The Green Approach to the Synthesis of Bio-Based Thermoplastic Polyurethane Elastomers with Partially Bio-Based Hard Blocks
Abstract
:1. Introduction
2. Materials and Methods
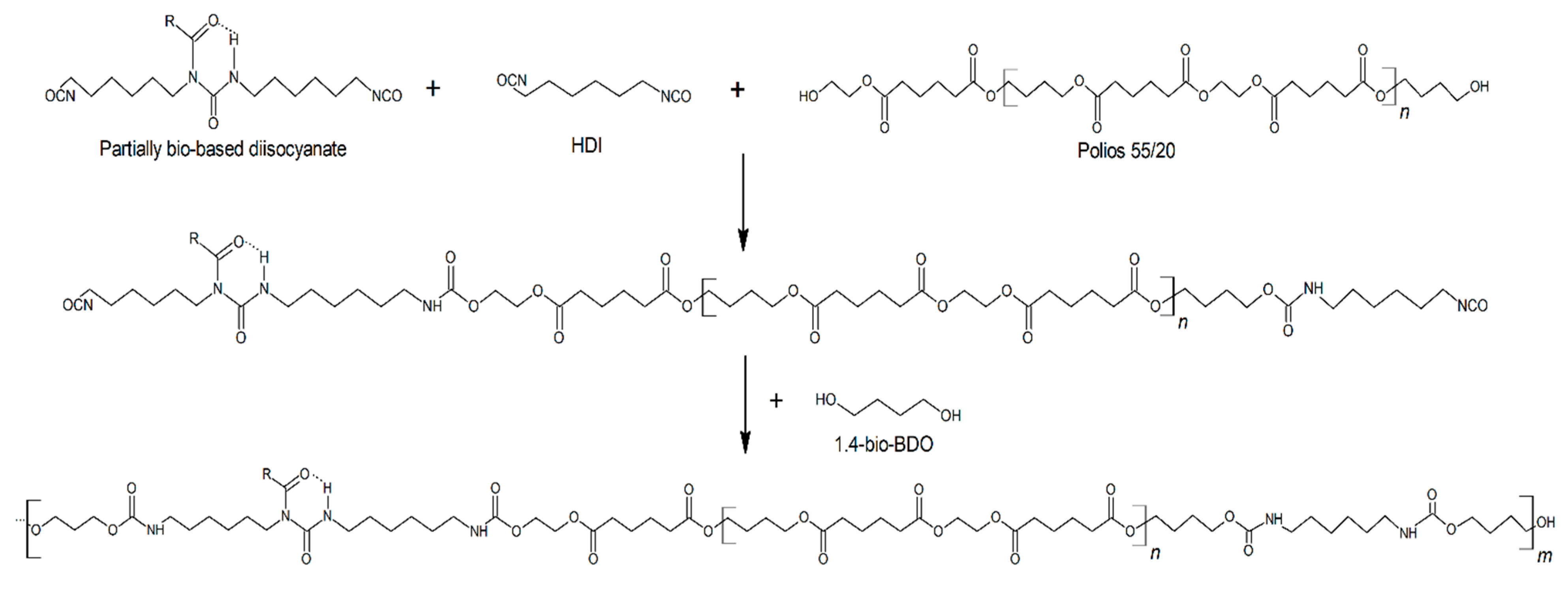
2.1. Samples Preparation
2.2. Measurement
2.2.1. Fourier Transform Infrared Spectroscopy—FTIR
2.2.2. Differential Scanning Calorimetry—DSC
2.2.3. Dynamic Mechanical Analysis—DMA
2.2.4. Thermogravimetric Analysis—TGA
2.2.5. X-ray Diffraction (XRD)
2.2.6. Tensile Test
2.2.7. Hardness
3. Results and Discussion
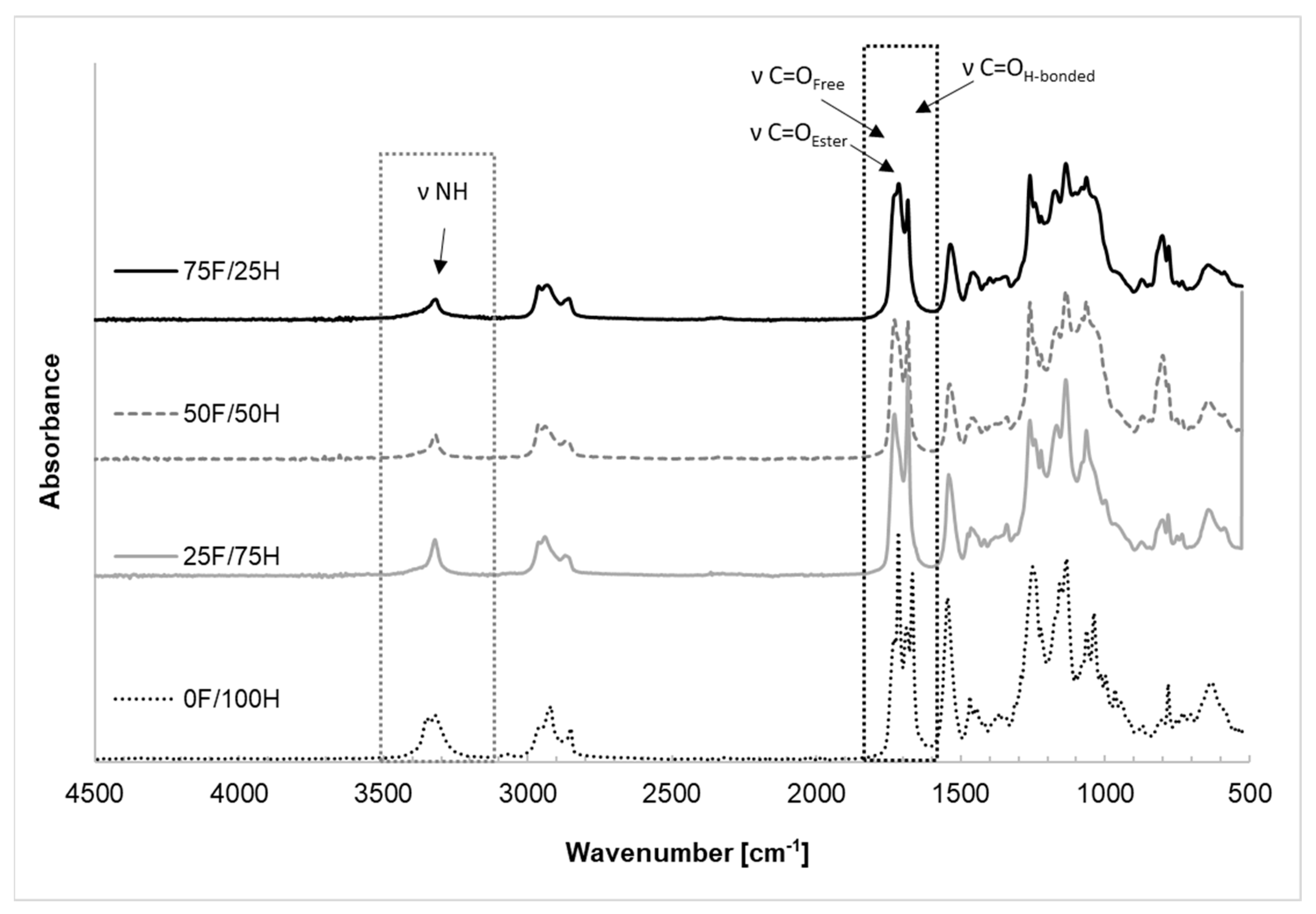
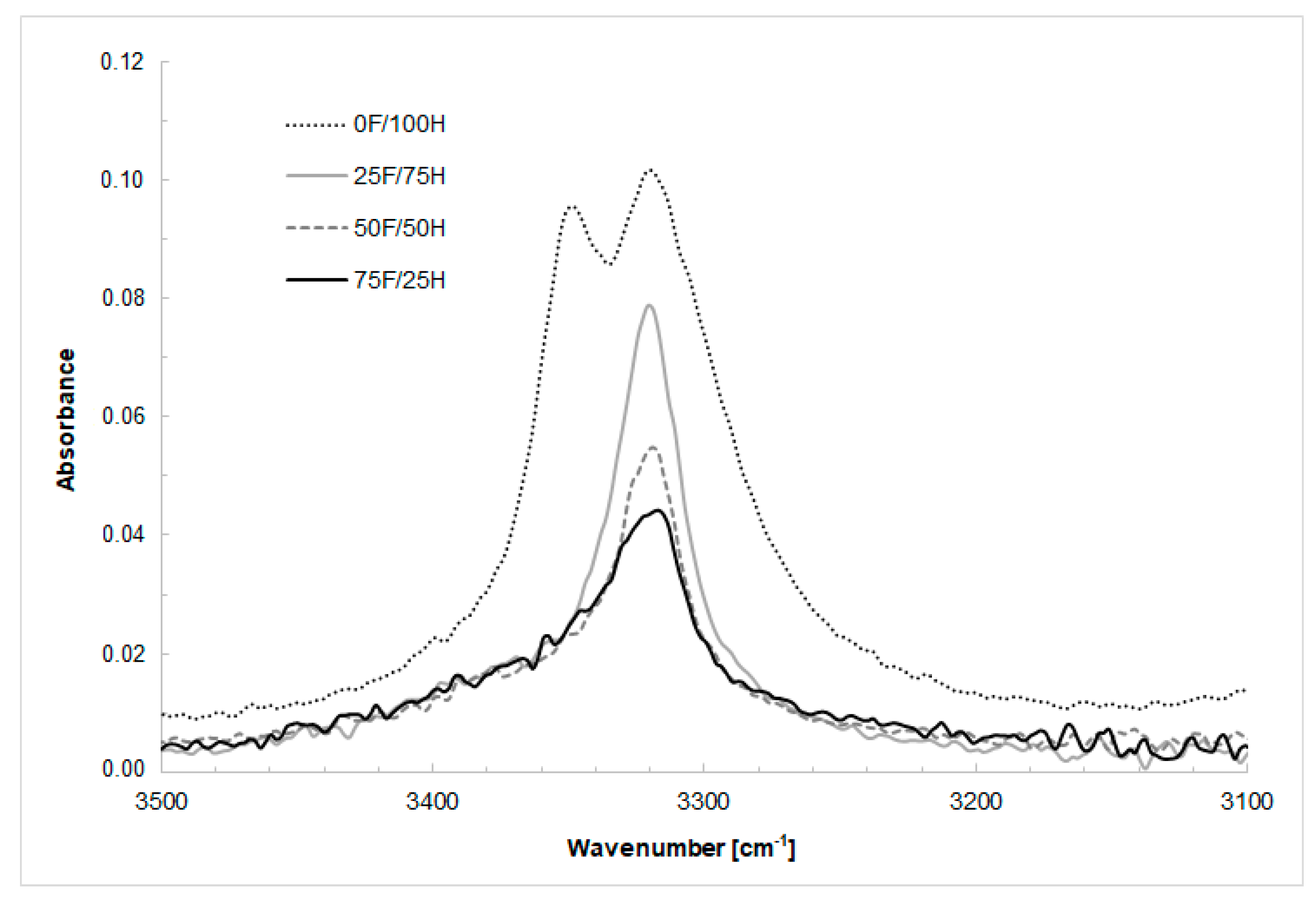
3.1. FT-IR Spectroscopy Analysis
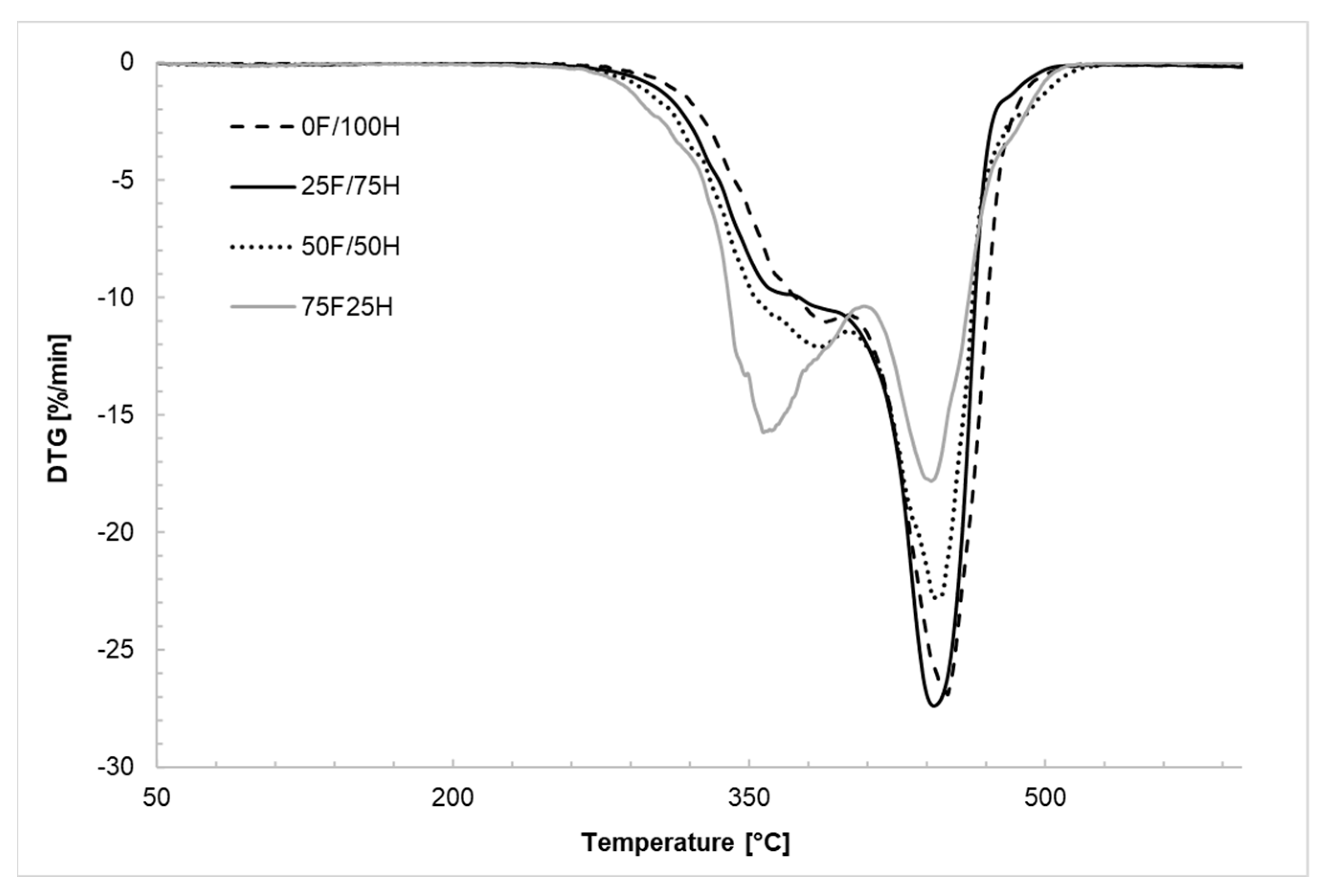
3.2. Thermogravimetric Analysis
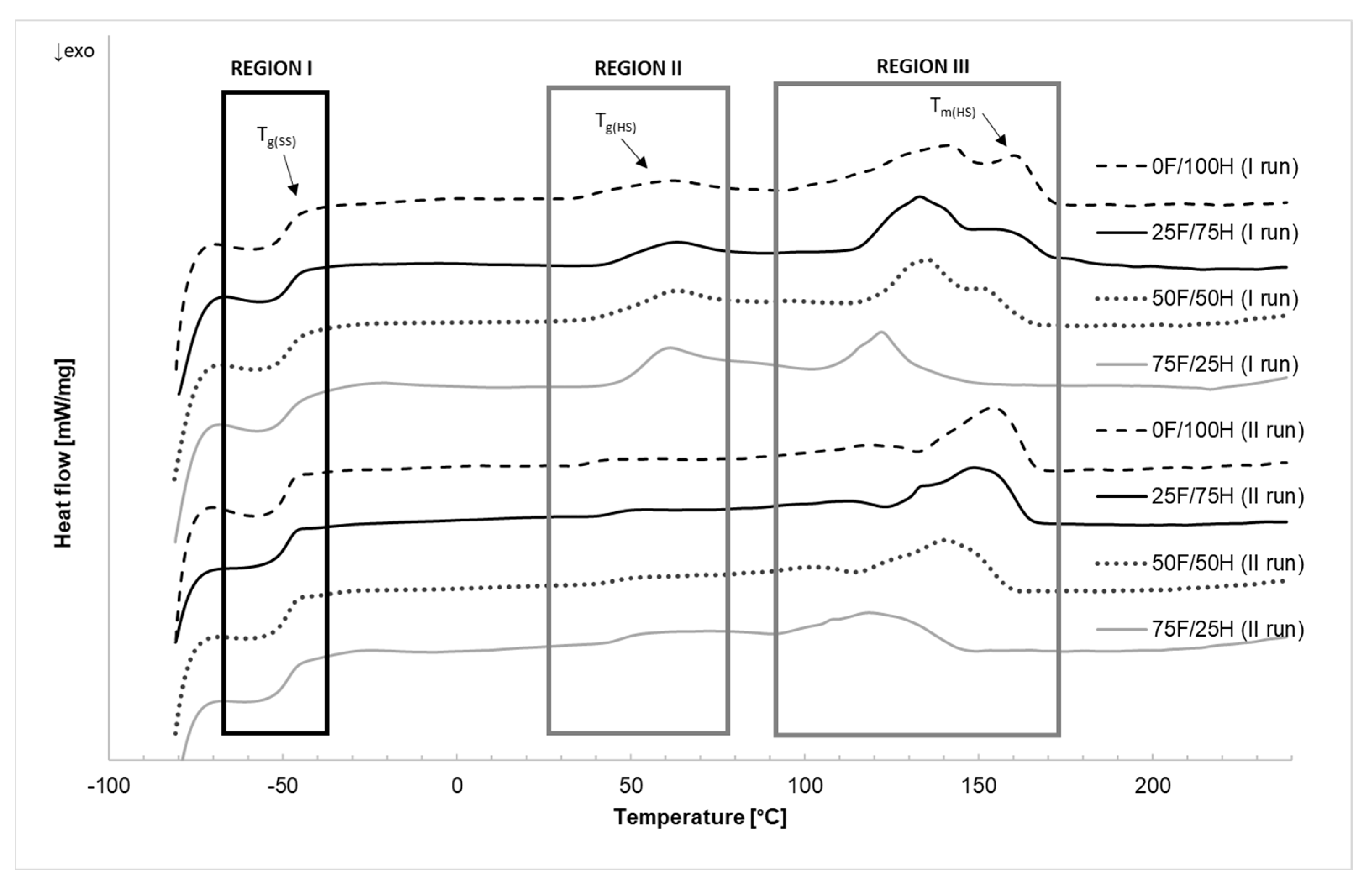
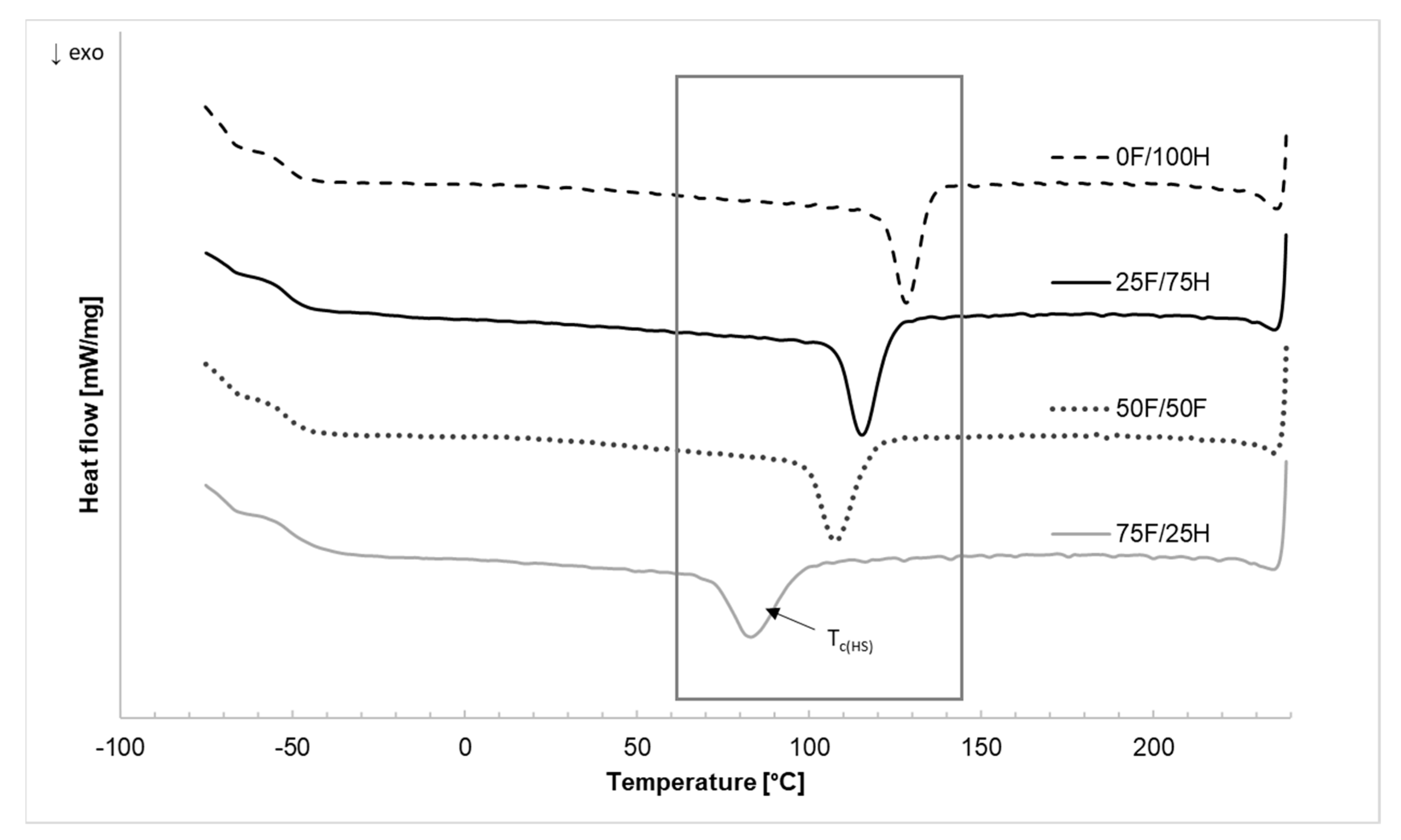
3.3. Differential Scanning Calorimetry
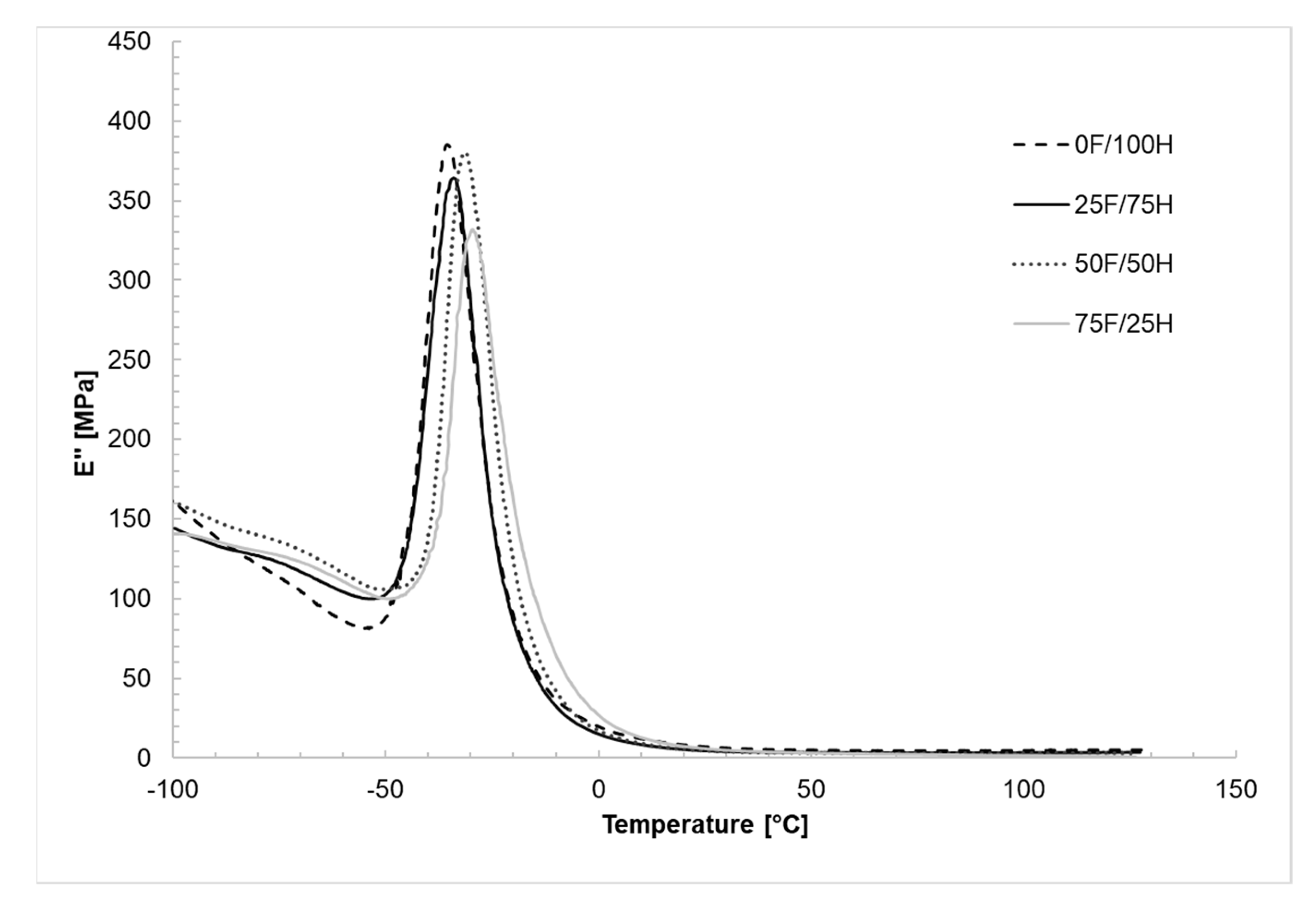
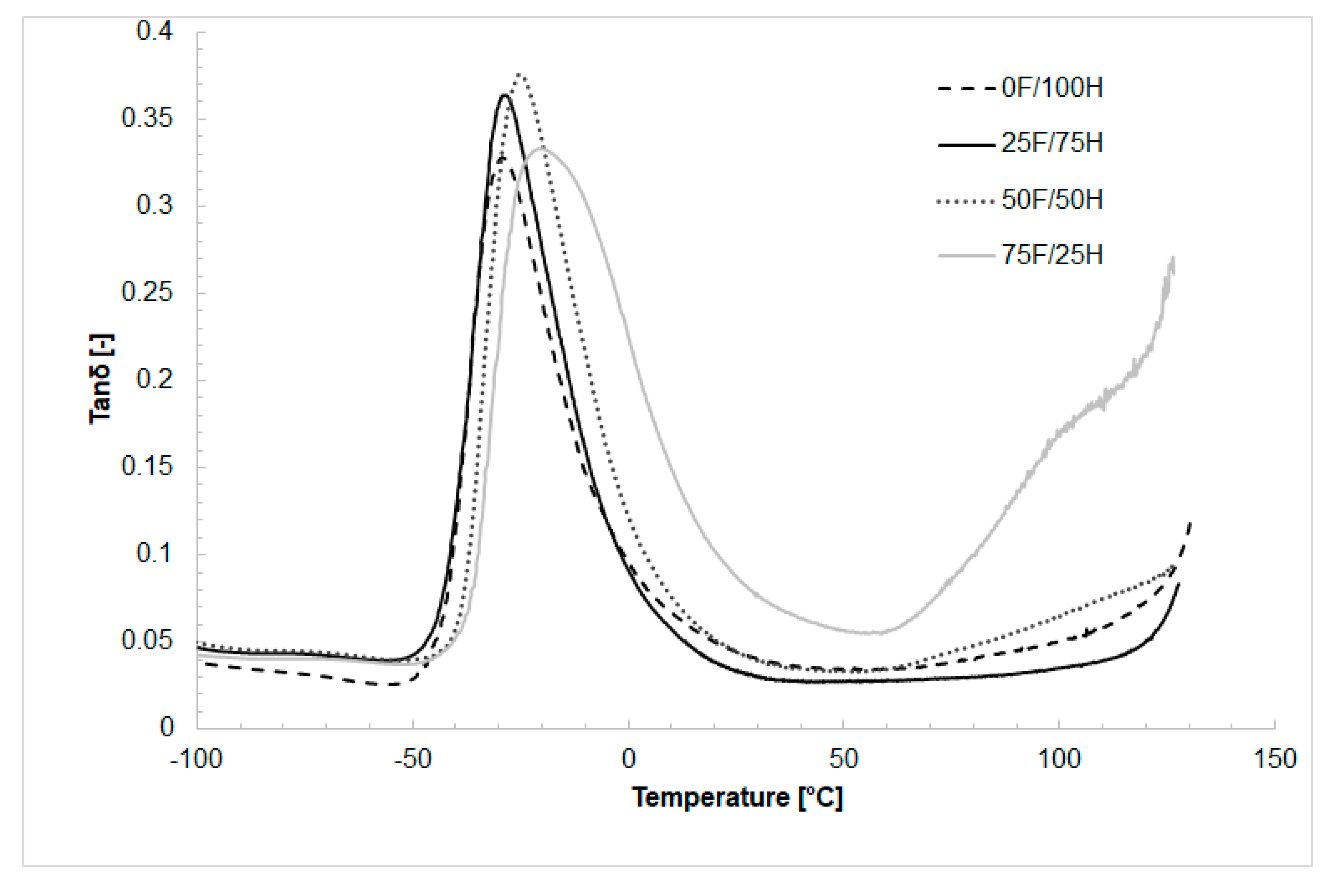
3.4. Dynamic Mechanical Analysis
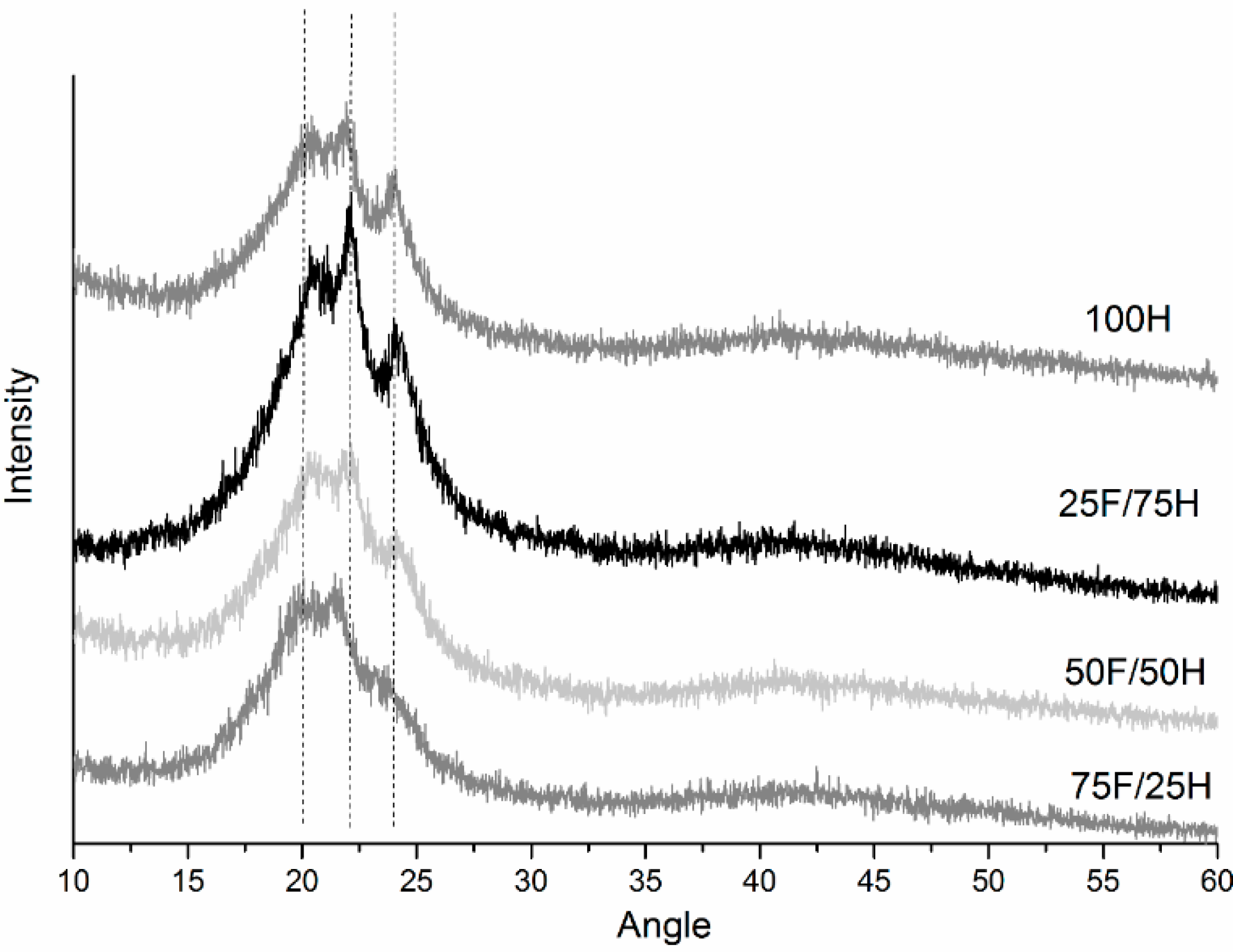
3.5. X-ray Diffraction (XRD)
3.6. Tensile Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beneš, H.; Vlček, T.; Černá, R.; Hromádková, J.; Walterová, Z.; Svitáková, R. Polyurethanes with bio-based and recycled components. Eur. J. Lipid Sci. Technol. 2012, 114, 71–83. [Google Scholar] [CrossRef]
- Calvo-Correas, T.; Santamaria-Echart, A.; Saralegi, A.; Martin, L.; Valea, Á.; Corcuera, M.A.; Eceiza, A. Thermally-responsive biopolyurethanes from a biobased diisocyanate. Eur. Polym. J. 2015, 70, 173–185. [Google Scholar] [CrossRef]
