Abstract
Research efforts have been focused on developing multivalent ion batteries because they hold great promise and could be a major advancement in energy storage, since two or three times more charge per ion can be transferred as compared with lithium. However, their application is limited because of the lack of suitable cathode materials to reversibly intercalate multivalent ions. From that perspective, vanadium pentoxide is a promising cathode material because of its low toxicity, ease of synthesis, and layered structure, which provides huge possibilities for the development of energy storage devices. In this mini review, the general strategies required for the improvement of reversibility, capacity value, and stability of the cathodes is presented. The role of nanostructural morphologies, structure, and composites on the performance of vanadium pentoxide in the last five years is addressed. Finally, perspectives on future directions of the cathodes are proposed.
1. Introduction
The imminent global energy crisis, with increasing consumption of fossil energy and growing ecological concerns related to the greenhouse gases emitted into the atmosphere, has led to the intensive development of new energy technologies to secure energy supply for the future. To address the rapid depletion of fossil fuels and meet the growing demand for green energies (i.e., renewable energy sources, such as solar and wind energy), an effective medium to store and transfer energy is required. Thus, the need for clean and efficient energy storage has moved to the center of attention.
Among them, organic lithium ion batteries are widely used in portable devices due to their high energy density, cycle stability, and energy efficiency, leading to the unbelievable development of smartphones, personal computers, and smart grids. However, their enlargement for the electrical grid is hindered by the safety and cost, mainly of organic electrolyte and electrode material [1]. Taking this into consideration, aqueous lithium ion batteries are promising alternatives not only in the field of grid-scale energy storage, but also for wearable applications [2]. In the last case, it was found that a flexible LiVPO4F material can act as both cathode and anode in a “water-in-salt” gel polymer electrolyte exhibiting energy density of 141 Wh kg−1, with power density of 20,600 W kg−1, and output voltage of 2.4 V during >4000 cycles. The use of aqueous electrolytes offers tremendous competitiveness in terms of low cost, environmental benignity, and high tolerance against electrical and mechanical mishandling [3]. Nevertheless, they suffer from a limited operating window, which is <2 V due to water electrolysis. It has been indicated that this can be extended up to 3–4 V through “water-in-salt” electrolytes [2] and hydrate-melt electrolytes [4].
Lithium ion batteries may deliver both high power and energy density, but the limited lithium resource, and increased cost, safety, and environmental issues cannot be neglected [5]. From that perspective, multivalent batteries tend to increase the energy density, since multivalent ions can transfer two or three electrons per ion [6].
Among the multivalent batteries, the magnesium ion battery (MIB) is the most studied, because magnesium metal is considered an ideal anode material due to its low reduction potential (−2.37 V), low price, and high abundance in the earth’s crust [7]. Aqueous magnesium ion batteries exhibit high ionic conductivity, absence of magnesium oxide formation, and fast mobility of magnesium ions in water solution [8]. Nevertheless, in some cases the diffusion process in cathode materials is observed to be slow due to the strong electrostatic interaction between the Mg2+ and the host material, resulting in the absence of high-performance electrode materials [9].
Zinc batteries have also attracted attention because they present high theoretical capacity (820 mAh g−1), low electrochemical potential, two-electron transfer during the redox reactions leading to a high energy density, high natural abundance, low toxicity, and intrinsic safety [10,11]. Issues of dendritic zinc have almost been sorted, replacing the alkaline electrolyte with mild neutral pH.
On the other hand, aluminum ion batteries can theoretically provide three times more charge per transferred ion as compared to lithium. If successful, multivalent systems such as aluminum could be a major advancement in the storage of energy due to the high theoretical specific capacity of 2.98 Ah g−1, which is the second highest compared with Li (3.86 Ah g−1), and much higher than magnesium (2.20 Ah g−1) and zinc (0.82 Ah g−1) [12]. Besides, it suffers from corrosion in aqueous electrolytes, which leads to the formation of a passive surface layer on the electrode, reducing the material’s efficiency. In addition, it is hard to develop aqueous aluminum ion batteries due to the low reduction potential of Al3+ (~1.68 V vs. standard hydrogen electrode, which is lower than H+) in aqueous solutions. Recently, Pan et al. reported a novel aqueous cell consisting of Al metal and graphite as electrodes, and “water-in-salt” high concentration aqueous AlCl3 solution as electrolyte [13]. It reached a capacity of 165 mAh g−1 at 500 mA g−1, exhibiting 95% coulombic efficiency over 1000 cycles.
