Zn- and Ti-Doped SnO2 for Enhanced Electroreduction of Carbon Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Electrode Preparation
2.3. Characterization of Materials
2.4. Electrochemical Characterization
3. Results and Discussion
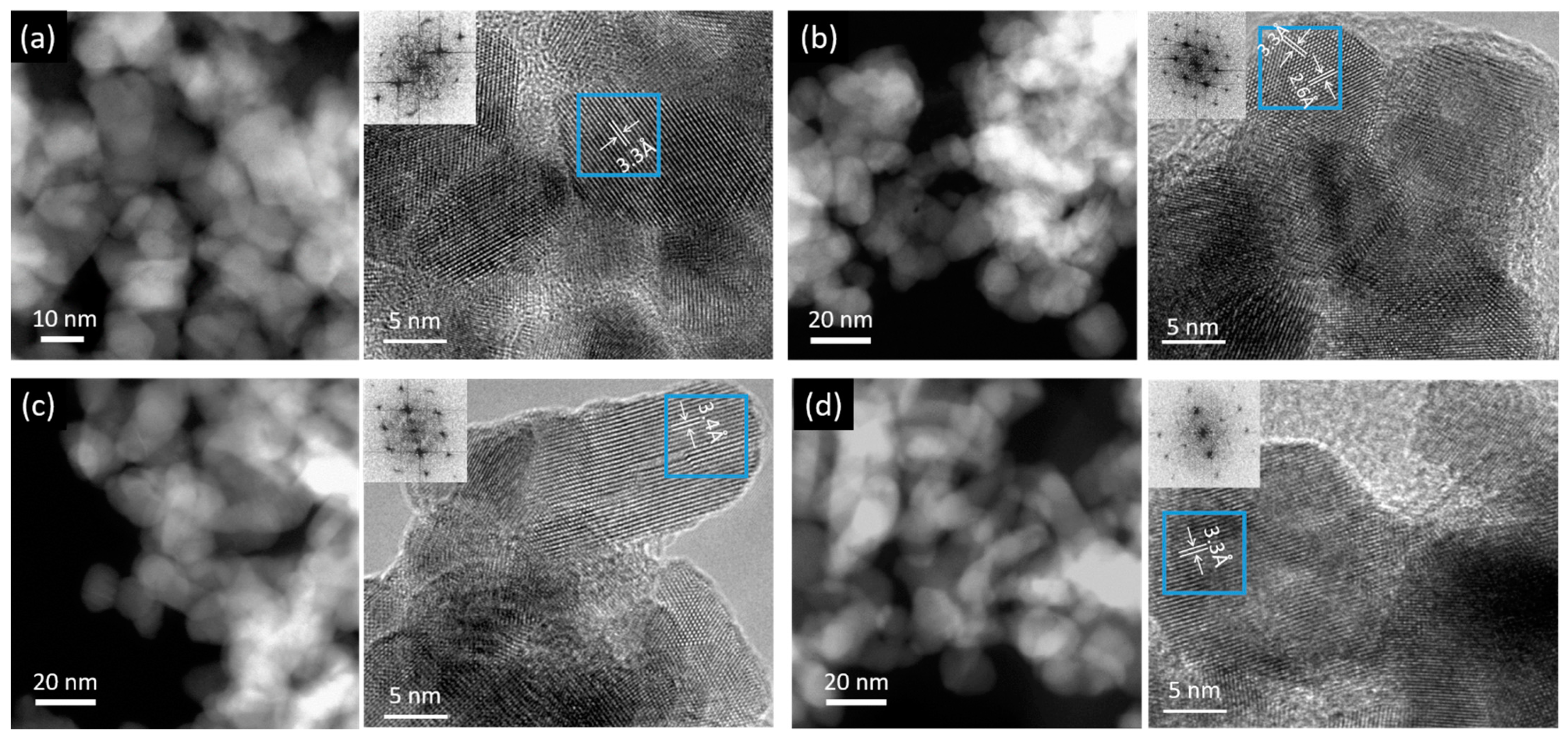
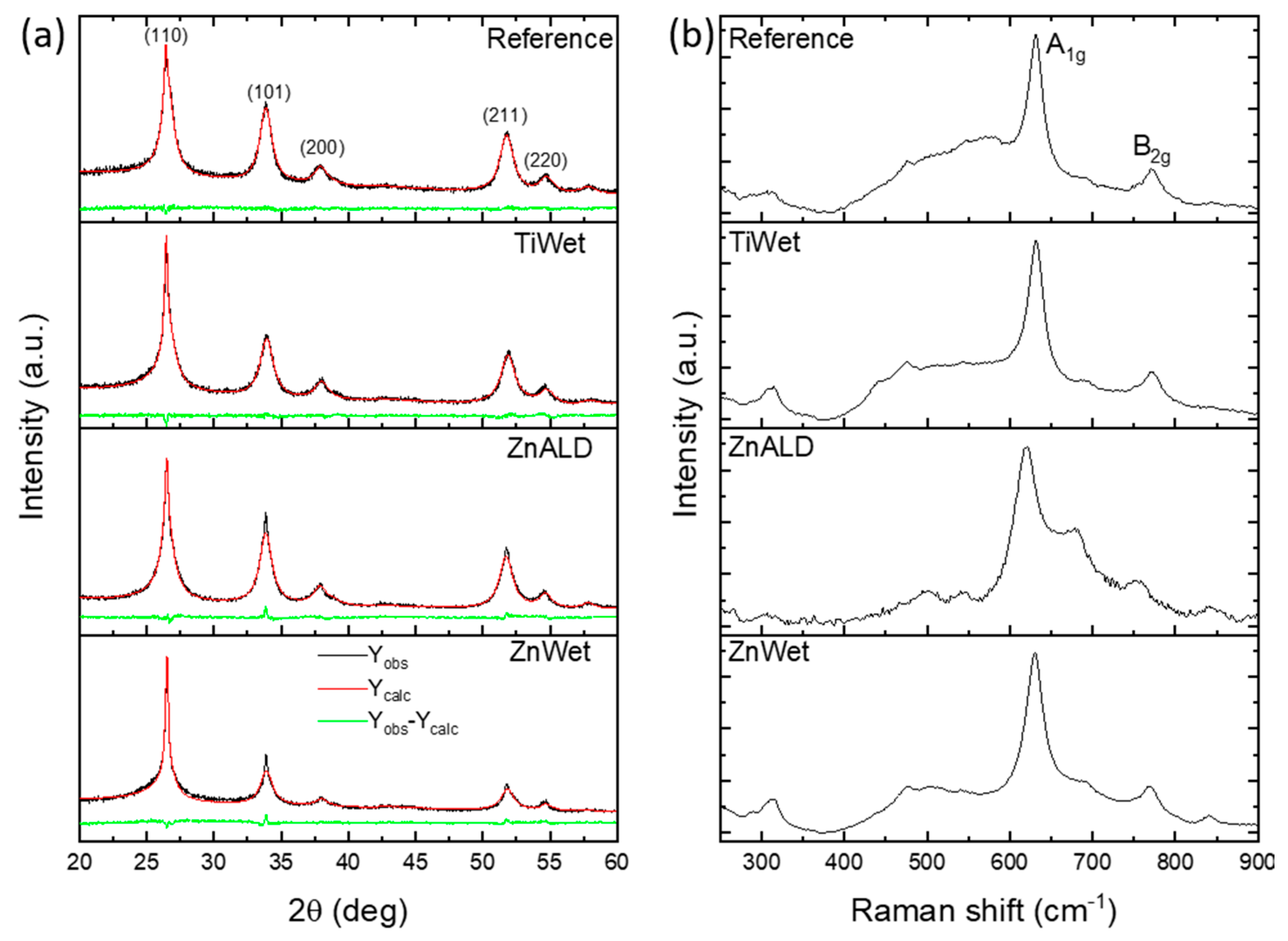
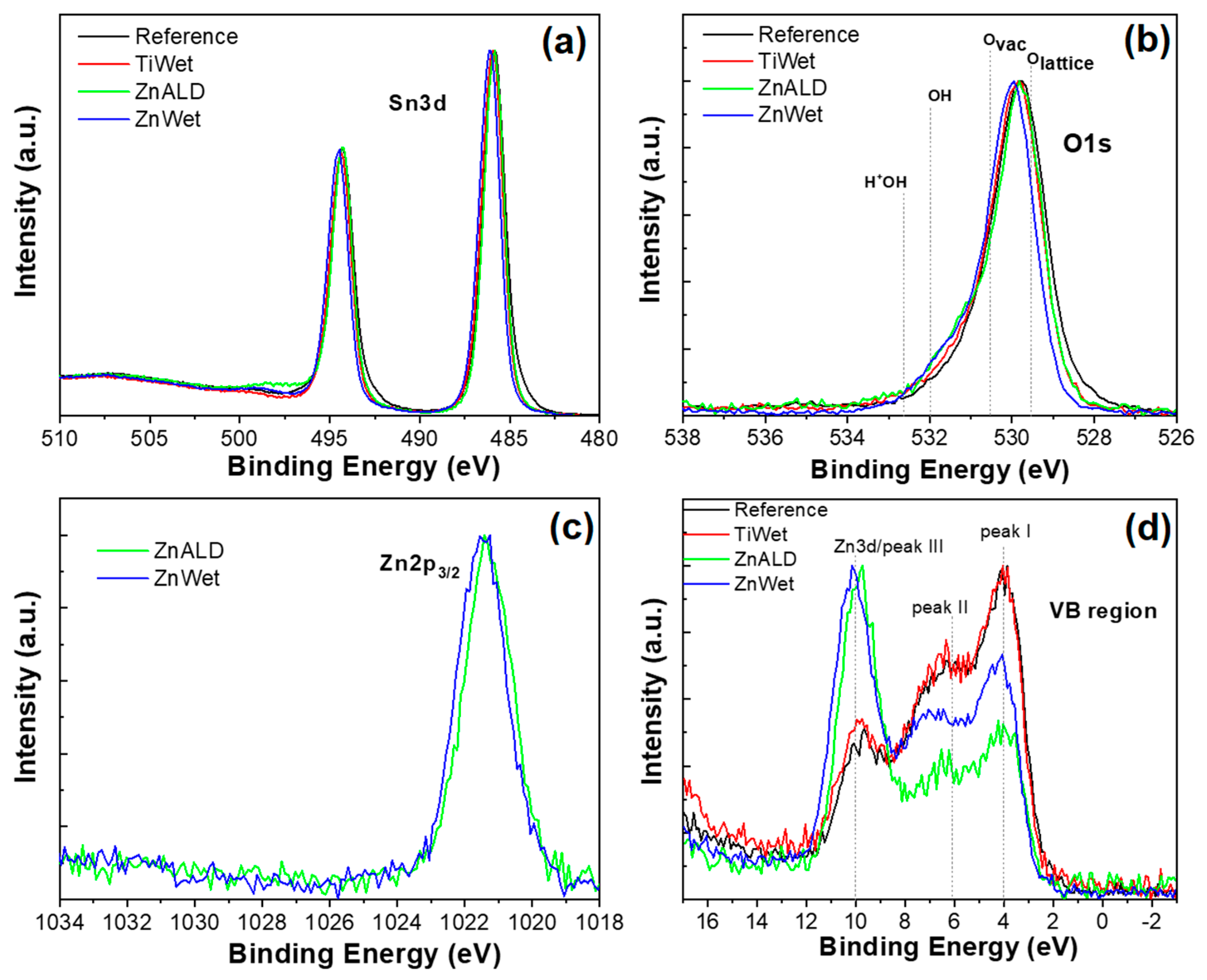
3.1. Characterization of the Prepared SnO2 Nanocatalysts Doped with Zn and Ti
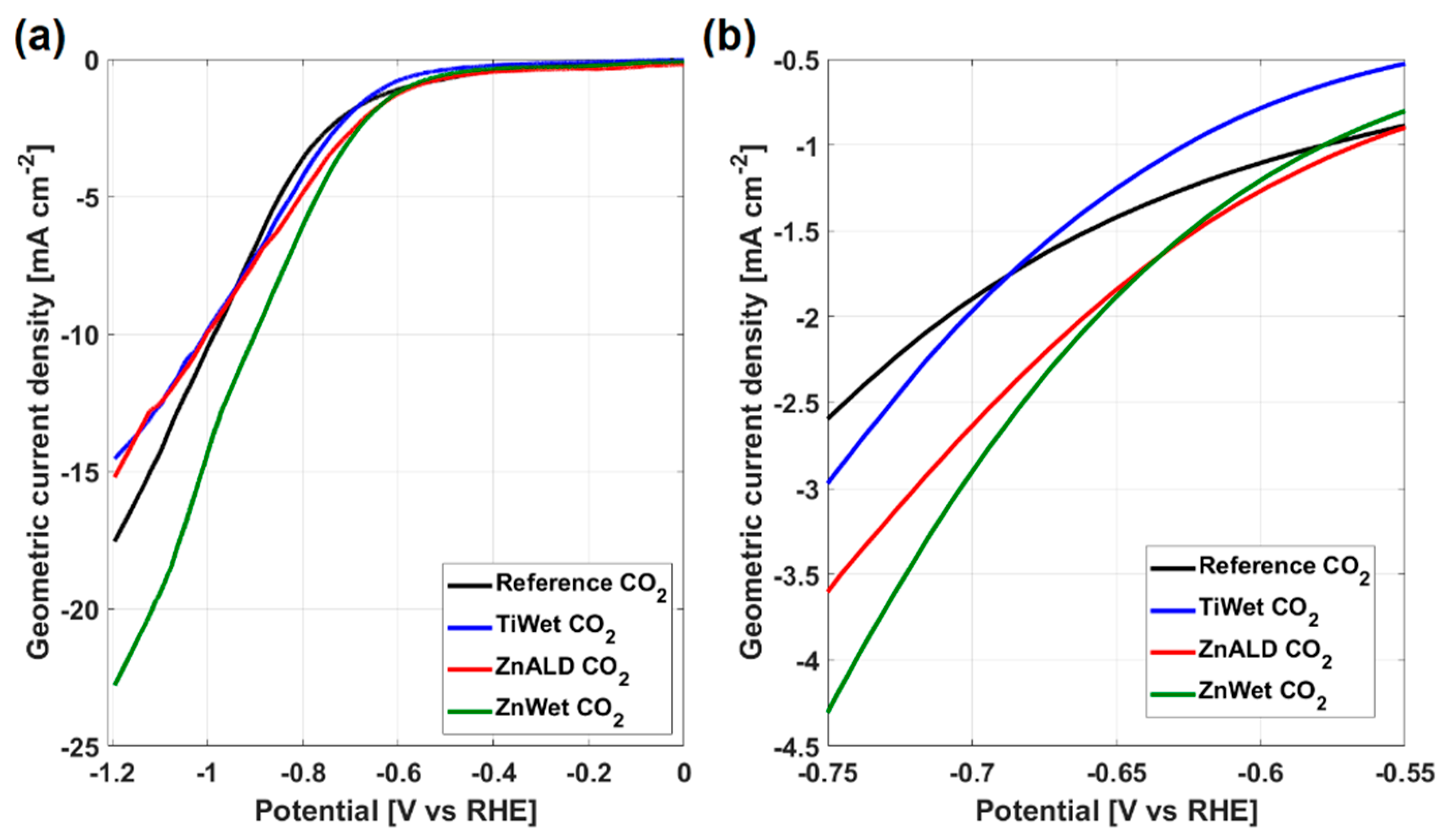
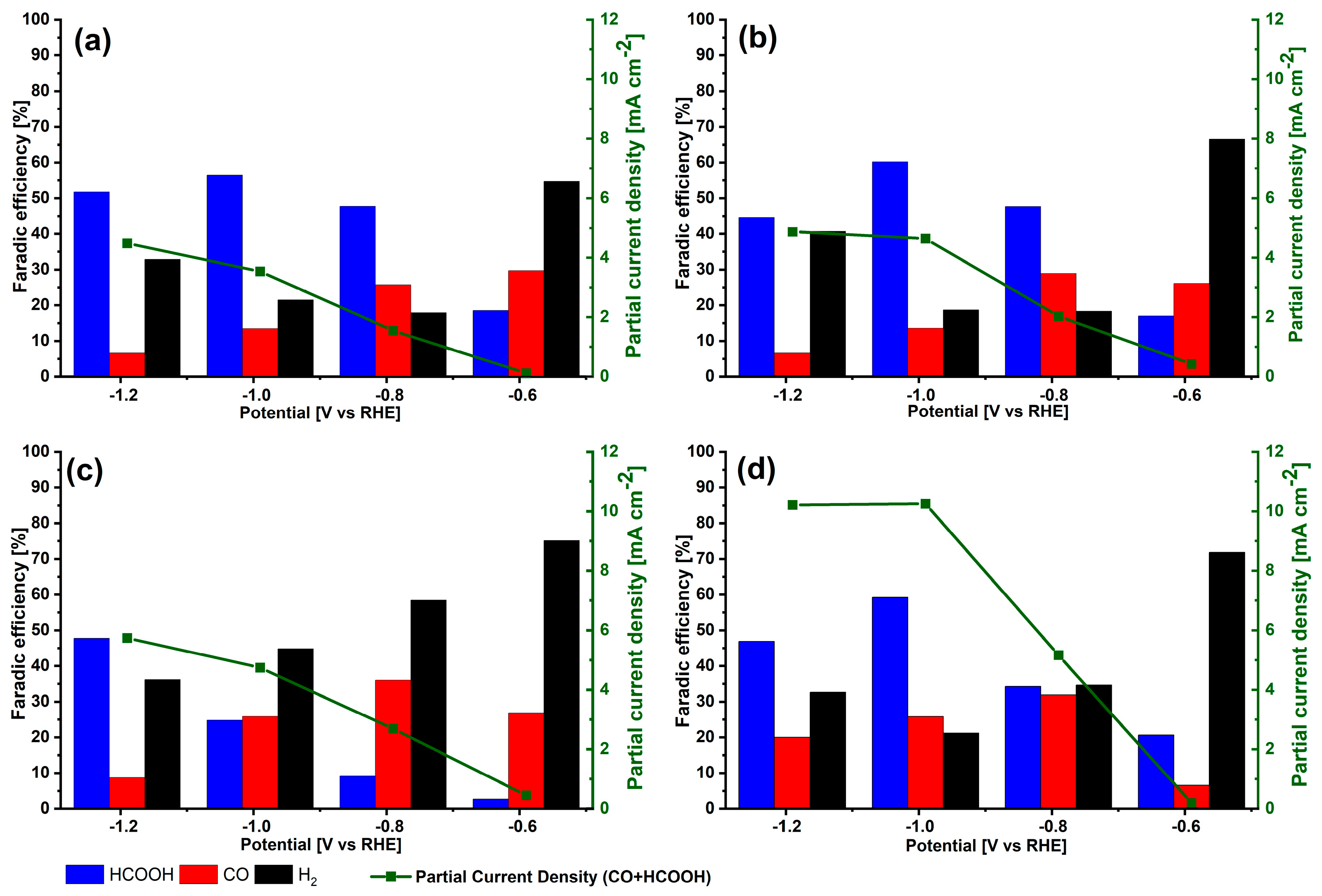
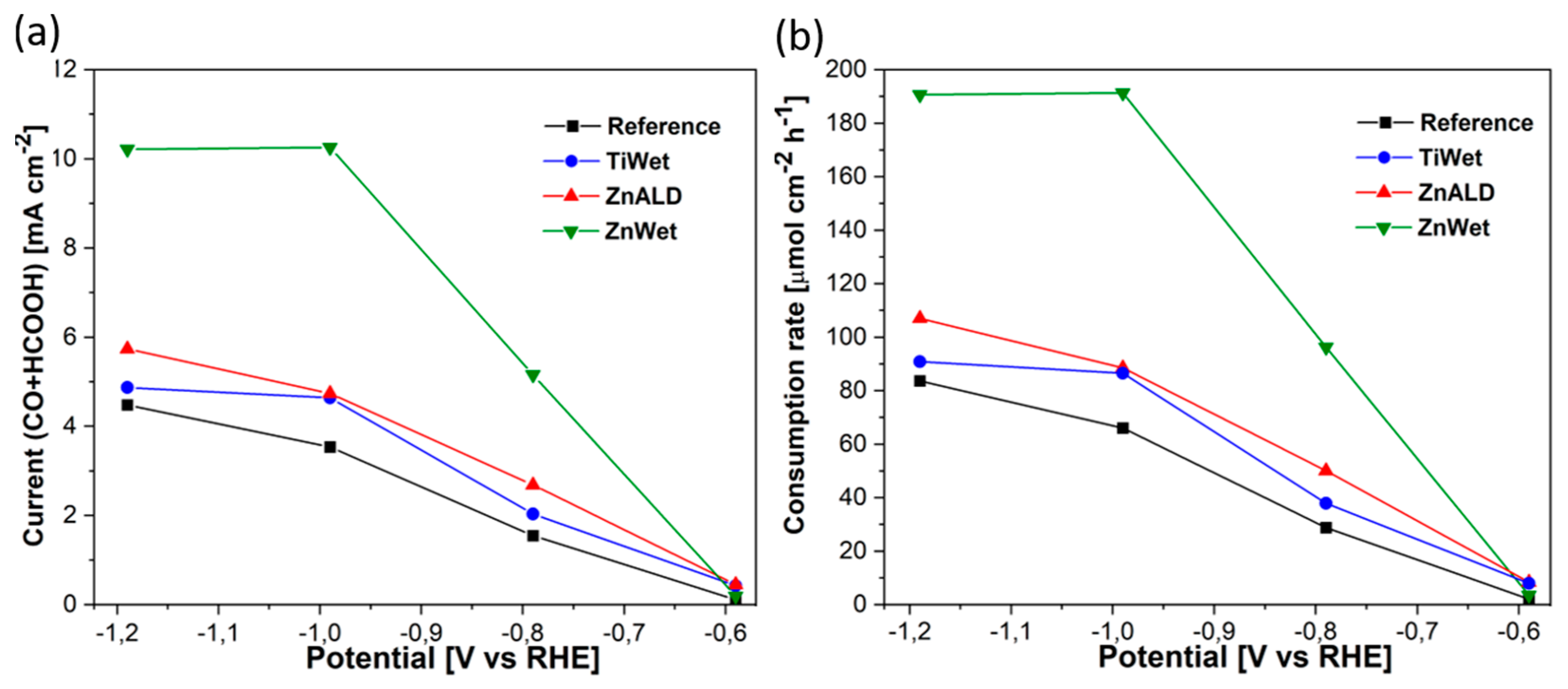
3.2. Electrochemical Behavior for CO2 Electroreduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durst, J.; Rudnev, A.; Dutta, A.; Fu, Y.; Herranz, J.; Kaliginedi, V.; Kuzume, A.; Permyakova, A.A.; Paratcha, Y.; Broekmann, P.; et al. Electrochemical CO2 Reduction—A Critical View on Fundamentals, Materials and Applications. Chimia 2015, 69, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Rumayor, M.; Dominguez-Ramos, A.; Irabien, A. Formic Acid Manufacture: Carbon Dioxide Utilization Alternatives. Appl. Sci. 2018, 8, 914. [Google Scholar] [CrossRef]
- Perez-Fortes, M.; Tzimas, E. Techno-Economic and Environmental Evaluation of CO2 Utilisation for Fuel Production. Synthesis of Methanol and Formic Acid; Publications Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-59133-4. [Google Scholar]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What should we make with CO2 and how can we make it? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Knowles, G.P.; MacFarlane, D.R.; Zhang, J. Hierarchical Mesoporous SnO2 Nanosheets on Carbon Cloth: A Robust and Flexible Electrocatalyst for CO2 Reduction with High Efficiency and Selectivity. Angew. Chem. Int. Ed. 2017, 56, 505–509. [Google Scholar] [CrossRef]
- He, Y.; Jiang, W.-J.; Zhang, Y.; Huang, L.-B.; Hu, J.-S. Pore-structure-directed CO2 electroreduction to formate on SnO2/C catalysts. J. Mater. Chem. A 2019, 7, 18428–18433. [Google Scholar] [CrossRef]