- Charlon, M.; Heinrich, B.; Matter, Y.; Couzigné, E.; Donnio, B.; Avérous, L. Synthesis, structure and properties of fully biobased thermoplastic polyurethanes, obtained from a diisocyanate based on modified dimer fatty acids, and different renewable diols. Eur. Polym. J. 2014, 61, 197–205. [Google Scholar] [CrossRef]
- Calle, M.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Non-isocyanate route to biobased polyurethanes and polyureas. Eur. Polym. J. 2016, 84, 837–848. [Google Scholar] [CrossRef]
- Abdolhosseini, F.; Kazem, M.; Givi, B. Characterization of a biodegradable polyurethane elastomer derived from castor oil. Am. J. Polym. Sci. 2016, 6, 18–27. [Google Scholar] [CrossRef]
- Karami, Z.; Zohuriaan-Mehr, M.; Rostami, A. Bio-based thermo-healable non-isocyanate polyurethane DA network in comparison with its epoxy counterpart. J. CO2 Util. 2017, 18, 294–302. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lebarbé, T.; Grau, E.; Cramail, H. Structure–properties relationship of fatty acid-based thermoplastics as synthetic polymer mimics. Polym. Chem. 2013, 4, 5472–5517. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Ferrer, C.; Hablot, E.; Garrigós, M.D.C.; Bocchini, S.; Averous, L.; Jiménez, A. Relationship between morphology, properties and degradation parameters of novative biobased thermoplastic polyurethanes obtained from dimer fatty acids. Polym. Degrad. Stab. 2012, 97, 1964–1969. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Datta, J. New plant-derived monomers in the synthesis of thermoplastic polyurethane elastomers. Elastomery 2018, 22, 200–213. [Google Scholar]
- Głowińska, E.; Datta, J. Possibilities for using modified soya and palm oils in synthesis of biopolyurethanes. Przem. Chem. 2012, 91, 1234–1236. [Google Scholar]
