Mechanochemical Synthesis of Pt/Nb2CTx MXene Composites for Enhanced Electrocatalytic Hydrogen Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
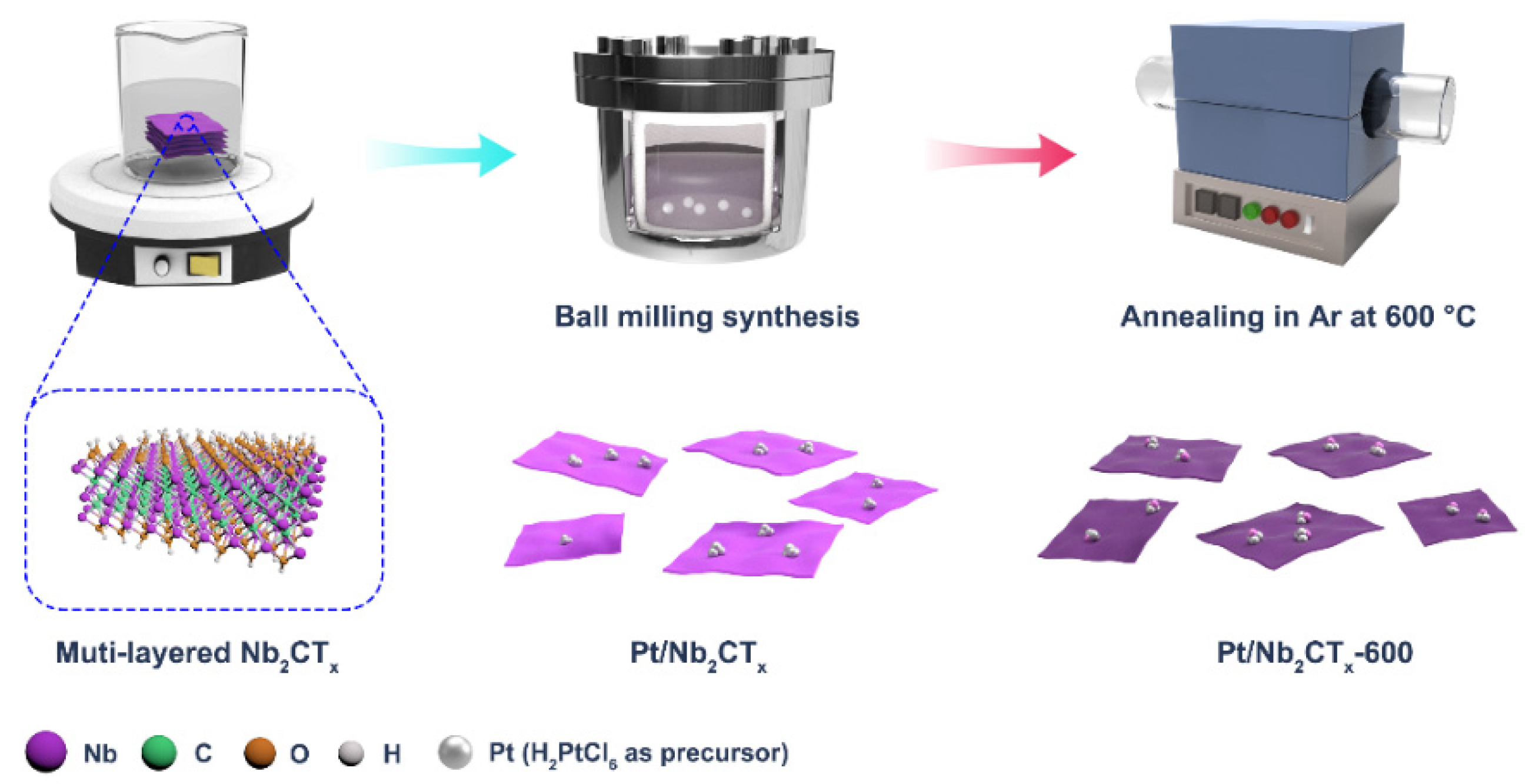
2.2. Preparation of Nb2CTx Based Catalysts
2.3. Characterizations of Nb2CTx Based Catalysts
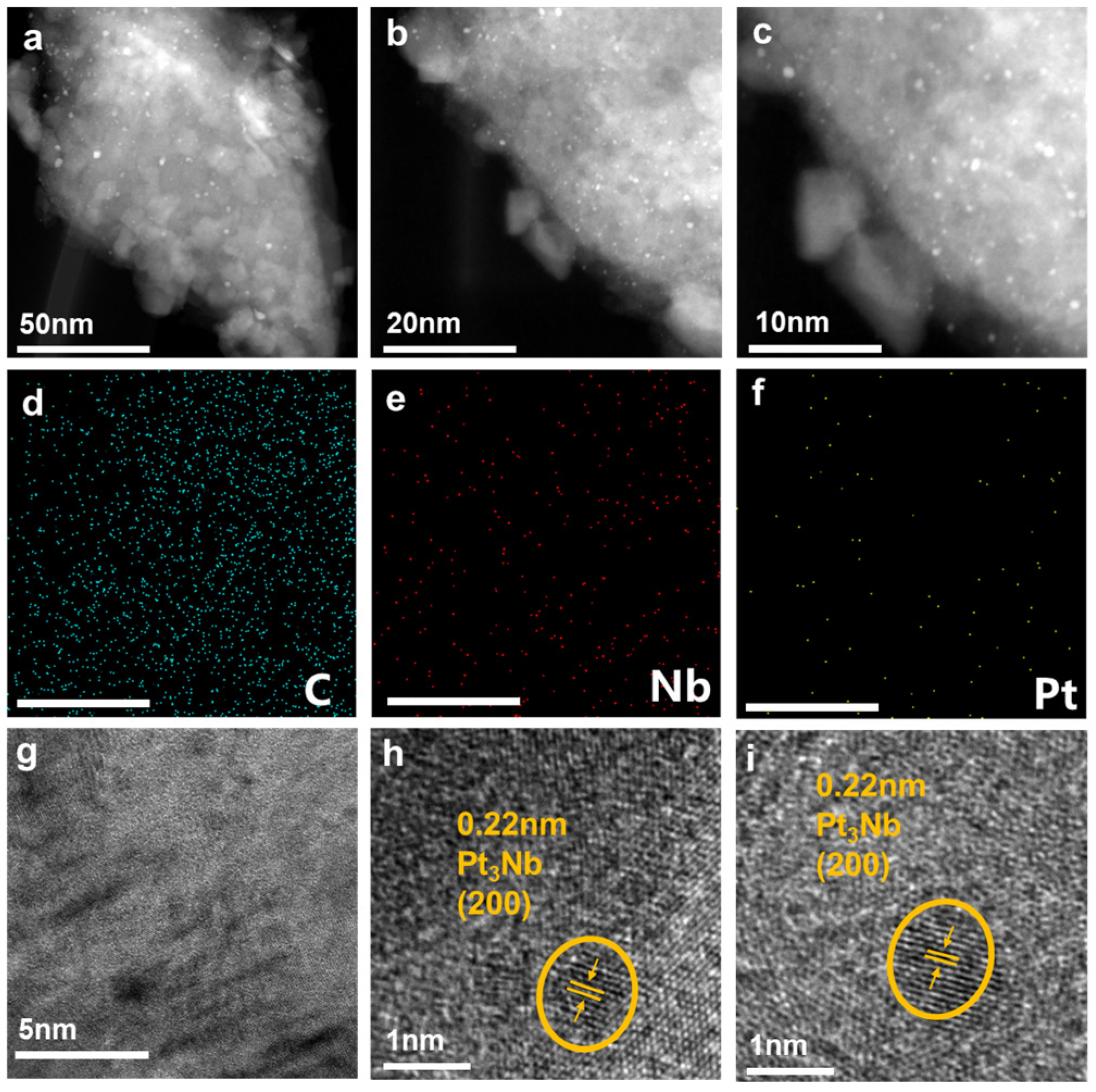
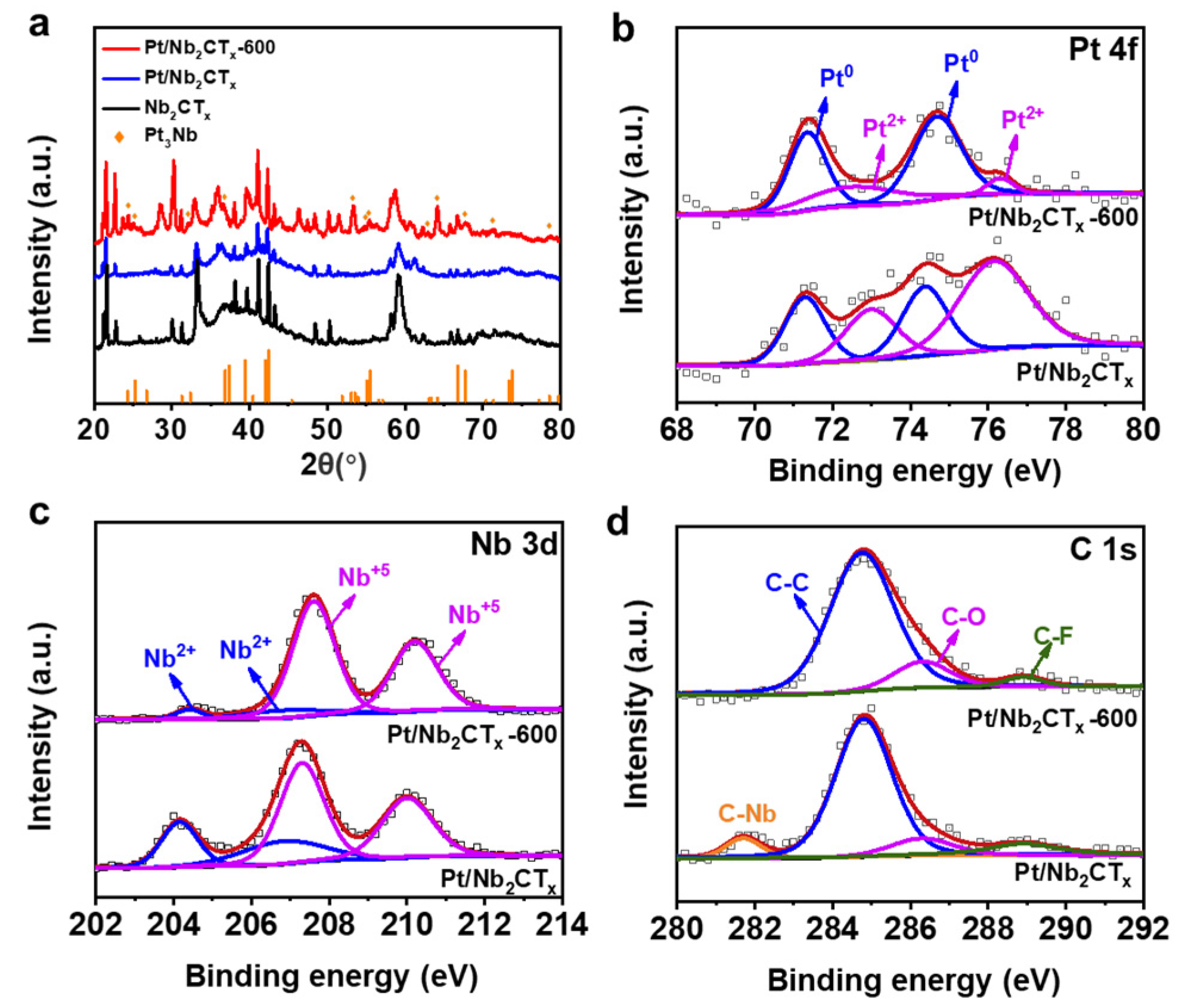
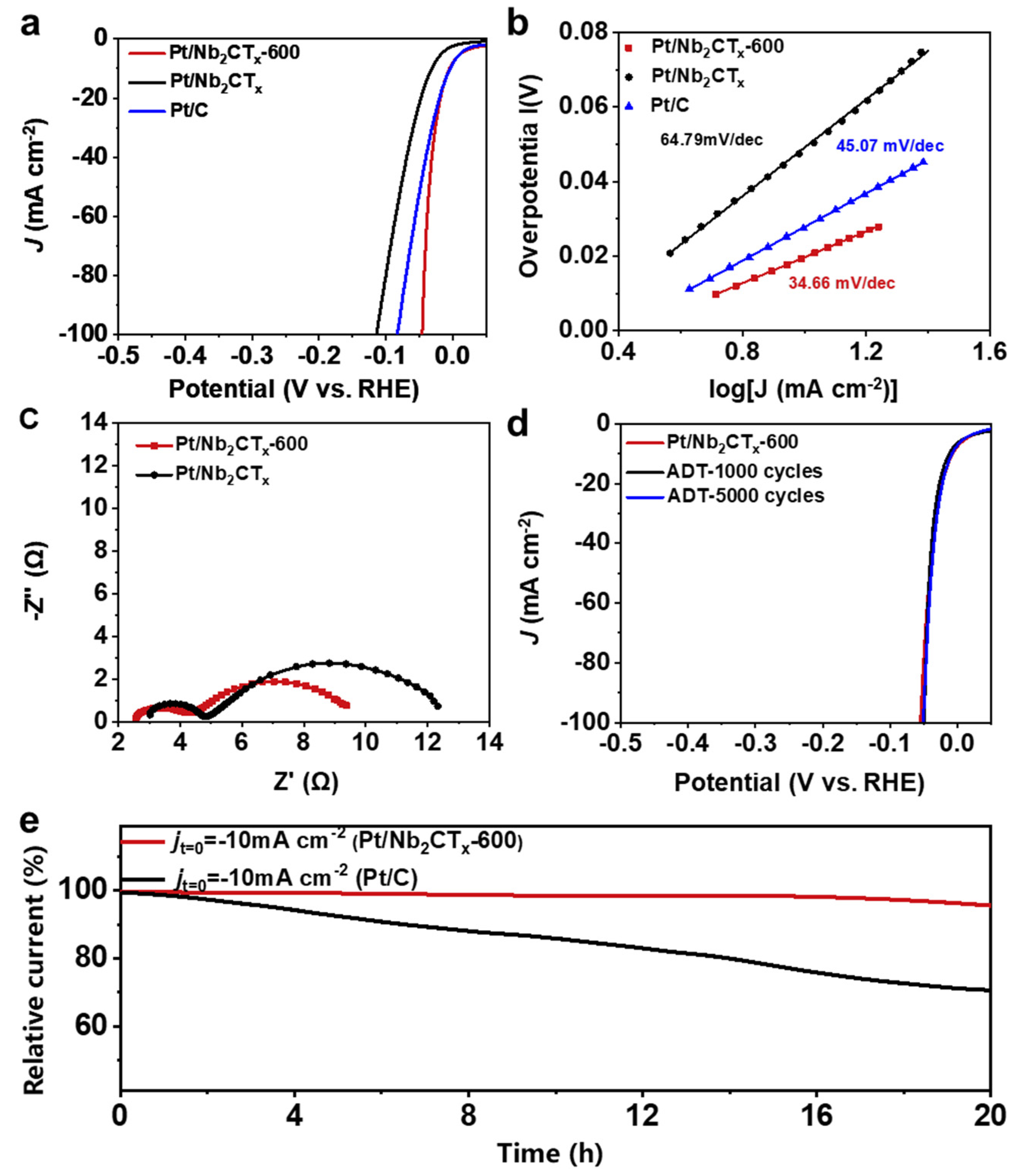
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Yang, C.; Rousse, G.; Svane, K.L.; Pearce, P.E.; Abakumov, A.M.; Deschamps, M.; Cibin, G.; Chadwick, A.V.; Corte, D.A.D.; Hansen, H.A.; et al. Cation insertion to break the activity/stability relationship for highly active oxygen evolution reaction catalyst. Nat. Commun. 2020, 11, 1378. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Huang, K.; Wang, R.; Zhao, S.; Du, P.; Wang, H.; Wei, H.; Long, Y.; Deng, B.; Lei, M.; Ge, B.; et al. Atomic species derived CoOx clusters on nitrogen doped mesoporous carbon as advanced bifunctional electro-catalysts for Zn-air battery. Energy Storage Mater. 2020, 29, 156–162. [Google Scholar] [CrossRef]
