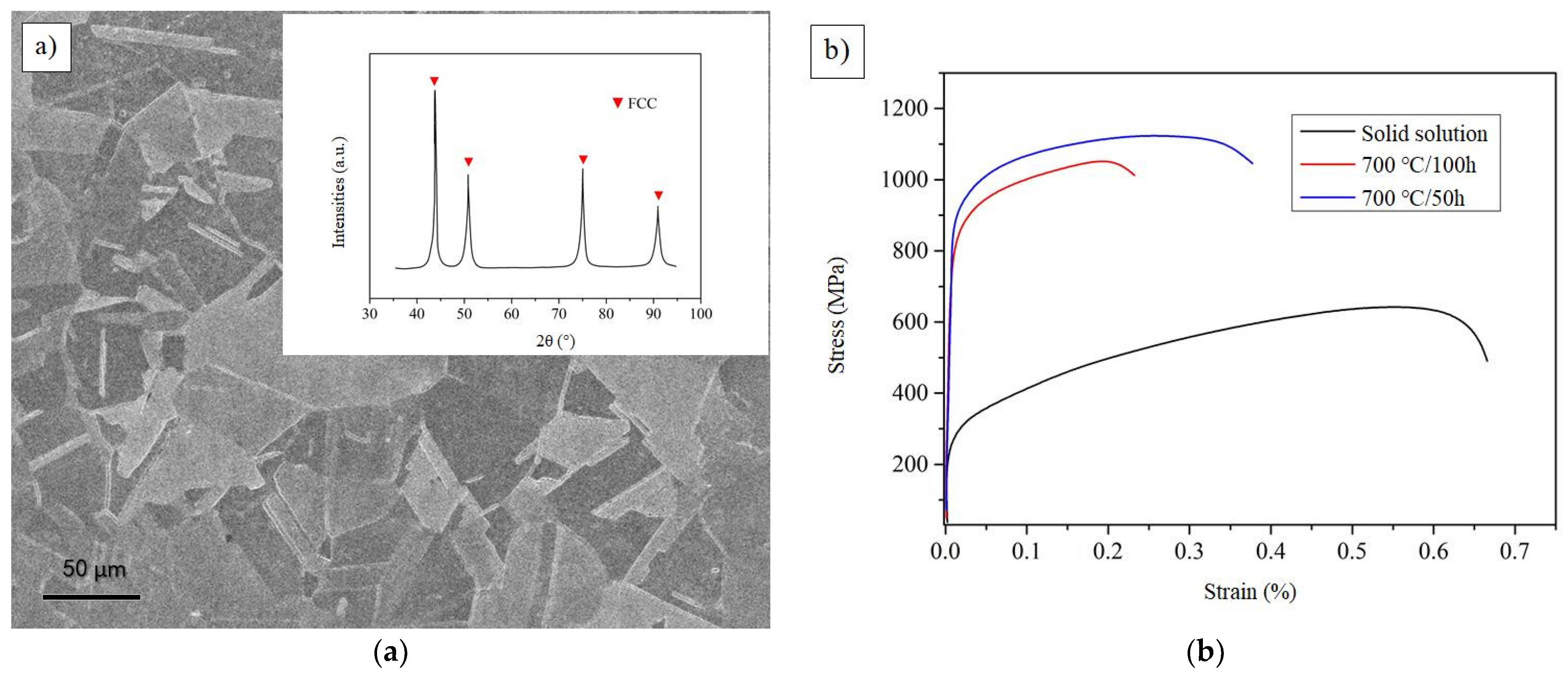
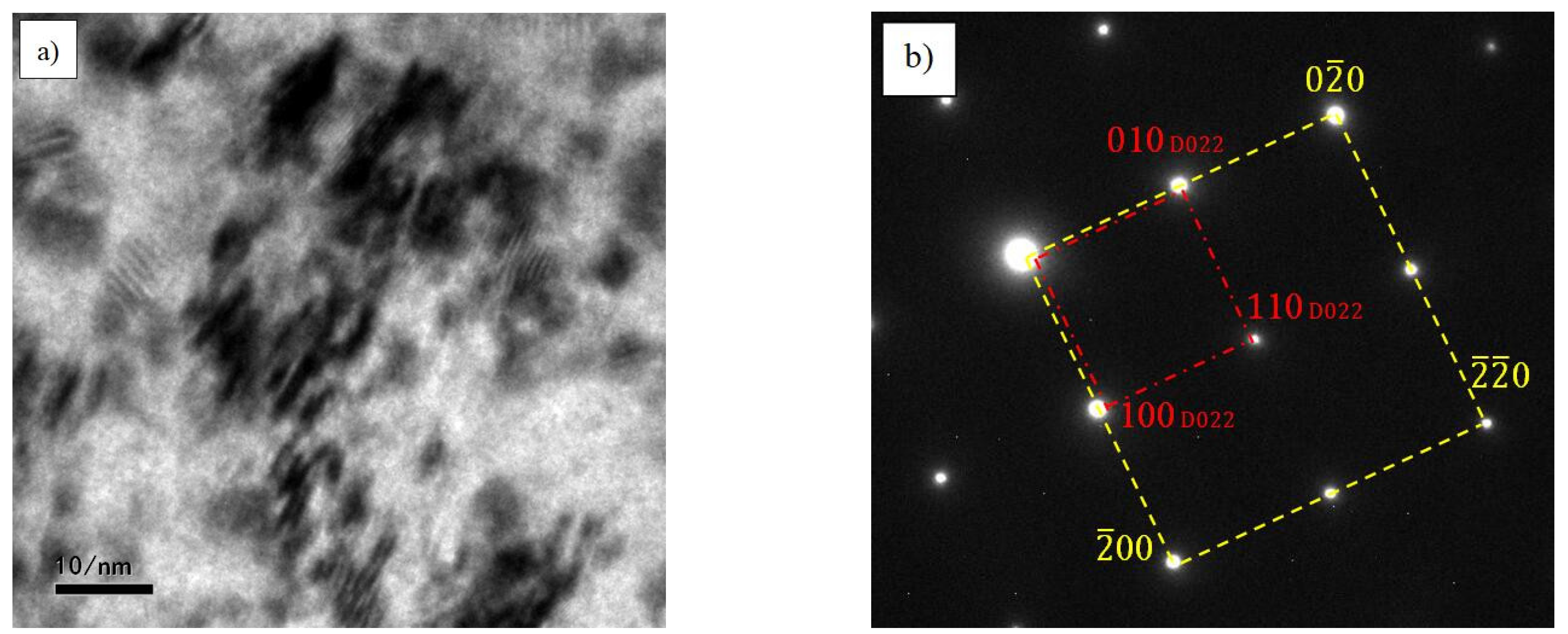
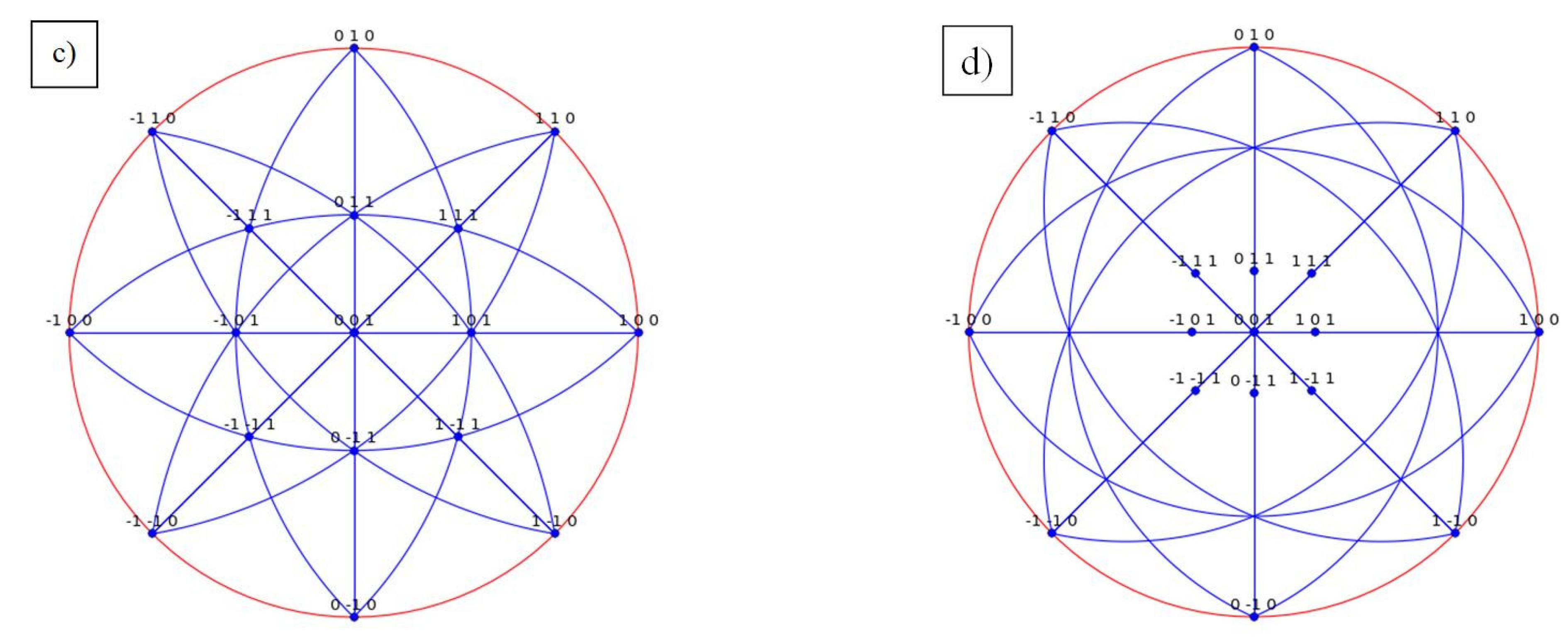
| Species | Atomic Number | a | c | ρ | V | ∆H | ∆E |
|---|---|---|---|---|---|---|---|
| Al3Sc | 21 | 4.024 | 8.840 | 2.920 | 143.173 | −0.366 | −4.221 |
| 4.021 [13] | 8.822 [13] | ||||||
| Al3Ti | 22 | 3.850 | 8.630 | 3.346 | 127.892 | −0.396 | −4.793 |
| 3.851 [13] | 8.576 [13] | ||||||
| Al3V | 23 | 3.773 | 8.324 | 3.697 | 118.484 | −0.283 | −4.846 |
| 3.766 [13] | 8.312 [13] | ||||||
| 3.773 [21] | 8.325 [21] | 118.510 [21] | −0.277 [21] | ||||
| Al3Y | 39 | 4.194 | 9.260 | 3.464 | 162.863 | −0.295 | −4.178 |
| Al3Zr | 40 | 3.963 | 9.027 | 4.034 | 141.757 | −0.464 | −4.916 |
| Al3Nb | 41 | 3.854 | 8.641 | 4.497 | 128.382 | −0.419 | −5.473 |
| 3.855 [21] | 8.645 [21] | 128.470 [21] | −0.409 [21] | ||||
| Al3La | 57 | 4.454 | 9.240 | 3.984 | 183.279 | −0.134 | −3.934 |
| Al3Hf | 72 | 3.946 | 8.918 | 6.206 | 138.842 | −0.406 | −4.906 |
| 3.946 [33] | 8.924 [33] | 138.900 [33] | −0.404 [33] | −5.040 [33] | |||
| Al3Ta | 73 | 3.862 | 8.591 | 6.789 | 128.121 | −0.322 | −5.151 |
| 3.857 [34] | 8.598 [34] | −0.318 [34] |
| Species | C11 | C12 | C13 | C33 | C44 | C66 |
|---|---|---|---|---|---|---|
| Al3Sc | 156.86 | 56.88 | 42.17 | 145.14 | 67.20 | 95.17 |
| 158.00 [13] | 60.00 [13] | 42.00 [13] | 158.00 [13] | 63.00 [13] | 93.00 [13] | |