- Hu, H.; Gui, L.; Zhou, W.; Sun, J.; Xu, J.; Wang, Q.; He, B.; Zhao, L. Partially reduced Sn/SnO2 porous hollow fiber: A highly selective, efficient and robust electrocatalyst towards carbon dioxide reduction. Electrochim. Acta 2018, 285, 70–77. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, K.; Fan, S.; Kanan, M.W. A Direct Grain-Boundary-Activity Correlation for CO Electroreduction on Cu Nanoparticles. ACS Cent. Sci. 2016, 2, 169–174. [Google Scholar] [CrossRef]
- Li, C.; Ciston, J.; Kanan, M. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 2014, 508, 504–507. [Google Scholar] [CrossRef]
- Kumar, B.; Atla, V.; Brian, J.P.; Kumari, S.; Nguyen, T.Q.; Sunkara, M.; Spurgeon, J.M. Reduced SnO2 Porous Nanowires with a High Density of Grain Boundaries as Catalysts for Efficient Electrochemical CO2-into-HCOOH Conversion. Angew. Chem. Int. Ed. 2017, 56, 3645–3649. [Google Scholar] [CrossRef]
- Bejtka, K.; Zeng, J.; Sacco, A.; Castellino, M.; Hernández, S.; Farkhondehfal, M.A.; Savino, U.; Ansaloni, S.; Pirri, C.F.; Chiodoni, A. Chainlike Mesoporous SnO2 as a Well-Performing Catalyst for Electrochemical CO2 Reduction. ACS Appl. Energy Mater. 2019, 2, 3081–3091. [Google Scholar] [CrossRef]
- Saravanan, K.; Basdogan, Y.; Dean, J.; Keith, J.A. Computational investigation of CO2 electroreduction on tin oxide and predictions of Ti, V, Nb and Zr dopants for improved catalysis. J. Mater. Chem. A 2017, 5, 11756–11763. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Cheng, F.; Chen, J. Mn-doped atomic SnO2 layers for highly efficient CO2 electrochemical reduction. J. Mater. Chem. A 2019, 7, 19651–19656. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Hu, X.; Tian, H.; Gao, M.; Zhang, D.; Li, Z.; Yang, D. Surface Modification of Tin Dioxide via (Bi, S) Co-Doping for Photoelectrocatalytic Reduction of CO2 to Formate. ChemElectroChem 2019, 6, 3782–3790. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Zhang, M.; Hou, P.; Kang, P. Nitrogen doped tin oxide nanostructured catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Energy Chem. 2017, 26, 825–829. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Guo, M.; Gao, M.; Zhang, E.; Tian, H.; Liang, Z.; Liu, X. Synthesis and Characterization of (Cu, S) Co-doped SnO2 for Electrocatalytic Reduction of CO2 to Formate at Low Overpotential. ChemElectroChem 2018, 5, 1330–1335. [Google Scholar] [CrossRef]
- Medhi, R.; Li, C.-H.; Lee, S.-H.; Marquez, M.D.; Jacobson, A.J.; Lee, T.-C.; Lee, T.R. Uniformly Spherical and Monodisperse Antimony- and Zinc-Doped Tin Oxide Nanoparticles for Optical and Electronic Applications. ACS Appl. Nano Mater. 2019, 2, 6554–6564. [Google Scholar] [CrossRef]
- Tian, S.; Gao, Y.; Zeng, D.; Xie, C. Effect of Zinc Doping on Microstructures and Gas-Sensing Properties of SnO2 Nanocrystals. J. Am. Ceram. Soc. 2012, 95, 436–442. [Google Scholar] [CrossRef]
- Ran, L.; Yin, L. Highly Crystalline Ti-doped SnO2 Hollow Structured Photocatalyst with Enhanced Photocatalytic Activity for Degradation of Organic Dyes. CrystEngComm 2015, 17, 4225–4237. [Google Scholar] [CrossRef]
- Wang, C.-T.; Lai, D.-L.; Chen, M.-T. Surface and catalytic properties of doped tin oxide nanoparticles. Appl. Surf. Sci. 2010, 257, 127–131. [Google Scholar] [CrossRef]
- Laurenti, M.; Canavese, G.; Sacco, A.; Fontana, M.; Bejtka, K.; Castellino, M.; Pirri, C.F.; Cauda, V. Nanobranched ZnO Structure: P-Type Doping Induces Piezoelectric Voltage Generation and Ferroelectric–Photovoltaic Effect. Adv. Mater. 2015, 27, 4218–4223. [Google Scholar] [CrossRef]
- Tjugito, L. Deposition of Titanium Dioxide Coating on Plastic for Medical Purposes. Master’s Thesis, University of New South Wales, Sydney, Australia, 2010. Available online: https://www.library.unsw.edu.au/ (accessed on 6 December 2020).
