Influence of Zirconia and Organic Additives on Mechanical and Electrochemical Properties of Silica Sol-Gel Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sol-Gel Materials
2.2. Preparation of Steel Substrates
2.3. Obtained Coatings and Stabilization
2.4. SEM and EDX
2.5. Raman Spectroscopy
2.6. Scratch Test
2.7. Nanointendation Test
2.8. Electrochemical Test
3. Results and Discussion
3.1. SEM and EDX
3.2. Raman Spectroscopy
3.3. Scratch Test
- for SiO2/ZrO2, the characteristic coating cracks were observed at the edges of scratches; they seemed to conform to the groove, which meant conformal cracking (PN-EN 1071-3:2007).
- for SiO2/GPTMS/ZrO2, the cracks were observed within the groove, which indicated Hertz cracking (PN-EN 1071-3:2007).
3.4. Nanointendation Test
3.5. Electrochemical Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yazıcı, M.; Çomaklı, O.; Yetim, T.; Yetim, A.F.; Çelik, A. Effect of sol aging time on the wear properties of TiO2–SiO2 composite films prepared by a sol–gel method. Tribol. Int. 2016, 104, 175–182. [Google Scholar] [CrossRef]
- Tlili, B.; Barkaoui, A.; Walock, M. Tribology and wear resistance of the stainless steel. The sol–gel coating impact on the friction and damage. Tribol. Int. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Thai, T.T.; Druart, M.-E.; Paint, Y.; Trinh, A.T.; Olivier, M.-G. Influence of the sol-gel mesoporosity on the corrosion protection given by an epoxy primer applied on aluminum alloy 2024–T3. Prog. Org. Coat. 2018, 121, 53–63. [Google Scholar] [CrossRef]
- Torrico, R.F.A.O.; Harb, S.V.; Trentin, A.; Uvida, M.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Structure and properties of epoxy-siloxane-silica nanocomposite coatings for corrosion protection. J. Colloid Interface Sci. 2018, 513, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, R.; Jiang, J.; Wang, Z. Environmentally-friendly superhydrophobic surface based on Al2O3@KH560@SiO2 electrokinetic nanoparticle for long-term anti-corrosion in sea water. Appl. Surf. Sci. 2019, 484, 307–316. [Google Scholar] [CrossRef]
- Gąsiorek, J.; Szczurek, A.; Babiarczuk, B.; Kaleta, J.; Jones, W.; Krzak, J. Functionalizable Sol-Gel Silica Coatings for Corrosion Mitigation. Materials 2018, 11, 197. [Google Scholar] [CrossRef] [Green Version]
- Pehkonen, S.O.; Yuan, S. General Background of Sol-Gel Coatings for Corrosion Mitigation. In Tailored Thin Coatings for Corrosion Inhibition Using a Molecular Approach; Elsevier: London, UK; Oxford, UK; Cambridge, UK; San Diego, CA, USA, 2018; Volume 23, pp. 63–112. [Google Scholar]
- Liu, M.M.; Hu, H.X.; Zheng, Y.G.; Wang, J.Q.; Gan, Z.H.; Qiu, S. Effect of sol-gel sealing treatment loaded with different cerium salts on the corrosion resistance of Fe-based amorphous coating. Surf. Coat. Technol. 2019, 367, 311–326. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Aruna, S.T.; Sampath, S. Ceria nanoparticles vis-à-vis cerium nitrate as corrosion inhibitors for silica-alumina hybrid sol-gel coating. Appl. Surf. Sci. 2017, 393, 397–404. [Google Scholar] [CrossRef]