| Al3Ti | 192.06 | 84.53 | 44.60 | 215.66 | 94.46 | 126.42 |
| 192.00 [13] | 84.00 [13] | 49.00 [13] | 216.00 [13] | 94.00 [13] | 122.00 [13] | |
| Al3V | 227.01 | 89.03 | 45.95 | 258.69 | 99.37 | 130.55 |
| 233.00 [13] | 77.00 [13] | 47.00 [13] | 258.00 [13] | 104.00 [13] | 129.00 [13] | |
| 220.87 [21] | 92.69 [21] | 45.26 [21] | 256.95 [21] | 98.57 [21] | 130.25 [21] | |
| Al3Y | 132.51 | 58.63 | 38.77 | 128.45 | 51.60 | 84.84 |
| Al3Zr | 185.96 | 85.34 | 43.13 | 202.08 | 90.00 | 125.22 |
| Al3Nb | 244.54 | 92.28 | 45.94 | 268.13 | 104.57 | 134.22 |
| 242.57 [21] | 92.85 [21] | 45.78 [21] | 266.84 [21] | 102.41 [21] | 134.03 [21] | |
| Al3La | 100.51 | 55.51 | 39.49 | 70.85 | 12.67 | 58.57 |
| Al3Hf | 192.46 | 88.32 | 48.29 | 217.76 | 92.91 | 127.96 |
| 193.60 [33] | 87.10 [33] | 47.40 [33] | 217.80 [33] | 92.40 [33] | 123.30 [33] | |
| Al3Ta | 249.05 | 88.99 | 52.89 | 274.55 | 104.96 | 130.05 |
| Species | BV | BR | B | GV | GR | G | E | B/G | Hv | |
|---|---|---|---|---|---|---|---|---|---|---|
| Al3Sc | 82.37 | 81.91 | 82.14 | 67.09 | 63.88 | 65.49 | 155.21 | 0.185 | 1.25 | 14.72 |
| 84.00 [13] | ||||||||||
| 92.1 [25] | 91.8 [25] | 91.9 [25] | 74.1 [25] | 71.7 [25] | 72.9 [25] | 173.1 [25] | 0.19 [25] | 1.26 [25] | ||
| Al3Ti | 105.25 | 105.14 | 105.19 | 91.47 | 84.71 | 88.09 | 206.60 | 0.173 | 1.19 | 19.32 |
| 107.00 [13] | ||||||||||