- Porro, S.; Jasmin, A.; Bejtka, K.; Conti, D.; Perrone, D.; Guastella, S.; Pirri, C.F.; Chiolerio, A.; Ricciardi, C. Low-temperature atomic layer deposition of TiO2 thin layers for the processing of memristive devices. J. Vac. Sci. Technol. A 2016, 34, 01A147. [Google Scholar] [CrossRef]
- Porro, S.; Bejtka, K.; Jasmin, A.; Fontana, M.; Milano, G.; Chiolerio, A.; Pirri, C.F.; Ricciardi, C. A multi-level memristor based on atomic layer deposition of iron oxide. Nanotechnology 2018, 29, 495201. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, C.; Ricciardi, C.; Pirri, C.F.; Chiolerio, A.; Porro, S. Effects of single-pulse Al2O3 insertion in TiO2 oxide memristors by low temperature ALD. Appl. Phys. A 2018, 124, 686. [Google Scholar] [CrossRef]
- Palacios-Padrós, A.; Altomare, M.; Lee, K.; Díez-Pérez, I.; Sanz, F.; Schmuki, P. Controlled Thermal Annealing Tunes the Photoelectrochemical Properties of Nanochanneled Tin-Oxide Structures. ChemElectroChem 2014, 1, 1133–1137. [Google Scholar] [CrossRef]
- Jain, K.; Pant, R.P.; Lakshmikumar, S.T. Effect of Ni doping on thick film SnO2 gas sensor. Sens. Actuators B 2006, 113, 823–829. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD (Material Analysis Using Diffraction): A User friendly Java program for texture analysis and more. Proc. Int. Conf. Textures Mater. 1999, 2, 1599–1604. [Google Scholar]
- Lutterotti, L. Total pattern fitting for the combined size-strain-stress-texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Ge, H.; Gu, Z.; Han, P.; Shen, H.; Al-Enizi, A.M.; Zhang, L.; Zheng, G. Mesoporous tin oxide for electrocatalytic CO2 reduction. J. Colloid. Interface Sci. 2018, 531, 564–569. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sahay, P.P. Zn-doped and undoped SnO2 nanoparticles: A comparative structural, optical and LPG sensing properties study. Mater. Res. Bull. 2012, 47, 4112–4118. [Google Scholar] [CrossRef]