Nevertheless, the availability of cathode materials for Mg2+/Al3+ ions is limited, owing to the numerous interactions within the lattice of the host material and with other species present in the electrolyte, making the diffusion of such high-charge-density ions slow [14,15]. Regarding the Zn-ion batteries, there are still challenges to overcome. The greater positive charge and the slow mobility and kinetics during the intercalation process imply that identifying suitable cathodes for aqueous Zn-ion batteries is difficult compared with Li-ion batteries. Critical characteristics, such as specific capacity and cycling stability, are primarily determined by the intrinsic electrochemical properties of electrode materials, and especially cathode materials, which to a great extent determine the energy density of a battery [16]. However, the research on aqueous batteries is in continuous progress in the development of high-energy-density cathode materials. In this review, we will highlight the progressive advancements in developing vanadium pentoxide (V2O5) cathodes with their respective performances for Mg-, Zn-, and Al-ion aqueous batteries in the last five years. There are numerous advantages of V2O5 as a cathode material. It has low cost, high abundance, ease of synthesis, low toxicity, and high theoretical capacity (~437 mAh g−1) compared with LiCoO2 (~274 mAh g−1) [17]. Furthermore, it consists of layers that hold together by van der Waals interactions, giving excellent possibilities for the development of energy storage devices. Finally, we aim to underline the future prospects of the cathode.
2. Storage Mechanism
For storage systems based on Mg and Al, the intercalation/deintercalation process is based on three mechanisms: intercalation, alloying, and conversion [3]. Among the three mechanisms, intercalation holds the credit in the majority of batteries. In a typical electrochemical intercalation process, the mobile ions enter into the lattice structure of the host. Regarding the Zn-ion batteries, the reaction mechanisms are complex and unclear. However, based on the literature, the following four main reaction mechanisms have been proposed [18]: reversible Zn2+ insertion/extraction, chemical conversion reactions, reversible Zn2+ and H+ co-intercalation/deintercalation, and the dissolution-deposition reaction mechanism.
Cathodes undergo both electronic and ionic transfers in the electrode/electrolyte interface and the electrode bulks during the process. In the case of multivalent cations, the diffusion suffers from slow kinetics due to the high electrostatic interactions when compared to monovalent ions [6]. Among Mg2+ and Zn2+, Al+3 presents more kinetic issues [19] due to the following:
- (1)
- Strong electrostatic interactions.
- (2)
- Lack of feasibility to tolerate massive electrons injection resulting in lattice structural changes.
- (3)
- Stronger salvation and bonding issues taking place as compared with Mg2+ and Zn2+.
- (4)
- Proceeding of the electrochemical reactions, along with conversion path driven by thermodynamics and low reversibility.
3. Vanadium Oxides
Vanadium-based compounds exhibit a range of oxidation states, including V5+, V4+, V3+, and V2+, making them feasible to composite with many other anions and cations to form vanadium oxides, vanadium carbides, vanadium nitrides, vanadium sulphides, vanadium phosphates, and metal vanadates. Among them, vanadium oxides have attracted interest for energy storage in the past decades. Due to the distortion of V-O coordination polyhedral and the conversion of diverse oxidation states, vanadium oxide compounds present higher specific capacity compared with the other vanadium-based materials when they are used as electrodes [20]. The most common phases are orthorhombic V2O5, bilayered V2O5·nH2O, VO2, V3O7·H2O, V6O13, and V2O3. Orthorhombic V2O5 and bilayered V2O5·nH2O possess layered structures with open ion diffusion channels between layers [21], strengthening the electrochemical performance. Nevertheless, they show low stability in aqueous electrolytes, poor conductivity, and a low ionic diffusion coefficient, which consequently results in poor long-term cycling performance. In the following sections, the general strategies undertaken to improve the cathode performance are indicated, and the structure–performance relationship as reported in the last five years is discussed.
3.1. Approaches to Improve the Electrochemical Performance of V2O5
3.1.1. Nanostructure Engineering
Nanostructure engineering is a promising approach to enhance the electrochemical performance of cathode materials, dealing with all aspects of design and structures on the nanoscale. Nanostructured electrode materials (tens of nanometers) offer large surface area, more active sites, and short electron/ion transport paths, which is beneficial to accelerate reaction kinetics enabling high rate capability. The nano-sized hollow structure cannot only alleviate the structural stress upon cycling, but it can also provide shortened ion and electron transport paths, and enhance the surface capacitive behavior. V2O5 nanospheres with a diameter of about 450 nm and shell thickness of 50 nm delivered ultrahigh reversible capacity (327 mAh g−1 at 0.1 A g−1), and excellent rate (146 mAh g−1 at 20 A g−1) and cycling behavior (147 mAh g−1 after 6000 cycles at 10 A g−1; 122 mAh g−1 after 10,000 cycles at 15 A g−1), indicating superior performance as compared with commercial V2O5, when applied for aqueous zinc ion batteries [22]. In contrast with aqueous zinc ion batteries, there are more recent reports on aqueous lithium ion batteries (i.e., mesoporous nanoflowers [23] and triple hollow shell [24] V2O5 structures), giving motivation to researchers to work more intensively on multivalent systems.