| 102.4 [25] | 102.2 [25] | 102.3 [25] | 81.8 [25] | 81.7 [25] | 81.8 [25] | 193.8 [25] | 0.18 [25] | 1.25 [25] | ||
| Al3V | 119.40 | 119.35 | 119.37 | 101.31 | 97.05 | 99.18 | 233.01 | 0.175 | 1.20 | 20.70 |
| 118.00 [13] | ||||||||||
| 118.32 [21] | 97.22 [21] | 228.95 [21] | 0.178 [21] | 1.217 [21] | ||||||
| Al3Y | 73.98 | 73.55 | 73.76 | 54.76 | 50.91 | 52.83 | 127.95 | 0.211 | 1.40 | 10.78 |
| Al3Zr | 101.91 | 101.61 | 101.76 | 87.87 | 80.58 | 84.23 | 198.04 | 0.176 | 1.21 | 18.45 |
| 100.8 [25] | 100.6 [25] | 100.7 [25] | 84.6 [25] | 83.3 [25] | 84.0 [25] | 197.2 [25] | 0.17 [25] | 1.20 [25] | ||
| Al3Nb | 125.06 | 124.89 | 124.98 | 106.88 | 103.25 | 105.06 | 246.20 | 0.172 | 1.19 | 21.85 |
| 124.45 [21] | 103.71 [21] | 243.50 [21] | 0.174 [21] | 1.200 [21] | ||||||
| Al3La | 60.09 | 56.78 | 58.43 | 25.94 | 18.88 | 22.41 | 59.62 | 0.330 | 2.61 | 1.02 |
| Al3Hf | 108.05 | 107.96 | 108.01 | 90.61 | 83.30 | 86.95 | 205.67 | 0.183 | 1.24 | 18.15 |
| 106.5 [25] | 106.0 [25] | 106.3 [25] | 77.8 [25] | 77.4 [25] | 77.6 [25] | 187.2 [25] | 0.21 [25] | 1.37 [25] | ||
| 107.60 [33] | 86.60 [33] | 204.80 [33] | 0.180 [33] | 1.24 [33] | ||||||
| Al3Ta | 130.22 | 130.18 | 130.21 | 109.53 | 106.85 | 108.19 | 254.17 | 0.175 | 1.20 | 21.94 |
| Species | AU | AB | AG | A1 | A2 | A3 |
|---|---|---|---|---|---|---|
| Al3Sc | 0.257 | 0.282 | 2.448 | 1.235 | 1.235 | 1.904 |
| Al3Ti | 0.400 | 0.053 | 3.838 | 1.186 | 1.186 | 2.351 |