- Sun, Y.; Alimohammadi, F.; Zhang, D.; Guo, G. Enabling colloidal synthesis of edge-oriented MoS2 with expanded interlayer spacing for enhanced HER catalysis. Nano Lett. 2017, 17, 1963–1969. [Google Scholar] [CrossRef]
- Huang, K.; Xu, Y.; Song, Y.; Wang, R.; Wei, H.; Long, Y.; Lei, M.; Tang, H.; Guo, J.; Wu, H. NiPS3 quantum sheets modified nitrogen-doped mesoporous carbon with boosted bifunctional oxygen electrocatalytic performance. J. Mater. Sci. Technol. 2021, 65, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; He, P.; Yang, Y.; Pan, Y.; Jin, Z.; Ling, R. Heterostructure Co3O4@ NiWO4 nanocone arrays with enriched active area for efficient hydrogen evolution reaction. J. Alloys Compd. 2020, 844, 156095. [Google Scholar] [CrossRef]
- Huang, K.; Guo, S.; Wang, R.; Lin, S.; Hussain, N.; Wei, H.; Deng, B.; Long, Y.; Lei, M.; Tang, H.; et al. Two-dimensional MOF/MOF derivative arrays on nickel foam as efficient bifunctional coupled oxygen electrodes. Chin. J. Catal. 2020, 41, 1754–1760. [Google Scholar] [CrossRef]