- Aragon, F.H.; Coaquira, J.A.H.; Hidalgo, P.; Da Silva, S.W.; Brito, S.L.M.; Gouvea, D.; Morais, P.C. Evidences of the evolution from solid solution to surface segregation in Ni-doped SnO2 nanoparticles using Raman spectroscopy. J. Raman Spectr. 2010, 42, 1081–1086. [Google Scholar] [CrossRef]
- McBride, J.C.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Synthesis, Properties, and Applications of Oxide Nanomaterials. J. Appl. Phys. 1994, 76, 2435. [Google Scholar] [CrossRef]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.W.; Herman, I.P. Size-dependent properties of CeO2−y nanoparticles as studied by Raman scattering. Phys. Rev. B 2001, 64, 245407. [Google Scholar] [CrossRef]
- Pagnier, T.; Boulova, M.; Galerie, A.; Gaskov, A.; Lucazeau, G. Reactivity of SnO2–CuO nanocrystalline materials with H2S: A coupled electrical and Raman spectroscopic study. Sens. Actuators B 2000, 71, 134–139. [Google Scholar] [CrossRef]
- Ansell, R.O.; Dickinson, T.; Povey, A.F.; Sherwood, P.M.A. Quantitative use of the angular variation technique in studies of tin by X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1977, 11, 301–313. [Google Scholar] [CrossRef]
- Loh, J.Y.Y.; Kherani, N.P. X-ray Photospectroscopy and Electronic Studies of Reactor Parameters on Photocatalytic Hydrogenation of Carbon Dioxide by Defect-Laden Indium Oxide Hydroxide Nanorods. Molecules 2019, 24, 3818. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Nose, K.; Suzuki, A.Y.; Oda, N.; Kamiko, M.; Mitsuda, Y. Oxidation of SnO to SnO2 thin films in boiling water at atmospheric pressure. Appl. Phys. Lett. 2014, 104, 091905. [Google Scholar] [CrossRef]
- Egdell, R.G.; Rebane, J.; Walker, T.J.; Law, D.S.L. Competition between initial- and final-state effects in valence- and core-level X-ray photoemission of Sb-doped SnO2. Phys. Rev. B 1999, 59, 1792–1799. [Google Scholar] [CrossRef]
- Zeng, J.; Rino, T.; Bejtka, K.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Chiodoni, A.; Drago, F.; Pirri, C.F. Coupled Copper–Zinc Catalysts for Electrochemical Reduction of Carbon Dioxide. ChemSusChem 2020, 13, 4128–4139. [Google Scholar] [CrossRef]