| Al3V | 0.220 | 0.019 | 2.149 | 1.009 | 1.009 | 1.892 |
| Al3Y | 0.385 | 0.294 | 3.648 | 1.125 | 1.125 | 2.297 |
| Al3Zr | 0.455 | 0.148 | 4.328 | 1.193 | 1.193 | 2.489 |
| Al3Nb | 0.177 | 0.067 | 1.727 | 0.994 | 0.994 | 1.763 |
| Al3La | 1.927 | 2.839 | 15.745 | 0.549 | 0.549 | 2.603 |
| Al3Hf | 0.440 | 0.043 | 4.203 | 1.185 | 1.185 | 2.457 |
| Al3Ta | 0.126 | 0.015 | 1.238 | 1.005 | 1.005 | 1.625 |
| Species | B | G | E | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [100] | [010] | [001] | [100] | [010] | [001] | [100] | [010] | [001] | ||||
| Al3Sc | 266.74 | 266.74 | 212.26 | 266.74 | 78.78 | 78.78 | 67.20 | 57.33 | 130.84 | 130.84 | 128.50 | 189.79 |
| Al3Ti | 325.45 | 325.45 | 297.08 | 325.45 | 108.13 | 108.13 | 94.46 | 68.53 | 151.81 | 151.81 | 201.28 | 255.45 |
| Al3V | 364.46 | 364.46 | 345.91 | 364.46 | 112.85 | 112.85 | 99.37 | 81.44 | 188.96 | 188.96 | 245.33 | 279.07 |
| Al3Y | 240.26 | 240.26 | 189.66 | 240.26 | 64.17 | 64.17 | 51.60 | 43.06 | 102.58 | 102.58 | 112.72 | 168.70 |
| Al3Zr | 321.50 | 321.50 | 276.17 | 321.50 | 104.73 | 104.73 | 90.00 | 64.54 | 143.97 | 143.97 | 188.37 | 251.67 |
| Al3Nb | 387.46 | 387.46 | 351.47 | 387.46 | 117.56 | 117.56 | 104.57 | 88.11 | 206.57 | 206.57 | 255.60 | 292.41 |
| Al3La | 253.02 | 253.02 | 103.00 | 253.02 | 20.84 | 20.84 | 12.67 | 16.21 | 64.20 | 64.20 | 50.86 | 114.52 |