- Datta, J.; Głowińska, E. Chemical modifications of natural oils and examples of their usage for polyurethane synthesis. J. Elastomers Plast. 2012, 46, 33–42. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J. Structure, morphology and mechanical behaviour of novel bio-based polyurethane composites with microcrystalline cellulose. Cellulose 2015, 22, 2471–2481. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J. Bio polyetherurethane composites with high content of natural ingredients: Hydroxylated soybean oil based polyol, bio glycol and microcrystalline cellulose. Cellulose 2016, 23, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Corcuera, M.A.; Rueda, L.; Saralegui, A.; Martín, M.D.; Fernández-D’Arlas, B.; Mondragon, I.; Eceiza, A. Effect of diisocyanate structure on the properties and microstructure of polyurethanes based on polyols derived from renewable resources. J. Appl. Polym. Sci. 2011, 122, 3677–3685. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, M. Function of silicon oil in the castor oil based rigid polyurethane foams. J. Polym. Eng. 2013, 33, 875–880. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R.S. PU foam derived from renewable sources: Perspective on properties enhancement: An overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Trzebiatowska, P.J.; Deuter, I.; Datta, J. Cast polyurethanes obtained from reactive recovered polyol intermediates via crude glycerine decomposition process. React. Funct. Polym. 2017, 119, 20–25. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Simón, D.; De Lucas, A.; Rodríguez, J.F.; Borreguero, A.M. Flexible polyurethane foams synthesized employing recovered polyols from glycolysis: Physical and structural properties. J. Appl. Polym. Sci. 2017, 134, 45087. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.; de Lucas, A.; Rodríguez, J. Glycolysis of viscoelastic flexible polyurethane foam wastes. Polym. Degrad. Stab. 2015, 116, 23–35. [Google Scholar] [CrossRef]
- McCarthy, P.; Rasul, M.; Moazzem, S. Comparison of the performance and emissions of different biodiesel blends against petroleum diesel. Int. J. Low-Carbon Technol. 2011, 6, 255–260. [Google Scholar] [CrossRef]
- Zain, N.M.; Ahmad, S.H.; Ahad, N.A.; Ali, E.S. Influence of isocyanate structures on mechanical performance of aluminum bonded with green polyurethane adhesive. Adv. Mater. Res. 2015, 879, 119–127. [Google Scholar] [CrossRef]
- Javni, I.; Zhang, W.; Petrović, Z.S. Effect of different isocyanates on the properties of soy-based polyurethanes. J. Appl. Polym. Sci. 2002, 88, 2912–2916. [Google Scholar] [CrossRef]
- AqilahHamuzan, H.; Badri, K.H. The role of isocyanates in determining the viscoelastic properties of polyurethane. AIP Conf. Proc. 2016. [Google Scholar] [CrossRef]
- Rahmawati, R.; Nozaki, S.; Kojio, K.; Takahara, A.; Shinohara, N.; Yamasaki, S. Microphase-separated structure and mechanical properties of cycloaliphatic diisocyanate-based thiourethane elastomers. Polym. J. 2019, 51, 265–273. [Google Scholar] [CrossRef]
- Terban, M.W.; Dabbous, R.; Debellis, A.D.; Pöselt, E.; Billinge, S.J.L. Structures of Hard Phases in Thermoplastic Polyurethanes. Macromolecules 2016, 49, 7350–7358. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. DSC annealing study of microphase separation and multiple endothermic behavior in polyether-based polyurethane block copolymers. Macromolecules 1986, 19, 706–713. [Google Scholar] [CrossRef]