3.1.2. Structural Stability Enhancement
The cycling stability of the cathode is affected by irreversible phase changes due to structural degradation. It has been proved that preintercalation of guest species such as Li ions, water molecules, and organic molecules can stabilize the host structure and enhance the ions’ diffusion [24,25]. In particular, LixV2O5·nH2O had a larger spacing of 13.77 A and higher diffusion coefficient of Zn2+ than in V2O5·nH2O [26]. In that case, the enlarged layer spacing and fast Zn2+ diffusion favors the rate capacity and cycling performance, reaching 192 mAh g−1 after 1000 cycles at 10 A g−1.
3.1.3. Surface Coating
The surface coating acts as a protective layer on the electrode surface, which can prevent volume changes and suppress the dissolution of active materials. In that case, graphene is a promising candidate to improve the electronic conductivity and inhibit dissolution. The exceptional conductivity, abundant adsorption sites, and short diffusion paths are expected to enhance the transport processes within the oxide nanomaterials. A major advantage of graphene over other carbon materials, such as graphite and carbon nanotubes, is the presence of oxygen-containing groups on the edges and surfaces, which strongly influence the size, shape, and distribution of metal oxide nanostructures on graphene.
3.1.4. Formation of Solid Electrolyte Interface Film
Solid electrolyte interface (SEI) has been proved to effectively prevent the dissolution of electrode materials [27]. For instance, Zhou et al. discovered that CaSO4·2H2O protects manganese from dissolution in the case of Ca2MnO4, decreases impedance, lowers activation energy, and facilitates the intercalation/deintercalation of zinc ions without fluctuations of discharge capacity after 1000 cycles at 1 A g−1 [28]. This approach could also be applied for V2O5 cathode.
4. Multivalent Ion Properties
4.1. Magnesium Ion Intercalation Properties
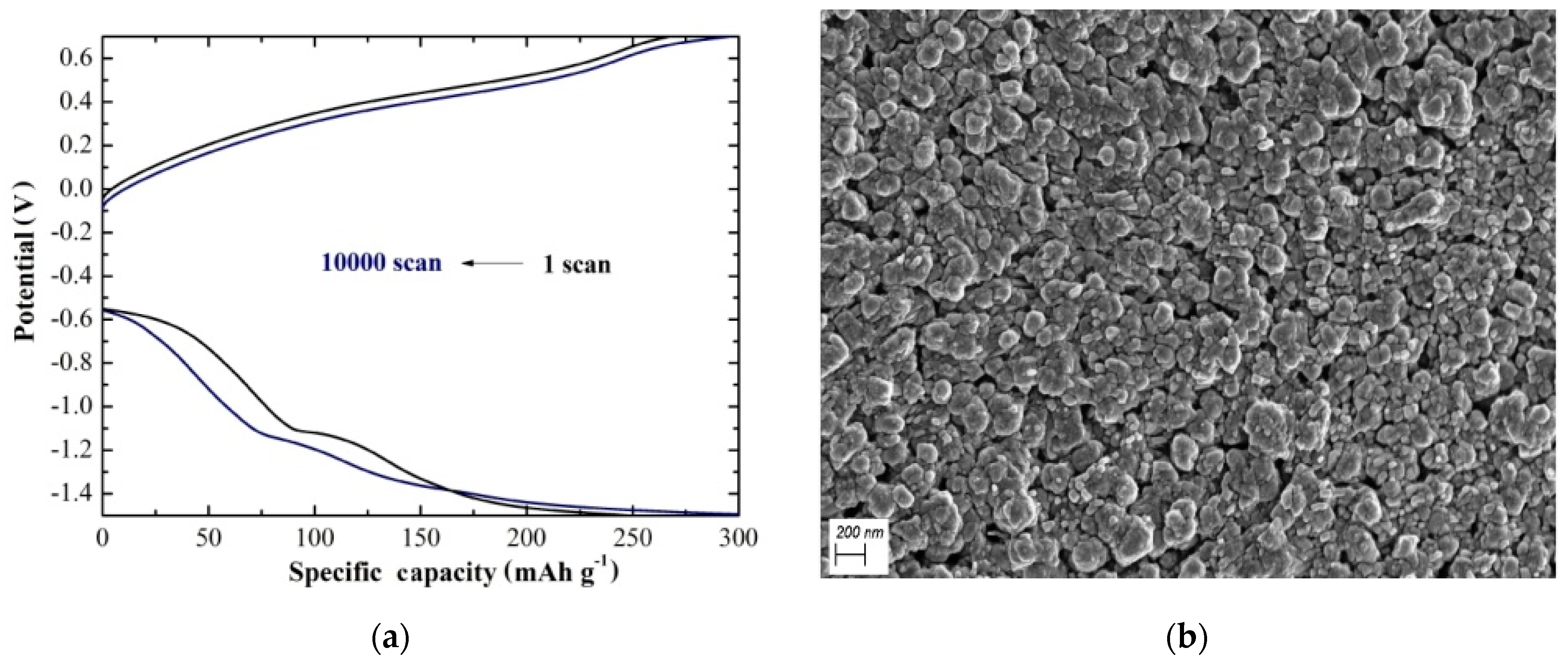
The strong electrostatic force between Mg2+ and V2O5 is one of the major challenges for V2O5 as cathode material for magnesium ion batteries. An efficient approach to solve this problem is the co-intercalation of water. For instance, in the case of aqueous MgCl2 electrolyte solution, a high magnesium storage capacity of 427 mAh g−1 was estimated for aerosol-assisted chemical-vapor-deposited (AACVD) V2O5 at 500 °C [29]. Further increasing the substrate temperature to 600 °C, the capacity retention was increased to 92% after 10,000 scans, with coulombic efficiency of 100%, noble structural