- Zeng, J.; Castellino, M.; Bejtka, K.; Sacco, A.; Di Martino, G.; Farkhondehfal, M.A.; Chiodoni, A.; Hernández, S.; Pirri, C.F. Facile synthesis of cubic cuprous oxide for electrochemical reduction of carbon dioxide. J. Mater. Sci. 2021, 56, 1255–1271. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Di Martino, G.; Sacco, A.; Castellino, M.; Re Fiorentin, M.; Risplendi, F.; Farkhondehfal, M.A.; Hernández, S.; Cicero, G.; et al. Microwave-Assisted Synthesis of Copper-Based Electrocatalysts for Converting Carbon Dioxide to Tunable Syngas. ChemElectroChem 2020, 7, 229–238. [Google Scholar] [CrossRef]
- Reid, O.; Saleh, F.S.; Easton, E.B. Determining electrochemically active surface area in PEM fuel cell electrodes with electrochemical impedance spectroscopy and its application to catalyst durability. Electrochim. Acta 2013, 114, 278–302. [Google Scholar] [CrossRef]
- Lee, C.W.; Cho, N.H.; Yang, K.D.; Nam, K.T. Reaction Mechanisms of the Electrochemical Conversion of Carbon Dioxide to Formic Acid on Tin Oxide Electrodes. ChemElectroChem 2017, 4, 2130–2136. [Google Scholar] [CrossRef]
- Lu, J.; Zhan, C.; Wu, T.; Wen, J.; Lei, Y.; Kropf, A.J.; Wu, H.; Miller, D.J.; Elam, J.W.; Sun, Y.-K.; et al. Effectively suppressing dissolution of manganese from spinel lithium manganate via a nanoscale surface-doping approach. Nat. Commun. 2014, 5, 5693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Merino-Garcia, I.; Albo, J.; Sánchez-Sánchez, C.M. Electrochemical CO2 reduction reaction on cost-effective oxide-derived copper and transition metal-nitrogen-carbon catalysts. Curr. Opin. Electrochem. 2020, 23, 65–73. [Google Scholar] [CrossRef]
- Deng, P.; Yang, F.; Wang, Z.; Chen, S.; Zhou, Y.; Zaman, S.; Xia, B.Y. Metal-Organic Framework-Derived Carbon Nanorods Encapsulating Bismuth Oxides for Rapid and Selective CO2 Electroreduction to Formate. Angew. Chem. Int. Ed. 2020, 59, 10807–10813. [Google Scholar] [CrossRef]