- Ashrafi-Shahri, S.M.; Ravari, F.; Seifzadeh, D. Smart organic/inorganic sol-gel nanocomposite containing functionalized mesoporous silica for corrosion protection. Prog. Org. Coat. 2019, 133, 44–54. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bera, P.; Anandan, C.; Basu, B.J. Effect of the size of silica nanoparticles on wettability and surface chemistry of sol–gel superhydrophobic and oleophobic nanocomposite coatings. Appl. Surf. Sci. 2014, 320, 780–786. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. Application of novel sol–gel composites on magnesium alloy. J. Magnes. Alloy. 2019, 7, 419–432. [Google Scholar] [CrossRef]
- Krzak, J.; Szczurek, A.; Babiarczuk, B.; Gąsiorek, J.; Borak, B. Sol–Gel Surface Functionalization Regardless of Form and Type of Substrate. In Handbook of Nanomaterials for Manufacturing Applications; Elsevier: London, UK; Oxford, UK; Cambridge, UK; San Diego, CA, USA, 2020; pp. 111–147. [Google Scholar]
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G.S. Sol–gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099–5111. [Google Scholar] [CrossRef]
- Krzak, J.; Borak, B.; Łukowiak, A.; Donesz-Sikorska, A.; Babiarczuk, B.; Marycz, K.; Szczurek, A. Advancement of Surface by Applying a Seemingly Simple Sol-gel Oxide Materials. In Advanced Surface Engineering Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 33–96. [Google Scholar]
- Figueira, R.B. Hybrid sol-gel coatings for corrosion mitigation: A critical review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idumah, C.I.; Obele, C.M.; Emmanuel, E.O.; Hassan, A.; Azikiwe, N. Recently Emerging Nanotechnological Advancements in Polymer Nanocomposite Coatings for Anti-corrosion, Anti-fouling and Self-healing. Surf. Interfaces 2020, 100734. [Google Scholar] [CrossRef]
- Sanaullah, I.; Bukhari, B.S.; Batool, T.; Riaz, S.; Khan, H.N.; Sabri, A.N.; Naseem, S. Antibacterial performance of glucose-fructose added MW based zirconia coatings—Possible treatment for bone infection. J. Mech. Behav. Biomed. Mater. 2020, 104, 103621. [Google Scholar] [CrossRef] [PubMed]
- Shanaghi, A.; Souri, A.R.; Chu, P.K. EIS and noise study of zirconia-alumina- benzotriazole nano-composite coating applied on Al2024 by the sol-gel method. J. Alloys Compd. 2020, 816, 152662. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Umeda, K. The friction and wear characteristics of low-pressure plasma-sprayed ZrO2-BaCrO4 composite coatings at elevated temperatures. Surf. Coat. Technol. 2002, 154, 131–139. [Google Scholar] [CrossRef]
- Celik, E.; Islamoglu, Y.; Akin, Y.; Hascicek, Y.S. Thermal analysis of high temperature ZrO2 insulation ceramic coatings on Ag tapes used as sheath of Bi-2212 superconducting materials using finite element method. Mater. Des. 2003, 24, 543–546. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.B.; Zhan, J.; Chen, Y.Q.; Hu, J.M. Electrodeposited superhydrophobic mesoporous silica films co-embedded with template and corrosion inhibitor for active corrosion protection. Appl. Surf. Sci. 2020, 508, 145242. [Google Scholar] [CrossRef]