- Li, M.; Duanmu, K.; Wan, C.; Cheng, T.; Zhang, L.; Dai, S.; Chen, W.; Zhao, Z.; Li, P.; Fei, H.; et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis. Nat. Catal. 2019, 2, 495–503. [Google Scholar] [CrossRef]
- Duan, H.; Li, D.; Tang, Y.; He, Y.; Ji, S.; Wang, R.; Lv, H.; Lopes, P.P.; Paulikas, A.P.; Li, H.; et al. High-performance Rh2P electrocatalyst for efficient water splitting. J. Am. Chem. Soc. 2017, 139, 5494–5502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, D.; Li, Y. Understanding of the major reactions in solution synthesis of functional nanomaterials. Sci. China Mater. 2016, 59, 938–996. [Google Scholar] [CrossRef]
- Campbell, C.T.; Parker, S.C.; Starr, D.E. The effect of size-dependent nanoparticle energetics on catalyst sintering. Science 2002, 298, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ming, M.; Niu, S.; Zhang, Y.; Fan, G.; Hu, J.S. Scalable Solid-State Synthesis of Highly Dispersed Uncapped Metal (Rh, Ru, Ir) Nanoparticles for Efficient Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801698. [Google Scholar] [CrossRef]
- García-Peña, N.G.; Redón, R.; Herrera-Gomez, A.; Fernández-Osorio, A.L.; Bravo-Sanchez, M.; Gomez-Sosa, G. Solventless synthesis of ruthenium nanoparticles. Appl. Surf. Sci. 2015, 340, 25–34. [Google Scholar] [CrossRef]
- Shi, D.; Yang, M.; Chang, B.; Ai, Z.; Zhang, K.; Shao, Y.; Wang, S.; Wu, Y.; Hao, X. Ultrasonic-Ball Milling: A Novel Strategy to Prepare Large-Size Ultrathin 2D Materials. Small 2020, 16, 1906734. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A new family of promising catalysts for the hydrogen evolution reaction. ACS Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Xiu, L.; Pei, W.; Zhou, S.; Wang, Z.; Yang, P.; Zhao, J.; Qiu, J. Multilevel Hollow MXene Tailored Low-Pt Catalyst for Efficient Hydrogen Evolution in Full-pH Range and Seawater. Adv. Funct. Mater. 2020, 30, 1910028. [Google Scholar] [CrossRef]