stability, and high reversibility [30] (Figure 1a). Studying this result, one needs to highlight the significance of AACVD to grow strongly adhered V2O5 coatings with good conformal coverage, optimized materials, and inexpensive up-scale. In addition, the temperature increase resulted in the co-existence of α-V2O5 and β-V2O5 with a compact structure of well-distributed grains (Figure 1b) as compared with α-V2O5 grown at 500 °C, which was the reason for the electrochemical enhancement. This cathode needs to be further tested in a cell to evaluate its respective performance. Sa et al. studied the magnesium storage performance of V2O5 in 1 M, magnesium bis(trifluoromethane sulfonyl)imide electrolyte, varying the water content [31]. It was found that with 2600 ppm water in electrolyte, the V2O5 cathode achieved a capacity of approximately 260 mAh g−1. Nevertheless, there was no evidence for reversible Mg plating/stripping limiting the use of Mg metal anode. In this work, it was also indicated that the proton involvement in the capacity value is of vital importance and should be considered with caution for future studies of possible cathode materials.
Figure 1.
(a) Specific capacity for the AACVD V2O5 at 600 °C at a potential ranging from −1.5 V to +0.7 V under constant specific current of 15 A g−1 for 1 and 10,000 scans. (b) FE-SEM image of the same material at ×50,000 magnification.
4.2. Zinc Ion Intercalation Properties
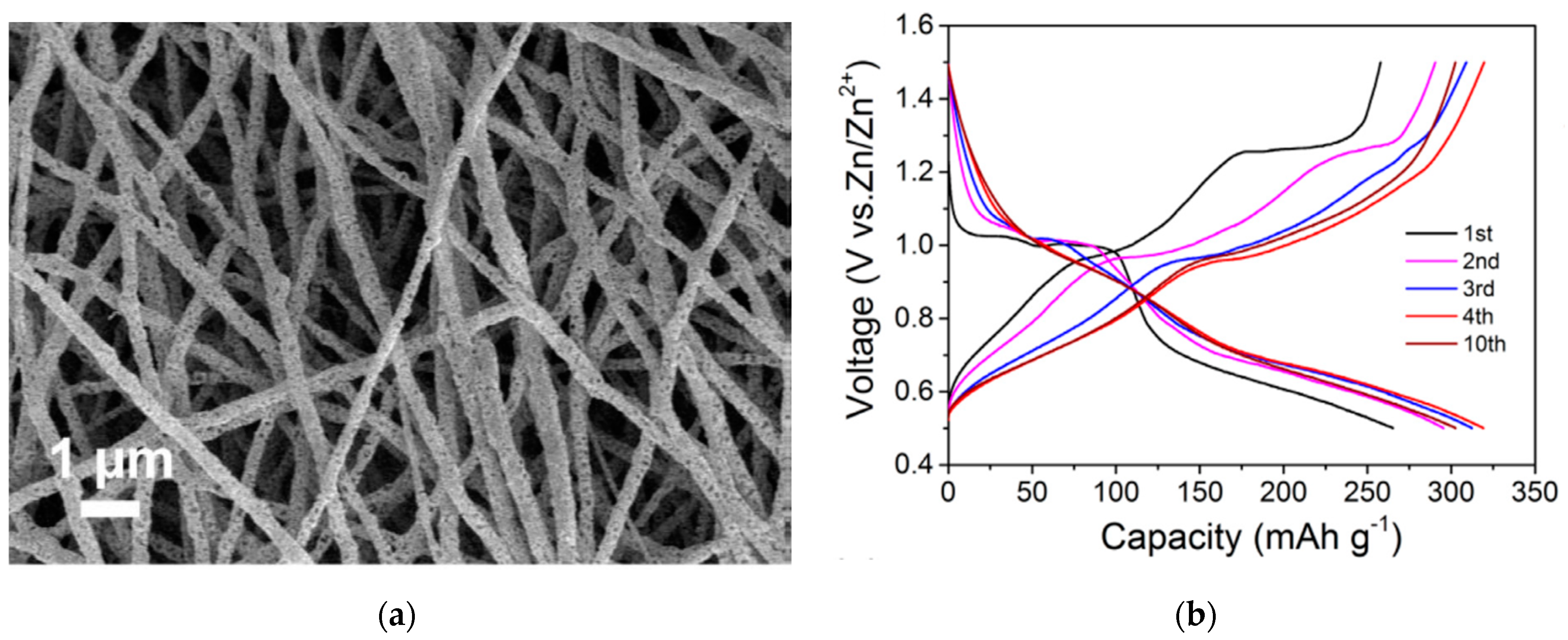
V2O5 was also investigated in aqueous zinc ion batteries. In this direction, Hu et al. developed an aqueous hybrid-ion battery using a mixed Zn ions/Li ions electrolyte and metallic Zn as anode [32]. In this work, it was shown that both ions can be intercalated into the porous V2O5 structure, but with a dominance of Li ions. Such a battery reached a discharge capacity of 238 mAh g−1 and capacity retention of 80% for 2000 cycles. This study presented the employment of “water-in-salt” electrolyte to effectively boost the energy density and the cyclability of the system. Layered Mg2+-intercalated V2O5 was reported to deliver high capacities of 353 and 264 mAh g−1 at 100 and 1000 mA g−1, along with long-term durability utilizing Zn foil as anode. The large radius of hydrated Mg2+ resulted in an interlayer spacing as large as 13.4 Å, which allowed efficient zinc ion intercalation/deintercalation [33]. Porous V2O5 nanofibers (Figure 2a) with high Zn-storage performance in an aqueous electrolyte of Zn(CF3SO3)2 and Zn foil as anode were reported in [34], enabling a high reversible capacity of 319 mAh g−1 at 20 mA g−1 (Figure 2b) and a capacity retention of 81% over 500 cycles. This work proposed a phase transition from orthorhombic V2O5 to zinc pyrovanadate on the first cycle, resulting in open-structured hosts enabling the particular performance.
