Single-Crystal Diamond Needle Fabrication Using Hot-Filament Chemical Vapor Deposition
Abstract
1. Introduction
2. Materials and Methods
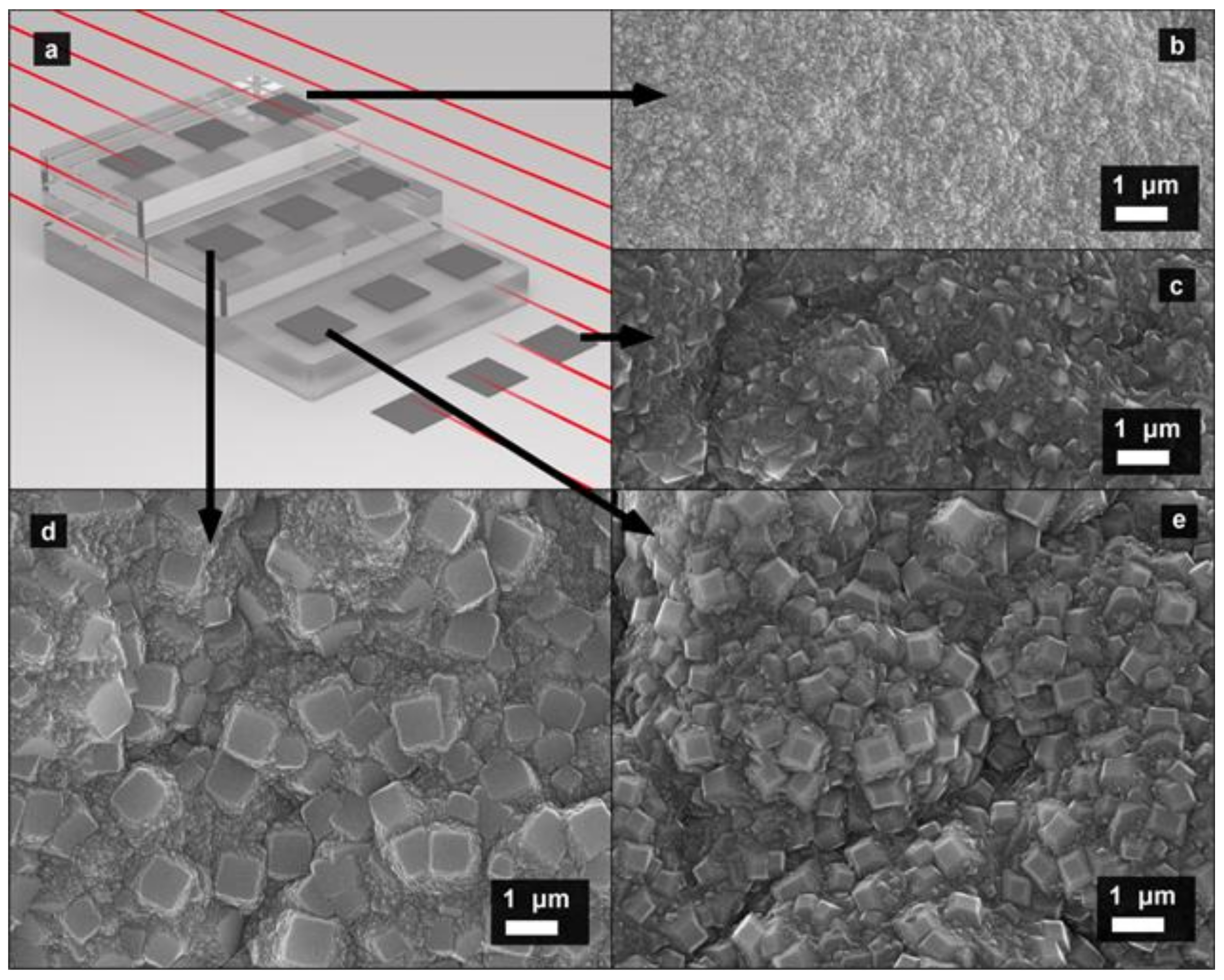
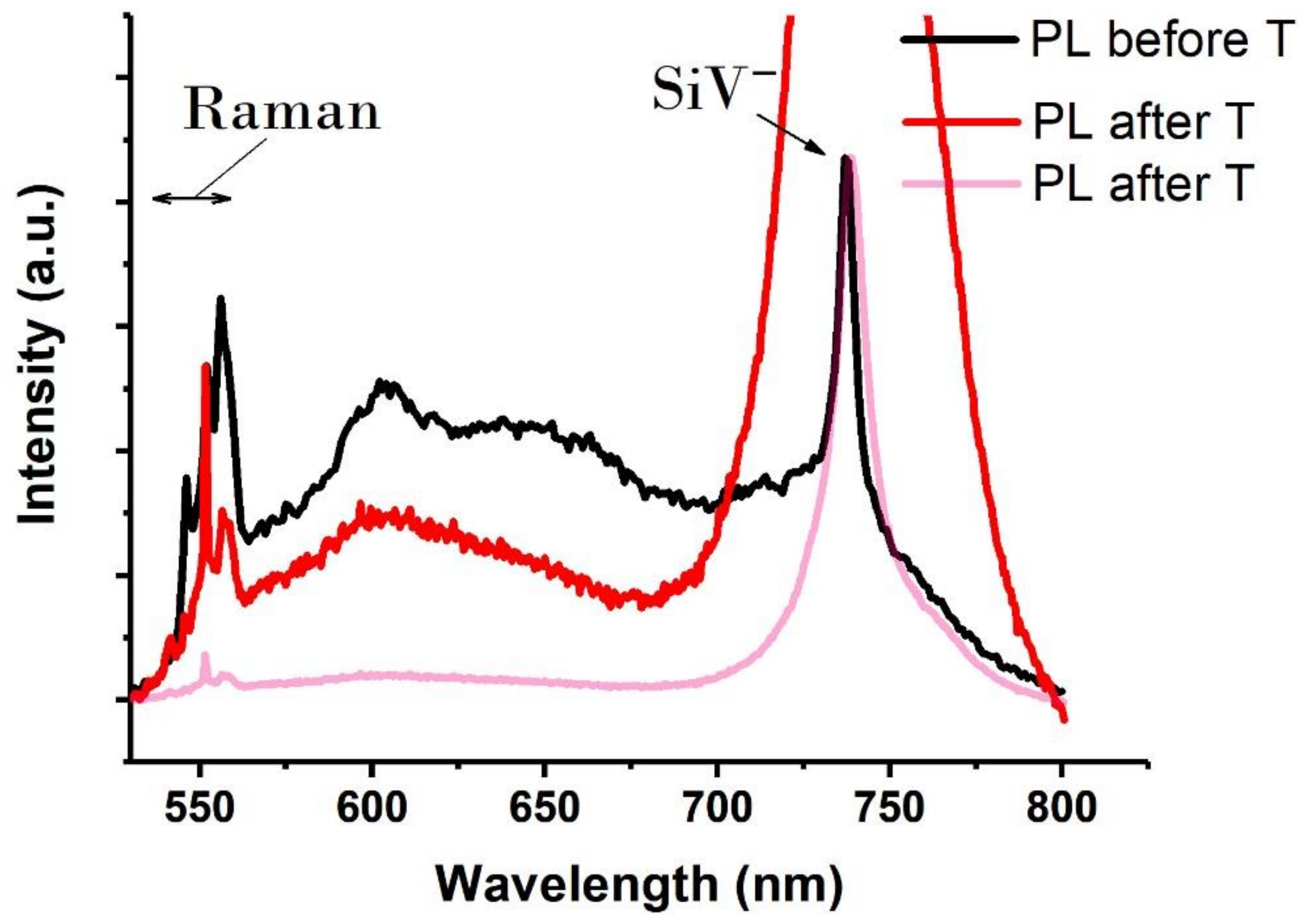
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wrachtrup, J.; Jelezko, F. Processing quantum information in diamond. J. Phys. Condens. Matter 2006, 18, S807–S824. [Google Scholar] [CrossRef]
- Shenderova, O.; Gruen, D. Ultrananocrystalline Diamond: Synthesis, Properties, and Applications, 2nd ed.; William Andrew: New York, NY, USA, 2012; pp. 20–584. [Google Scholar]
- Schmitt, S.; Gefen, T.; Stürner, F.M.; Unden, T.; Wolff, G.; Müller, C.; Scheuer, J.; Naydenov, B.; Markham, M.; Pezzagna, S.; et al. Submillihertz magnetic spectroscopy performed with a nanoscale quantum sensor. Science 2017, 356, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Mrozek, M.; Schabikowski, M.; Mitura-Nowak, M.; Lekki, J.; Marszałek, M.; Wojciechowski, A.; Gawlik, W. Nitrogen-vacancy color centers created by proton implantation in a diamond. Materials 2021, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.; Dutta, M.; Koeck, F.A.; Benipal, M.; Brown, J.; Fox, B.; Hathwar, R.; Johnson, H.; Malakoutian, M.; Saremi, M.; et al. A 4.5 μm PIN diamond diode for detecting slow neutrons. Nuc. Instrum. Meth. A 2018, 903, 297–301. [Google Scholar] [CrossRef]
- Dutta, M.; Mandal, S.; Hathwar, R.; Fisher, A.; Koeck, F.; Nemanich, R.; Goodnick, S.; Chowdhury, S. Determination of minority carrier lifetime of holes in diamond p-i-n diodes using reverse recovery method. IEEE Electron. Device Lett. 2018, 39, 552–555. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; Van De Walle, C.G.; Bellotti, E.; et al. Ultrawide-bandgap semiconductors: Research opportunities and challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Kleshch, V.; Porshyn, V.; Lutzenkirchen-Hecht, D.; Obraztsov, A. Coulomb blockade and quantum confinement in field electron emission from heterostructured nanotips. Phys. Rev. B 2020, 102, 235437. [Google Scholar] [CrossRef]