- Dutta, A.; Kuzume, A.; Rahaman, M.; Vesztergom, S.; Broekmann, P. Monitoring the Chemical State of Catalysts for CO2 Electroreduction: An In Operando Study. ACS Catal. 2015, 5, 7498–7502. [Google Scholar] [CrossRef]
- Baruch, M.F.; Pander, J.E.; White, J.L.; Bocarsly, A.B. Mechanistic Insights into the Reduction of CO2 on Tin Electrodes Using In Situ ATR-IR Spectroscopy. ACS Catal. 2015, 5, 3148–3156. [Google Scholar] [CrossRef]
- Pander, J.E., III; Ren, D.; Huang, Y.; Wei, N.; Loo, X.; Hui, S.; Hong, L.; Yeo, B.S. Understanding the Heterogeneous Electrocatalytic Reduction of Carbon Dioxide on Oxide-Derived Catalysts. ChemElectroChem 2018, 5, 219–237. [Google Scholar] [CrossRef]
- Feaster, J.T.; Shi, C.; Cave, E.R.; Hatsukade, T.; Abram, D.N.; Kuhl, K.P.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes. ACS Catal. 2017, 7, 4822–4827. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.-J.; Hu, C.; Yang, P.; Yuan, X.; Wang, Y.; Zhang, L.; Moskaleva, L.; Gong, J. Tuning Oxygen Vacancies of Oxides to Promote Electrocatalytic Reduction of Carbon Dioxide. ACS Energy Lett. 2020, 5, 552–558. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, N.; Han, P.; Kuang, M.; Mei, B.; Jiang, Z.; Zhong, J.; Li, L.; Zheng, G. Oxygen Vacancy Tuning toward Efficient Electrocatalytic CO2 Reduction to C2H4. Small Methods 2019, 3, 1800449. [Google Scholar]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 Reduction: A Classification Problem. ChemPhysChem 2017, 18, 3266–3273. [Google Scholar] [CrossRef] [PubMed]
- Won, D.H.; Hyuck, C.; Jaehoon, C.; Min, C.; Chung, W.; Kim, E.-H.; Woo, S.I. Rational Design of a Hierarchical Tin Dendrite Electrode for Efficient Electrochemical Reduction of CO2. ChemSusChem 2015, 8, 3092–3098. [Google Scholar] [CrossRef]
- Qin, B.; Wang, H.; Peng, F.; Yu, H.; Cao, Y. Effect of the surface roughness of copper substrate on three-dimensional tin electrode for electrochemical reduction of CO2 into HCOOH. J. CO2 Util. 2017, 21, 219–223. [Google Scholar] [CrossRef]