- Cui, M.; Shen, Y.; Tian, H.; Yang, Y.; Feng, H.; Li, J. Influence of water adhesion of superhydrophobic surfaces on their anti-corrosive behavior. Surf. Coat. Technol. 2018, 347, 38–45. [Google Scholar] [CrossRef]
- Metroke, T.L.; Apblett, A. Effect of solvent dilution on corrosion protective properties of Ormosil coatings on 2024-T3 aluminum alloy. Prog. Org. Coat. 2004, 51, 36–46. [Google Scholar] [CrossRef]
- Khramov, A.N.; Johnson, J.A. Phosphonate-functionalized ORMOSIL coatings for magnesium alloys. Prog. Org. Coat. 2009, 65, 381–385. [Google Scholar] [CrossRef]
- Salazar-Hernández, C.; Salazar-Hernández, M.; Carrera-Cerritos, R.; Mendoza-Miranda, J.M.; Elorza-Rodríguez, E.; Miranda-Avilés, R.; Mocada-Sánchez, C.D. Anticorrosive properties of PDMS-Silica coatings: Effect of methyl, phenyl and amino groups. Prog. Org. Coat. 2019, 136, 105220. [Google Scholar] [CrossRef]
- Pehkonen, S.O.; Yuan, S. Inorganic-Organic Hybrid Coatings. In Interface Science and Technology; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 23, pp. 115–132. [Google Scholar]
- Rodič, P.; Milošev, I.; Lekka, M.; Andreatta, F.; Fedrizzi, L. Corrosion behaviour and chemical stability of transparent hybrid sol-gel coatings deposited on aluminium in acidic and alkaline solutions. Prog. Org. Coat. 2018, 124, 286–295. [Google Scholar] [CrossRef]
- Soares, B.G.; Bezerra, B.M.; Barros, D.N.; Silva, A.A. Epoxy modified with urea-based ORMOSIL and isocyanate-functionalized polybutadiene: Viscoelastic and adhesion properties. Compos. Part B Eng. 2019, 168, 334–341. [Google Scholar] [CrossRef]
- Thai, T.T.; Trinh, A.T.; Olivier, M.G. Hybrid sol–gel coatings doped with cerium nanocontainers for active corrosion protection of AA2024. Prog. Org. Coat. 2020, 138, 105428. [Google Scholar] [CrossRef]
- Varma, P.C.R.; Colreavy, J.; Cassidy, J.; Oubaha, M.; McDonagh, C.; Duffy, B. Corrosion protection of AA 2024-T3 aluminium alloys using 3, 4-diaminobenzoic acid chelated zirconium-silane hybrid sol-gels. Thin Solid Film. 2010, 518, 5753–5761. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Zhang, D.; Hou, L.; Li, X.; Wei, Y. Understanding of the corrosion protection by V(IV) conversion coatings from a sol-gel perspective. Corros. Sci. 2019, 161, 108196. [Google Scholar] [CrossRef]
- Yu, M.; Liang, M.; Liu, J.; Li, S.; Xue, B.; Zhao, H. Effect of chelating agent acetylacetone on corrosion protection properties of silane-zirconium sol-gel coatings. Appl. Surf. Sci. 2016, 363, 229–239. [Google Scholar] [CrossRef]
- Bandeira, R.M.; van Drunen, J.; Garcia, A.C.; Tremiliosi-Filho, G. Influence of the thickness and roughness of polyaniline coatings on corrosion protection of AA7075 aluminum alloy. Electrochim. Acta 2017, 240, 215–224. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Dong, C.; Wu, J.; Li, X.; Huang, Y. In situ Raman spectroscopy study of corrosion products on the surface of carbon steel in solution containing Cl−and SO42. Eng. Fail. Anal. 2011, 18, 1981–1989. [Google Scholar] [CrossRef]