- Rueda, L.; D’Arlas, B.F.; Corcuera, M.; Eceiza, A. Biostability of polyurethanes. Study from the viewpoint of microphase separated structure. Polym. Degrad. Stab. 2014, 108, 195–200. [Google Scholar] [CrossRef]
- Bhausaheb, T.; Shingte Rahul, D.; Sachin, K.; Prakash, W.; Sadavarte, N.V.; Garg, K.; Maher, D.; Ichake, A.; More, A. Bio-based di/ polyisocyanates for polyurethanes: An overview. Polyureth. Today 2017, 41–46. [Google Scholar] [CrossRef]
- Konieczny, J.; Loos, K. Green polyurethanes from renewable isocyanates and biobased white dextrins. Polymers 2019, 11, 256. [Google Scholar] [CrossRef] [Green Version]
- More, A.S.; Lebarbé, T.; Maisonneuve, L.; Gadenne, B.; Alfos, C.; Cramail, H. Novel fatty acid based di-isocyanates towards the synthesis of thermoplastic polyurethanes. Eur. Polym. J. 2013, 49, 823–833. [Google Scholar] [CrossRef]
- Reddy, C.S.; Connor, K.O. The influence of biobased olegomeric diisocyanate on thermal and mechanical properties of poly(3-hydroxybutyrate). Macromol. Symp. 2016, 365, 223–229. [Google Scholar] [CrossRef]
- Schemmer, B.; Kronenbitter, C.; Mecking, S. Thermoplastic polyurethane elastomers with aliphatic hard segments based on plant-oil-derived long-chain diisocyanates. Macromol. Mater. Eng. 2018, 303, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Jose, J.; Bouzidi, L.; Leao, A.L.; Narine, S.S. Maximizing the utility of bio-based diisocyanate and chain extenders in crystalline segmented thermoplastic polyester urethanes: Effect of polymerization protocol. Polymer 2014, 55, 6764–6775. [Google Scholar] [CrossRef]