- Atature, M.; Englund, D.; Vamivakas, N.; Lee, S.; Wrachtrup, J. Material platforms for spin-based photonic quantum technologies. Nat. Rev. Mater. 2018, 3, 38–51. [Google Scholar] [CrossRef]
- Zolotukhin, A.; Kopylov, P.; Ismagilov, R.; Obraztsov, A. Thermal oxidation of CVD diamond. Diam. Relat. Mater. 2010, 19, 1007–1011. [Google Scholar] [CrossRef]
- Arnoldi, L.; Spies, M.; Houard, J.; Blum, I.; Etienne, A.; Ismagilov, R.; Obraztsov, A.; Vella, A. Thermal diffusivity of diamond nanowires studied by laser assisted atom probe tomography. Appl. Phys. Lett. 2018, 112, 143104. [Google Scholar] [CrossRef]
- Malykhin, S.; Houard, J.; Ismagilov, R.; Orekhov, A.; Vella, A.; Obraztsov, A. Luminescent characteristics of needle-like single crystal diamonds. Phys. Status Solidi B 2018, 255, 1700189. [Google Scholar] [CrossRef]
- Malykhin, S.A.; Ismagilov, R.R.; Tuyakova, F.T.; Obraztsova, E.A.; Fedotov, P.V.; Ermakova, A.; Siyushev, P.; Katamadze, K.G.; Jelezko, F.; Rakovich, Y.P.; et al. Photoluminescent properties of single crystal diamond microneedles. Opt. Mater. 2018, 75, 49–55. [Google Scholar] [CrossRef]
- Tuyakova, F.; Obraztsova, E.; Korostylev, E.; Klinov, D.; Prusakov, K.; Alekseev, A.; Ismagilov, R.; Obraztsov, A. Photo- and cathodo-luminescence of needle-like single crystal diamonds. J. Lumin. 2016, 179, 539–544. [Google Scholar] [CrossRef]
- Malykhin, S.; Mindarava, Y.; Ismagilov, R.; Orekhov, A.; Jelezko, F.; Obraztsov, A. Formation of GeV, SiV, and NV color centers in single crystal diamond needles grown by chemical vapor deposition. Phys. Status Solidi B 2019, 256, 1800721. [Google Scholar] [CrossRef]
- Borisov, V.; Ismagilov, R.; Zolotukhin, A.; Obraztsov, A. Fabrication of carbon nanomaterials by hot filament chemical vapor deposition. J. Nanoelectron. Optoelectron. 2013, 8, 100–105. [Google Scholar] [CrossRef]
- Kudarenko, I.; Malykhin, S.; Orekhov, A.; Puzyr, A.; Kleshch, V.; Ismagilov, R.; Obraztsov, A. Detonation nanodiamond-Assisted carbon nanotube growth by hot filament chemical vapor deposition. Phys. Status Solidi B 2018, 255, 1700286. [Google Scholar] [CrossRef]