- Colomban, P. 28 Potential and Drawbacks of Raman (Micro)Spectrometry for the Understanding of Iron and Steel Corrosion. New Trends and Developments in Automotive System Enginnering. In New Trends and Developments in Automotive System Engineering; Intech: Rijeka, Croatia, 2011; pp. 567–584. [Google Scholar]
- Jitianu, A.; Cadars, S.; Zhang, F.; Rodriguez, G.; Picard, Q.; Aparicio, M.; Mosa, J.; Klein, L.C. 29Si NMR and SAXS investigation of the hybrid organic-inorganic glasses obtained by consolidation of the melting gels. Dalt. Trans. 2017, 46, 3729–3741. [Google Scholar] [CrossRef] [Green Version]
- Pakjamsai, C.; Kobayashi, N.; Koyano, M.; Sasaki, S.; Kawakami, Y. Characterization of the benzene-insoluble fraction of the hydrolyzate of phenyltrimethoxysilanes in the presence of benzyltrimethylammonium hydroxide. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4587–4597. [Google Scholar] [CrossRef]
- Szczurek, A.; Paszkowski, M.; Lewandowski, D.; Gąsiorek, J.; Kaleta, J.; Krzak, J. Organically functionalized sol-gel silica network growth. Ceram. Int. 2020, 46, 13198–13204. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK; New York, NY, USA; Weinheim, Germany; Toronto, ON, Canada; Brisbane, Australia; Singapore, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Gizdavic-Nikolaidis, M.R.; Zujovic, Z.D.; Edmonds, N.R.; Bolt, C.J.; Easteal, A.J. Spectroscopic characterization of GPTMS/DETA and GPTMS/EDA hybrid polymers. J. Non-Cryst. Solids 2007, 353, 1598–1605. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Gozzi, G.; Chinaglia, D.L.; Oliveira, O.N.; de Vicente, F.S. Proton conduction mechanisms in GPTMS/TEOS-derived organic/silica hybrid films prepared by sol-gel process. Synth. Met. 2020, 267, 116448. [Google Scholar] [CrossRef]
- Rauter, A.; Slemenik Perše, L.; Orel, B.; Bengu, B.; Sunetci, O.; Šurca Vuk, A. Ex situ IR and Raman spectroscopy as a tool for studying the anticorrosion processes in (3-glycidoxypropyl) trimethoxysilane-based sol-gel coatings. J. Electroanal. Chem. 2013, 703, 97–107. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Condrate, R.A. The infrared and Raman spectra of ZrO2-SiO2 glasses prepared by a sol-gel process. J. Mater. Sci. 1988, 23, 2951–2959. [Google Scholar] [CrossRef]
- Ehrhart, G.; Capoen, B.; Robbe, O.; Boy, P.; Turrell, S.; Bouazaoui, M. Structural and optical properties of n-propoxide sol-gel derived ZrO 2 thin films. Thin Solid Film. 2006, 496, 227–233. [Google Scholar] [CrossRef]
- Naumenko, A.; Gnatiuk, I.; Smirnova, N.; Eremenko, A. Characterization of sol-gel derived TiO2/ZrO2 films and powders by Raman spectroscopy. In Thin Solid Films; Elsevier: Amsterdam, The Netherlands, 2012; Volume 520, pp. 4541–4546. [Google Scholar]