Figure 2.
(a) SEM image of as-prepared V2O5 nanofibers. (b) Galvanostatic discharge/charge profiles at 20 mA g−1 [34].
It was also shown that the pre-intercalation of transition metal ions such as Fe2+, Co2+, Ni2+, Mn2+, Zn2+, and Cu2+ into the interlayer of V2O5 with metallic Zn as anode exhibited improved capacities, as well as excellent rate capability and cyclic stability in a temperature range of 0–50 °C. This enhancement is due to the enlarged interlayer spacing and electrical conductivity [35]. Another interesting work is related to V2O5 nanopaper, consisting of V2O5 nanofibers and carbon nanotubes, along with Zn metal pellet with a thickness of 0.35 mm as the anode, as reversible Zn ion batteries, with a high capacity of 375 mAh g−1 and long cycle life up to 500 cycles [36]. The enhanced behavior is due to the spaces between the oxide layers, which serve as diffusion pathways for Zn ions. In addition, the nanofiber morphology provided a short diffusion distance tolerating the high volume change. Qin et al. reported that V2O5 hollow spheres displayed a high specific discharge capacity of 132 mAh g−1, with capacity retention of 82.5% after 6200 cycles at the current density of 10 A g−1 utilizing Zn metal anode [37]. This performance is better than the one reported for commercial V2O5 due to the hollow sphere structure and the high surface area that can buffer the volume expansion of V2O5 and improve the cycling behavior of the material. The as-fabricated V2O5 @PEDOT/CC electrode displayed a maximum capacity of 360 mAh g−1 at 0.1 A g−1 and a high rate capability, with a specific capacity of 232 mAh g−1 at a large current density of 20 A g−1 utilizing a Zn foil of 0.8 mm [38]. This storage performance resulted from the synergistic effects of the V2O5 nanosheet arrays providing enough Zn storage active sites and the PEDOT coating shell increasing Zn ion/electron transport kinetics, which further acted as a protective layer to restrain structural collapse during cycling. Wang et al. reported 2D amorphous V2O5/graphene heterostructures with high capacity of 447 mAh g−1 at 0.3 A g−1, high rate capability of 202 mAh g−1 at 30 A g−1, and excellent cycle life of 20,000 cycles at 30 A g−1 utilizing Zn foil as anode, due to the unique features and the synergistic effect of the materials [39].