- Sacco, A.; Zeng, J.; Bejtka, K.; Chiodoni, A. Modeling of bubble-induced dependent mass transport in the electrochemical reduction of carbon dioxide on nanostructured electrodes. J. Catal. 2019, 372, 39–48. [Google Scholar] [CrossRef]
- Ju, W.; Zeng, J.; Bejtka, K.; Ma, H.; Rentsch, D.; Castellino, M.; Sacco, A.; Pirri, C.F.; Battaglia, C. Sn-Decorated Cu for Selective Electrochemical CO2 to CO Conversion: Precision Architecture beyond Composition Design. ACS Appl. Energy Mater. 2019, 2, 867–872. [Google Scholar] [CrossRef]
- Alfath, M.; Lee, C.W. Recent Advances in the Catalyst Design and Mass Transport Control for the Electrochemical Reduction of Carbon Dioxide to Formate. Catalysts 2020, 10, 859. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejtka, K.; Monti, N.B.D.; Sacco, A.; Castellino, M.; Porro, S.; Farkhondehfal, M.A.; Zeng, J.; Pirri, C.F.; Chiodoni, A. Zn- and Ti-Doped SnO2 for Enhanced Electroreduction of Carbon Dioxide. Materials 2021, 14, 2354. https://doi.org/10.3390/ma14092354
Bejtka K, Monti NBD, Sacco A, Castellino M, Porro S, Farkhondehfal MA, Zeng J, Pirri CF, Chiodoni A. Zn- and Ti-Doped SnO2 for Enhanced Electroreduction of Carbon Dioxide. Materials. 2021; 14(9):2354. https://doi.org/10.3390/ma14092354
Chicago/Turabian StyleBejtka, Katarzyna, Nicolò B. D. Monti, Adriano Sacco, Micaela Castellino, Samuele Porro, M. Amin Farkhondehfal, Juqin Zeng, Candido F. Pirri, and Angelica Chiodoni. 2021. "Zn- and Ti-Doped SnO2 for Enhanced Electroreduction of Carbon Dioxide" Materials 14, no. 9: 2354. https://doi.org/10.3390/ma14092354
APA StyleBejtka, K., Monti, N. B. D., Sacco, A., Castellino, M., Porro, S., Farkhondehfal, M. A., Zeng, J., Pirri, C. F., & Chiodoni, A. (2021). Zn- and Ti-Doped SnO2 for Enhanced Electroreduction of Carbon Dioxide. Materials, 14(9), 2354. https://doi.org/10.3390/ma14092354