| Al3Hf | 333.26 | 333.26 | 306.61 | 333.26 | 107.66 | 107.66 | 92.91 | 66.74 | 148.61 | 148.61 | 201.15 | 257.63 |
| Al3Ta | 393.05 | 393.05 | 375.16 | 393.05 | 116.16 | 116.16 | 104.96 | 90.85 | 212.90 | 212.90 | 257.95 | 285.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Sun, F.; Liu, H.; Ren, X.; Xu, H.; Wang, M.; Fu, Y. Exploration of D022-Type Al3TM(TM = Sc, Ti, V, Zr, Nb, Hf, Ta): Elastic Anisotropy, Electronic Structures, Work Function and Experimental Design. Materials 2021, 14, 2206. https://doi.org/10.3390/ma14092206
Zhang G, Sun F, Liu H, Ren X, Xu H, Wang M, Fu Y. Exploration of D022-Type Al3TM(TM = Sc, Ti, V, Zr, Nb, Hf, Ta): Elastic Anisotropy, Electronic Structures, Work Function and Experimental Design. Materials. 2021; 14(9):2206. https://doi.org/10.3390/ma14092206
Chicago/Turabian StyleZhang, Guowei, Fenger Sun, Heping Liu, Xiaoyan Ren, Hong Xu, Mingjie Wang, and Yizheng Fu. 2021. "Exploration of D022-Type Al3TM(TM = Sc, Ti, V, Zr, Nb, Hf, Ta): Elastic Anisotropy, Electronic Structures, Work Function and Experimental Design" Materials 14, no. 9: 2206. https://doi.org/10.3390/ma14092206
APA StyleZhang, G., Sun, F., Liu, H., Ren, X., Xu, H., Wang, M., & Fu, Y. (2021). Exploration of D022-Type Al3TM(TM = Sc, Ti, V, Zr, Nb, Hf, Ta): Elastic Anisotropy, Electronic Structures, Work Function and Experimental Design. Materials, 14(9), 2206. https://doi.org/10.3390/ma14092206