4.3. Aluminium Ion Intercalation Properties
The first stable aluminum ion batteries based on V2O5 nanowires with extended life were reported in 2011 by Jayaprakash et al., exhibiting a discharge capacity of 305 mAh g−1 in the 1st cycle and 273 mAh g−1 in the 20th cycle at 125 mA g−1 utilizing Al metal as anode [40]. The energy density of this first aluminum ion battery began the search for materials that would result in sustained improvements. Following this effort, amorphous V2O5/C composite was synthesized as a cathode for aluminum ion batteries, utilizing 250 mg of vanadium powder that was dissolved into 25 mL of 30 wt.% hydrogen peroxide solution in an ice bath, followed by the addition of 750 mg Ketjen black in the solution [41]. The morphological analysis indicated that the spherical Ketjen black particle surface was uniformly covered by V2O5. The composite was measured in glass cells with Mo plate as the current collector [41]. At a current density of 11 mA g−1, the composite exhibited a discharge capacity of 200 mA g−1 in the first cycle and about 70 mAh g−1 in the 30th cycle. The reversible capacity of xerogel V2O5 was estimated to be 120 mAh g−1 [42], with higher capacity values at lower scan rates. The loss of crystallinity occurring during the electrochemical intercalation enhanced the chemical exchange between the electrode material and the electrolyte solution.
Recently, the reversible proton intercalation/deintercalation chemistry in aqueous aluminum electrochemical cells was investigated for orthorhombic V2O5 cathode and Al anode exhibiting a capacity of approximately 200 mAh g−1 with high reversibility over 50 cycles. In this work, the cathode coatings composed of cation selective membranes prevented anion cross-over in aqueous electrolytes, enhancing the cathode reversibility [43].
5. Conclusions
In this mini review, we presented strategic approaches to provide cathode V2O5 materials of unique morphologies and crystal structures with high electrochemical performance for aqueous multivalent ion batteries. The development of V2O5 cathodes for Mg-, Zn-, and Al-ion batteries has been addressed, indicating that there are still challenges to overcome.
First, the incorporation of graphene is known to optimize the electrical conductivity of electrode materials. Nevertheless, there is no study reported on the effects of surface modification on the electrochemical properties of V2O5 for aqueous magnesium ion batteries. It will be interesting to see if the interfacial properties of the cathode can be enhanced as a consequence of the Mg2+ diffusion.
Second, the superconcentration strategy would be useful to apply to the expansion of the electrochemical stability window to 2.0 V and to examine if the energy density can be increased, keeping a high rate capability more intensively in all multivalent systems.
Third, the key challenge for the optimization and discovery of new materials and structures, we believe, lies in the definition of the desired performance goals to find the structure, composition, and the electrode–electrolyte interfaces that best fulfill these targets, without a priori defining the starting materials.
Forth, finding a growth process with excellent control over the structure and morphology with up-scaling capabilities is an issue that needs to be resolved for applications in the electromotive field. There is little work on CVD, but more work needs to be done. Limitation for scaling up results can be overcome through computational fluid dynamics studies of materials’ properties in numerous tests and environments to maintain optimum performance of the cathode per surface area.
Finally, another matter for consideration is the maintenance of the electrodes’ performance after the assembly and during the function of aqueous batteries under real conditions. In this case, their potential at the prototype level will initially need to be proved, and then the feasibility of their up-scaling to the industrial process level will need to be assessed. This information will be useful for the necessary steps required between the creation of innovative materials and the design of the battery.