- Chiodini, N.; Meinardi, F.; Morazzoni, F.; Paleari, A.; Scotti, R.; Spinolo, G. Tin doped silica by sol-gel method: Doping effects on the SiO2 Raman spectrum. Solid State Commun. 1998, 109, 145–150. [Google Scholar] [CrossRef]
- Geringer, J.; Atmani, F.; Forest, B. Friction-corrosion of AISI 316L/bone cement and AISI 316L/PMMA contacts: Ionic strength effect on tribological behaviour. Wear 2009, 267, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Sudesh, T.; Wijesinghe, L.; Blackwood, J. Electrochemical & optical characterisation of passive films on stainless steels. J. Phys. Conf. Ser. 2006, 28, 74–78. [Google Scholar] [CrossRef]
- Keyvani, A.; Bahamirian, M.; Esmaeili, B. Sol-gel synthesis and characterization of ZrO2-25wt.%CeO2-2.5wt.%Y2O3 (CYSZ) nanoparticles. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Łęcka, K.M.; Gąsiorek, J.; Mazur-Nowacka, A.; Szczygieł, B.; Antończak, A.J. Adhesion and corrosion resistance of laser-oxidized titanium in potential biomedical application. Surf. Coat. Technol. 2019, 366, 179–189. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Ballarre, J.; Jimenez-Pique, E.; Anglada, M.; Pellice, S.A.; Cavalieri, A.L. Mechanical characterization of nano-reinforced silica based sol-gel hybrid coatings on AISI 316L stainless steel using nanoindentation techniques. Surf. Coat. Technol. 2009, 203, 3325–3331. [Google Scholar] [CrossRef]
- Díaz-Parralejo, A.; Díaz-Díez, M.Á.; Sánchez-González, J.; Macías-García, A.; Carrasco-Amador, J.P. Mechanical properties and thermal shock in thin ZrO2–Y2O3–Al2O3 films obtained by the sol-gel method. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Mazur, A.; Chęcmanowski, J.; Szczygieł, B. Corrosive resistance of 316L stainless Steel covered with SiO2 coatings deposited by sol-gel method in a simulated body fluid. Corros. Prot. 2016, 59, 160–168. [Google Scholar] [CrossRef]
- Kamiński, J.; Tarnowski, M.; Wierzchoń, T. Corrosion resistance of nitrocarburized layers produced on Ti6Al7Nb titanium alloy. Corros. Prot. 2017, 60, 91–95. [Google Scholar] [CrossRef]
- Mazur, A.; Szczurek, A.; Chęcmanowski, J.G.; Szczygieł, B. Corrosion resistance and bioactivity of SiO2-Y2O3 coatings deposited on 316L steel. Surf. Coat. Technol. 2018, 350, 502–510. [Google Scholar] [CrossRef]
- Su, Y.; Li, K.; Zhang, L.; Sun, J.; Liu, S.; Liu, G. Effect of microwave heating time on bonding strength and corrosion resistance of Ca-P composite layers for carbon/carbon composites. J. Alloys Compd. 2017, 713, 266–279. [Google Scholar] [CrossRef]
- Farahani, M.; Yousefnia, H.; Seyedraoufi, Z.S.; Shajari, Y. The effect of benzotriazole gradual change on the corrosion performance of nanocomposite multilayer self-healing coating based on Titania-Alumina-Benzotriazole on AA7075. Ceram. Int. 2019, 45, 16584–16590. [Google Scholar] [CrossRef]
- Croll, S.G. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
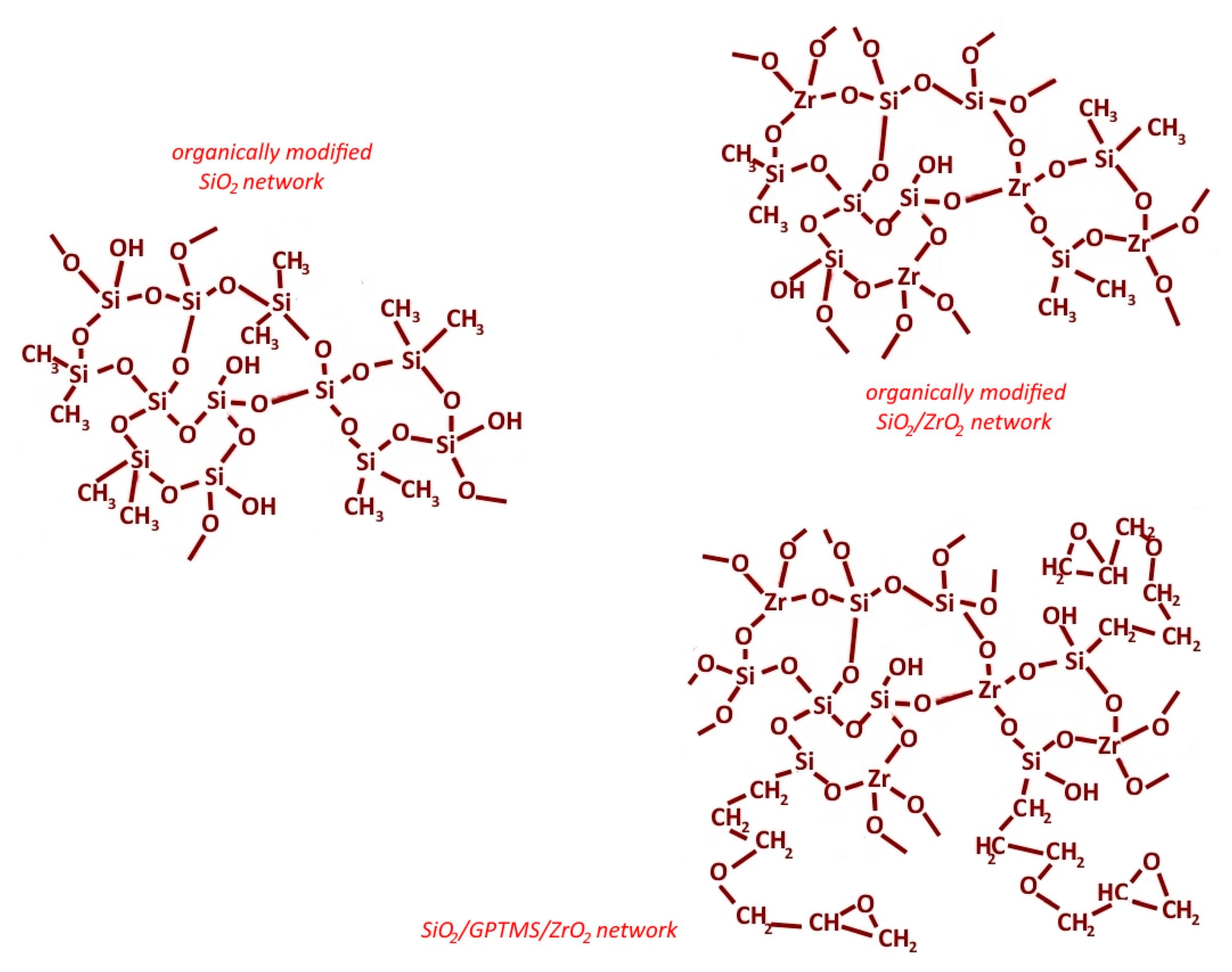















| Sol | Chemical Compounds |
|---|---|
| SiO2 | TEOS + dMdEOS + EtOH + HCl (1:1:4:0.02) |