- Głowińska, E.; Wolak, W.; Datta, J. Eco-friendly route for thermoplastic polyurethane elastomers with bio-based hard segments composed of bio-glycol and mixtures of aromatic–aliphatic and aliphatic–aliphatic diisocyanate. J. Polym. Environ. 2021, 1–10. [Google Scholar] [CrossRef]
- Teo, L.-S.; Chen, A.C.-Y.; Kuo, J.-F. Fourier transform infrared spectroscopy study on effects of temperature on hydrogen bonding in amine-containing polyurethanes and poly(urethane−urea)s. Macromolecules 1997, 30, 1793–1799. [Google Scholar] [CrossRef]
- He, Y.; Xie, D.; Zhang, X. The structure, microphase-separated morphology, and property of polyurethanes and polyureas. J. Mater. Sci. 2014, 49, 7339–7352. [Google Scholar] [CrossRef]
- Niemczyk, A.; Piegat, A.; Olalla, Á.S.; El Fray, M. New approach to evaluate microphase separation in segmented polyurethanes containing carbonate macrodiol. Eur. Polym. J. 2017, 93, 182–191. [Google Scholar] [CrossRef]
- Oliaie, H.; Haddadi-Asl, V.; Mirhosseini, M.M.; Jouibari, I.S.; Mohebi, S.; Shams, A. Role of sequence of feeding on the properties of polyurethane nanocomposite containing halloysite nanotubes. Des. Monomers Polym. 2019, 22, 199–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parcheta, P.; Głowińska, E.; Datta, J. Effect of bio-based components on the chemical structure, thermal stability and mechanical properties of green thermoplastic polyurethane elastomers. Eur. Polym. J. 2020, 123, 109422. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J.; Parcheta, P. Effect of sisal fiber filler on thermal properties of bio-based polyurethane composites. J. Therm. Anal. Calorim. 2017, 130, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Datta, J.; Kasprzyk, P. Thermoplastic polyurethanes derived from petrochemical or renewable resources: A comprehensive review. Polym. Eng. Sci. 2018, 58, E14–E35. [Google Scholar] [CrossRef] [Green Version]