Funding
This research received no funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
MDPI Research Data Policies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Delaporte, N.; Lajoie, G.; Collin-Martin, S.; Zaghib, K. Toward low-cost all-organic and biodegradable Li-ion batteries. Sci. Rep. 2020, 10, 3812. [Google Scholar] [CrossRef]
- Yang, C.; Ji, X.; Fan, X.; Gao, T.; Suo, L.; Wang, F.; Sun, W.; Chen, J.; Chen, L.; Han, F. Flexible aqueous Li-ion battery with high energy and power densities. Adv. Mater. 2017, 29, 1701972. [Google Scholar] [CrossRef] [PubMed]
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Sodeyama, K.; Ko, S.; Tateyama, Y.; Yamada, A. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 2016, 1, 16129. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Canepa, P.; Sai Gautam, G.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of multivalent cathode materials: Open questions and future challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef]
- Chen, L.; Bao, J.L.; Dong, X.; Truhlar, D.G.; Wang, Y.; Wang, C.; Xia, Y. Aqueous Mg-ion battery based on polyimide anode and prussian blue cathode. ACS Energy Lett. 2017, 2, 1115–1121. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, D.; Bai, X. High rate performance of aqueous magnesium-ion batteries based on the δ-MnO2@carbon molecular sieves composite as the cathode and nanowire VO2 as the anode. J. Power Source 2019, 444, 227299. [Google Scholar] [CrossRef]
- Wang, F.; Fan, X.; Gao, T.; Sun, W.; Ma, Z.; Yang, C.; Han, F.; Xu, K.; Wang, C. High-voltage aqueous magnesium ion batteries. ACS Cent. Sci. 2017, 3, 1121–1128. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, H.; Xu, W.; Wang, W.; Li, X. A long cycle life, self-healing zinc-iodine flow battry with high power density. Angew. Chem. 2018, 130, 11341–11346. [Google Scholar] [CrossRef]
- Mori, R. Electrochemical properties of a rechargeable aluminium-air battery with a metal-oraganic framework as air cathode material. RSC Adv. 2017, 7, 6389–6395. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Y.; Zhang, Y.; Kwok, H.Y.K.; Wu, M.; Zhao, X.; Leung, D.Y.C. A low-cost and dendrite-free rechargeable aluminium-ion battery with superior performance. J. Mater. Chem. 2019, 7, 17420–17425. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Nann, T. Trends in aluminium-based intercalation batteries. Adv. Energy Mater. 2017, 7, 1602093. [Google Scholar] [CrossRef]
- Sun, X.; Bonnick, P.; Nazar, L.F. Layered TiS2 positive electrode for Mg batteries. ACS Energy Lett. 2016, 1, 297–301. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.-L.; Dou, S.-X. Recent progress of layered transition metal oxide cathodes for sodium-ion batteries. Small 2019, 15, 1805381. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, J.; Yang, H.; Zhou, K.; Pan, D. V2O5-based nanomaterials: Synthesis and their applications. RSC Adv. 2018, 8, 4014–4031. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J. Recent advances in cathode materials of rechargeable aqueous zinc-ion batteries. Mater. Today Adv. 2020, 7, 100078. [Google Scholar] [CrossRef]
- Wu, F.; Yang, H.; Bai, Y.; Wu, C. Paving the path toward reliable cathode materials for aluminium-ion batteries. Adv. Mater. 2019, 31, 1806510. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Wu, L.; Xiong, F.; Pei, F.; An, Q.; Mai, L. Vanadium-based cathode materials for rechargeable multivalent batteries: Challenges and opportunities. Electrochem. Energy Rev. 2018, 1, 169–199. [Google Scholar] [CrossRef]
- Xu, X.; Xiong, F.; Meng, J.; Wang, X.; Niu, C.; An, Q.; Mai, L. Vanadium-based nanomaterials: A promising family for emerging metal-ion batteries. Adv. Funct. Mater. 2020, 30, 1904398. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Cui, F.; Meng, J.; Chen, H.; Zheng, X. Enhanced rate and cycling performances of hollow V2O5 nanospheres for aqueous zinc ion battery cathode. Appl. Surf. Sci. 2010, 507, 145137. [Google Scholar] [CrossRef]