- Vanyukov, V.; Mikheev, G.; Mogileva, T.; Puzyr, A.; Bondar, V.; Svirko, Y. Near-IR nonlinear optical filter for optical communication window. Appl. Opt. 2015, 54, 3290–3293. [Google Scholar] [CrossRef]
- Puzyr, A.; Burov, A.; Bondar, V. Modification and comparative study of commercial nanodiamonds. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 93–97. [Google Scholar] [CrossRef]
- Alexeev, A.; Ismagilov, R.; Ashkinazi, E.; Orekhov, A.; Malykhin, S.; Obraztsov, A. Diamond platelets produced by chemical vapor deposition. Diam. Relat. Mater. 2016, 65, 13–16. [Google Scholar] [CrossRef]
- Alexeev, A.; Ismagilov, R.; Obraztsov, A. Structural and morphological peculiarities of needle-like diamond crystallites obtained by chemical vapor deposition. Diam. Relat. Mater. 2018, 87, 261–266. [Google Scholar] [CrossRef]
- Krapivsky, P.; Nazarov, L.; Tamm, M. Geometrical selection in growing needles. J. Stat. Mech. Theory Exp. 2019, 2019, 073206. [Google Scholar] [CrossRef]
- Malykhin, S.; Alexeev, A.; Obraztsova, E.; Ismagilov, R.; Kleshch, V.; Obraztsov, A. Production and potential applications of needle-like diamonds. Mater. Today Proceed. 2018, 5, 26146–26152. [Google Scholar] [CrossRef]
- Van der Drift, A. Evolutionary selection, a principle governing growth orientation in vapour-deposited layers. Philips. Res. Rep. 1967, 22, 267–288. [Google Scholar]
- Kuzmany, H.; Pfeiffer, R.; Salk, N.; Günther, B. The mystery of the 1140 cm−1 Raman line in nanocrystalline diamond films. Carbon 2004, 42, 911–917. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Origin of the 1150cm−1 Raman mode in nanocrystalline diamond. Phys. Rev. B 2001, 63, 121405. [Google Scholar] [CrossRef]
- Jorio, A.; Dresselhaus, M.; Saito, R.; Dresselhaus, G. Raman Spectroscopy in Graphene Related Systems, 1st ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 3–334. [Google Scholar]