- Saralegi, A.; Rueda, L.; Fernández-D’Arlas, B.; Mondragon, I.; Eceiza, A.; Corcuera, M.A. Thermoplastic polyurethanes from renewable resources: Effect of soft segment chemical structure and molecular weight on morphology and final properties. Polym. Int. 2013, 62, 106–115. [Google Scholar] [CrossRef]
- Wirpsza, Z. Poliuretany: Chemia, Technologia, Zastosowanie; Wydawnictwa Naukowo-Techniczne: Warsaw, Poland, 1991. [Google Scholar]




| Parameter | Bio-Based Diisocyanate Content (wt %) | |||
|---|---|---|---|---|
| 0 | 25 | 50 | 75 | |
| R | 3.56 | 1.92 | 1.56 | 1.43 |
| R2 | 0.9931 | 0.9976 | 0.9963 | 0.9943 |
| DPS | 0.780 | 0.657 | 0.609 | 0.588 |
| DPM | 0.220 | 0.343 | 0.391 | 0.412 |
| HS [%] | 29.6 | 34.0 | 40.2 | 53.4 |
| Properties | Bio-Based Diisocyanate Content (wt %) | |||
|---|---|---|---|---|
| 0 | 25 | 50 | 75 | |
| T5% [°C] | 339 | 330 | 324 | 316 |
| T10% [°C] | 357 | 347 | 341 | 335 |
| T50% [°C] | 429 | 424 | 415 | 398 |
| Ash residue at 600 °C [%] | 1.4 | 1.7 | 1.7 | 0.6 |
| Properties | Bio-Based Diisocyanate Content (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | |||||
| I Run | II Run | I Run | II Run | I Run | II Run | I Run | II Run | |
| TgSS [°C] | −49.5 | −49.1 | −48.9 | −49.1 | −49.2 | −48.9 | −49.7 | −49.1 |
| TgHS [°C] | 43.9 | 38.0 | 51.8 | 44.2 | 54.0 | 44.7 | 54.2 | 47.1 |
| TmHS [°C] | 160.2 | 154.1 | 161.2 | 148.7 | 150.7 | 140.3 | 122.1 | 118.4 |
| ΔHmHS [J/g] | 16.67 | 12.34 | 20.68 | 13.27 | 16.19 | 11.92 | 5.34 | 10.01 |