- Karthik, K.; Pradeeswari, K.; Mohan Kumar, R.; Murugesan, R. Microwave-assisted V2O5 nanoflowers for efficient lithium-ion battery. Mater. Res. Innov. 2019, 24, 1. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Al-Mamun, M.; Dou, Y.; Liu, P.; Su, D.; Wang, G.; Zhang, S.; Wang, D.; Zhao, H. A hollow-shell structured V2O5 electrode-based symmetric full Li-ion battery with highest capacity. Adv. Energy Mater. 2019, 9, 1900909. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly stable aqueous zinc-ion storage using a layered calcium vanadium oxide bronze cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Fang, G.; Shan, L.; Guo, J.; Zhang, W.; Wang, C.; Wang, L.; Zhou, J.; Liang, S. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- Suo, L.; Oh, D.; Lin, Y.; Zhuo, Z.; Borodin, O.; Gao, T.; Wang, F.; Kushima, A.; Wang, Z.; Kim, H.C.; et al. How solid-electrolyte interphase forms in aqueous electrolytes. J. Am. Chem. Soc. 2017, 139, 18670–18680. [Google Scholar] [CrossRef]
- Guo, S.; Liang, S.; Zhang, B.; Fang, G.; Ma, D.; Zhou, J. Cathode interfacial layer formation via in situ electrochemically charging in aqueous zinc-ion battery. ACS Nano 2019, 13, 13456–13464. [Google Scholar] [CrossRef] [PubMed]
- Drosos, C.; Jia, C.; Mathew, S.; Palgrave, R.G.; Moss, B.; Kafizas, A.; Vernardou, D. Aerosol-assisted chemical vapor deposition of V2O5 cathodes with high rate capabilities for magnesium-ion batteries. J. Power Source 2018, 384, 355–359. [Google Scholar] [CrossRef]
- Drosos, C.; Moss, B.; Kafizas, A.; Vernardou, D. V2O5 as magnesium cathode material with extended cyclic stability. J. Electrochem. Sci. Eng. 2020, 10, 257–262. [Google Scholar] [CrossRef]
- Sa, N.; Wang, H.; Proffit, D.L.; Lipson, A.L.; Key, B.; Liu, M.; Feng, Z.; Fister, T.T.; Ren, Y.; Sun, C.-J.; et al. Is alpha-V2O5 a cathode material for Mg insertion batteries? J. Power Source 2016, 323, 44–50. [Google Scholar] [CrossRef]
- Hu, P.; Yan, M.; Zhu, T.; Wang, X.; Wei, X.; Li, J.; Zhou, L.; Li, Z.; Chen, L.; Mai, L. Zn/V2O5 aqueous hybrid-ion battery with high voltage platform and long cycle life. ACS Appl. Mater. Interfaces 2017, 9, 42717–42722. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Kandambeth, S.; Eddaoudi, M.; Alshareef, H.N. Layered MgxV2O5·nH2O as cathode material for high-performance aqueous zinc ion batteries. ACS Energy Lett. 2018, 3, 2602–2609. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, H.; Cheng, F.; Chen, J. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J. Energy Chem. 2019, 38, 20–25. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Liang, S.; Wu, Z.; Fang, G.; Cao, X.; Wang, C.; Lin, T.; Pan, A.; Zhou, J. Transition metal ion-preintercalated V2O5 as high-performance aqueous zinc-ion battery cathode with broad temperature adaptibility. Nano Energy 2019, 61, 617–625. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Lalambate, P.K.; Zhong, Y.; Huang, Z.; Xie, M.; Shen, Y.; Huang, Y. V2O5 nanopaper as a cathode material with high capacity and long cycle life for rechargeable aqueous zinc-ion battery. Nano Energy 2019, 60, 752–759. [Google Scholar] [CrossRef]
- Qin, H.; Chen, L.; Wang, L.; Chen, X.; Yang, Z. V2O5 hollow spheres as high rate and long life cathode for aqueous rechargeable zinc ion batteries. Electrochim. Acta 2019, 306, 307–316. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Li, F.; Guan, Z.; Wang, R.; He, B.; Gong, Y.; Hu, X. Conformal conducting polymer shells on V2O5 nanosheet arrays as a high-rate and stable zinc-ion battery cathode. Adv. Mater. Interfaces 2018, 6, 1801506. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Das, P.; Zheng, S.; Zhou, F.; Wu, Z.-S. Layer-by-layer stacked amorphous V2O5/Graphene 2D heterostructures with strong-coupling effect for high-capacity aqueous zinc-ion batteries with ultra-long cycle life. Energy Storage Mater. 2020, 31, 156–163. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Das, S.K.; Archer, L.A. The rechargeable aluminium-ion battery. Chem. Commun. 2011, 47, 12610–12612. [Google Scholar] [CrossRef]
- Chiku, M.; Takeda, H.; Matsumura, S.; Higuchi, E.; Inoue, H. Amorphous vanadium oxide/carbon composite positive electrode for rechargeable aluminium battery. ACS Appl. Mater. Interfaces 2015, 7, 24385–24389. [Google Scholar] [CrossRef] [PubMed]
- González, J.R.; Nacimiento, F.; Cabello, M.; Alcántara, R.; Lavela, P.; Tirado, J.L. Reversible intercalation of aluminium into vanadium pentoxide xerogel for aqueous rechargeable batteries. RSC Adv. 2016, 6, 62157–62164. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, L.; Yin, J.; Zheng, J.; Zhang, D.; Chen, J.; Archer, L.A. Proton intercalation/de-intercalation dynamics in vanadium oxides for aqueous aluminium electrochemical cells. Angew. Chem. Int. Ed. 2019, 59, 3048–3052. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).