- Tuyakova, F.; Obraztsova, E.; Ismagilov, R. Single-crystal diamond pyramids: Synthesis and application for atomic force microscopy. J. Nanophoton. 2015, 10, 012517. [Google Scholar] [CrossRef]
- Veprek, S.; Sarott, F. Electron-impact-induced anisotropic etching of silicon by hydrogen. Plasma Chem. Plasma Process. 1982, 2, 233–246. [Google Scholar] [CrossRef]
- Mankelevich, Y.; Ashfold, M.; Comerford, D.; Ma, J.; Richley, J. Boron doping: B/H/C/O gas-phase chemistry; H atom density dependences on pressure and wire temperature; puzzles regarding the gas-surface mechanism. Thin Solid Films 2011, 519, 4421–4425. [Google Scholar] [CrossRef]
- Boland, J. Structure of the H-saturated Si(100) surface. Phys. Rev. Lett. 1990, 65, 3325–3328. [Google Scholar] [CrossRef]
- Lu, Y.; Man, W.; Wang, B.; Rosenkranz, A.; Yang, M.; Yang, K.; Yi, J.; Song, H.; Li, H.; Jiang, N. (100) Oriented diamond film prepared on amorphous carbon buffer layer containing nano-crystalline diamond grains. Surf. Coat. Tech. 2020, 385, 125368. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismagilov, R.; Malykhin, S.; Puzyr, A.; Loginov, A.; Kleshch, V.; Obraztsov, A. Single-Crystal Diamond Needle Fabrication Using Hot-Filament Chemical Vapor Deposition. Materials 2021, 14, 2320. https://doi.org/10.3390/ma14092320
Ismagilov R, Malykhin S, Puzyr A, Loginov A, Kleshch V, Obraztsov A. Single-Crystal Diamond Needle Fabrication Using Hot-Filament Chemical Vapor Deposition. Materials. 2021; 14(9):2320. https://doi.org/10.3390/ma14092320
Chicago/Turabian StyleIsmagilov, Rinat, Sergei Malykhin, Aleksey Puzyr, Artem Loginov, Victor Kleshch, and Alexander Obraztsov. 2021. "Single-Crystal Diamond Needle Fabrication Using Hot-Filament Chemical Vapor Deposition" Materials 14, no. 9: 2320. https://doi.org/10.3390/ma14092320
APA StyleIsmagilov, R., Malykhin, S., Puzyr, A., Loginov, A., Kleshch, V., & Obraztsov, A. (2021). Single-Crystal Diamond Needle Fabrication Using Hot-Filament Chemical Vapor Deposition. Materials, 14(9), 2320. https://doi.org/10.3390/ma14092320