| SiO2/ZrO2 |
|
| |
| SiO2/GPTMS/ZrO2 |
|
|
| Element | P265GH | 316L |
|---|---|---|
| C | 0.20 | 0.03 |
| Mn | 1.40 | 2.00 |
| Si | 0.40 | 1.00 |
| P | 0.025 | 0.045 |
| S | 0.02 | 0.03 |
| Cr | 0.30 | 16.00–18.00 |
| Mo | 0.08 | 2.00–3.00 |
| Ni | 0.30 | 10.00–15.00 |
| Al | 0.02 | - |
| Cu | 0.30 | - |
| Nb | 0.01 | - |
| Ti | 0.04 | - |
| V | 0.02 | - |
| N | - | 0.11 |
| Fe | 96.89 | 60.79–68.79 |
| Coating | Value of the Characteristic Forces | ||
|---|---|---|---|
| LC1 (N) | LC2 (N) | LC3 (N) | |
| Substrate: P265GH Steel | |||
| SiO2/ZrO2 | 1.49 ± 0.59 | 4.17 ± 0.29 | 8.64 ± 0.81 |
| SiO2/GPTMS/ZrO2 | 1.29 ± 0.02 | 6.49 ± 0.94 | 15.76 ± 3.22 |
| Substrate: 316L Steel | |||
| SiO2/ZrO2 | 2.58 ± 0.66 | 5.10 ± 0.97 | 7.49 ± 3.20 |
| SiO2/GPTMS/ZrO2 | 1.92 ± 0.31 | 7.35 ± 0.29 | 12.46 ± 1.11 |
| Samples | EIT (GPa) | HIT (Vickers) |
|---|---|---|
| Uncoated steel | 119 ± 34 | 236 ± 22 |
| SiO2/ZrO2 | 51 ± 27 | 236 ± 14 |
| SiO2/GPTMS/ZrO2 | 7 ± 2 | 41 ± 12 |
| Material | OCP | ||
|---|---|---|---|
| Ecorr (mV) | Rp (kΩ·cm2) | jcorr (μA/cm2) | |
| After 0 h Duration in 3% NaCl | |||
| uncoated P265GH | −596.1 | 0.889 | 29 |
| uncoated P265GH heat at 250 °C | −646.3 | 0.319 | 82 |
| SiO2/ZrO2 | −570.2 | 1.706 | 15 |
| SiO2/GPTMS/ZrO2 | −497.4 | 7.273 | 3.6 |
| After 24 h Duration in 3% NaCl | |||
| uncoated P265GH | −692.7 | 0.597 | 44 |
| uncoated P265GH heat at 250 °C | −643.9 | 0.427 | 61 |
| SiO2/ZrO2 | −653.3 | 0.576 | 45 |
| SiO2/GPTMS/ZrO2 | −613.2 | 1.878 | 13 |
| Material | OCP | ||
|---|---|---|---|
| Ecorr (mV) | Rp (MΩ·cm2) | jcorr (nA/cm2) | |
| After 0 h Duration 3% NaCl | |||
| uncoated 316L | −328.5 | 0.31 | 85 |
| uncoated 316L heat at 250 °C | −294.9 | 1.4 | 18 |
| SiO2/ZrO2 | −344.3 | 2.0 | 13 |
| SiO2/GPTMS/ZrO2 | −239.8 | 1.5 | 17 |
| After 24 h Duration in 3% NaCl | |||
| uncoated 316L | −231.9 | 0.07 | 340 |
| uncoated 316L heat at 250 °C | −333.4 | 0.08 | 320 |
| SiO2/ZrO2 | −372.0 | 0.17 | 150 |
| SiO2/GPTMS/ZrO2 | −363.8 | 5.9 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gąsiorek, J.; Mazur-Nowacka, A.; Szczurek, A.; Babiarczuk, B.; Tic, W.J.; Guziałowska-Tic, J.; Kaleta, J.; Krzak, J. Influence of Zirconia and Organic Additives on Mechanical and Electrochemical Properties of Silica Sol-Gel Coatings. Materials 2021, 14, 2389. https://doi.org/10.3390/ma14092389
Gąsiorek J, Mazur-Nowacka A, Szczurek A, Babiarczuk B, Tic WJ, Guziałowska-Tic J, Kaleta J, Krzak J. Influence of Zirconia and Organic Additives on Mechanical and Electrochemical Properties of Silica Sol-Gel Coatings. Materials. 2021; 14(9):2389. https://doi.org/10.3390/ma14092389
Chicago/Turabian StyleGąsiorek, Jolanta, Anna Mazur-Nowacka, Anna Szczurek, Bartosz Babiarczuk, Wilhelm Jan Tic, Joanna Guziałowska-Tic, Jerzy Kaleta, and Justyna Krzak. 2021. "Influence of Zirconia and Organic Additives on Mechanical and Electrochemical Properties of Silica Sol-Gel Coatings" Materials 14, no. 9: 2389. https://doi.org/10.3390/ma14092389