| TcHS [°C] | 128.2 | - | 115.4 | - | 107.7 | - | 83.1 | - |
| ΔHc [J/g] | −11.39 | - | −14.82 | - | −13.78 | - | −14.62 | - |
| Properties | The Diisocyanate Mixture Type | |||
|---|---|---|---|---|
| 0F/100H | ||||
| [NCO]/[OH] | 0.95 | 1.0 | 1.05 | 1.1 |
| TSb (MPa) | 9.2 ± 0.6 | 16.8 ± 2.8 | 17.9 ± 2.6 | 25.8 ±4.5 |
| εb (%) | 218 ± 67 | 706 ± 139 | 613 ± 82 | 803 ± 86 |
| 25F/75H | ||||
| [NCO]/[OH] | 0.95 | 1.0 | 1.05 | 1.1 |
| TSb (MPa) | 10.8 ± 0.8 | 25.7 ± 1.9 | 29.8 ± 1.9 | 31.7 ± 2.2 |
| εb (%) | 449 ± 98 | 883 ± 52 | 890 ± 16 | 874 ± 29 |
| 50F/50H | ||||
| [NCO]/[OH] | 0.95 | 1.0 | 1.05 | 1.1 |
| TSb (MPa) | 8.7 ± 0.5 | 27.5 ± 3.1 | 24.9 ± 8.3 | 33.3 ± 1.8 |
| εb (%) | 449 ± 102 | 895 ± 16 | 809 ± 91 | 904 ± 15 |
| 75F/25H | ||||
| [NCO]/[OH] | 0.95 | 1.0 | 1.05 | 1.1 |
| TSb (MPa) | 6.5 ± 0.6 | 16.1 ± 0.9 | 18.9 ± 0.9 | 18.4 ± 1.2 |
| εb (%) | 421 ± 4 | 818 ± 41 | 845 ± 40 | 883 ± 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowińska, E.; Kasprzyk, P.; Datta, J. The Green Approach to the Synthesis of Bio-Based Thermoplastic Polyurethane Elastomers with Partially Bio-Based Hard Blocks. Materials 2021, 14, 2334. https://doi.org/10.3390/ma14092334
Głowińska E, Kasprzyk P, Datta J. The Green Approach to the Synthesis of Bio-Based Thermoplastic Polyurethane Elastomers with Partially Bio-Based Hard Blocks. Materials. 2021; 14(9):2334. https://doi.org/10.3390/ma14092334
Chicago/Turabian StyleGłowińska, Ewa, Paulina Kasprzyk, and Janusz Datta. 2021. "The Green Approach to the Synthesis of Bio-Based Thermoplastic Polyurethane Elastomers with Partially Bio-Based Hard Blocks" Materials 14, no. 9: 2334. https://doi.org/10.3390/ma14092334