- Yin, J.; Pan, S.; Guo, X.; Gao, Y.; Zhu, D.; Yang, Q.; Gao, J.; Zhang, C.; Chen, Y. Nb2C MXene-Functionalized Scaffolds Enables Osteosarcoma Phototherapy and Angiogenesis/Osteogenesis of Bone Defects. Nano-Micro Lett. 2021, 13, 30. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Mayorga-Martinez, C.C.; Vyskočil, J.; Sofer, Z.; Pumera, M. Integrated Biomonitoring Sensing with Wearable Asymmetric Supercapacitors Based on Ti3C2 MXene and 1T-Phase WS2 Nanosheets. Adv. Funct. Mater. 2020, 30, 2003673. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, C.; Siqueira, G.; Han, D.; Huch, A.; Abdolhosseinzadeh, S.; Heier, J.; Nüesch, F.; Zhang, C.; Nyström, G. Nanocellulose-MXene Biomimetic Aerogels with Orientation-Tunable Electromagnetic Interference Shielding Performance. Adv. Sci. 2020, 7, 2000979. [Google Scholar] [CrossRef] [PubMed]
- Penner, S.; Armbrüster, M. Formation of intermetallic compounds by reactive metal–support interaction: A frequently encountered phenomenon in catalysis. ChemCatChem 2015, 7, 374–392. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Milligan, C.; Ma, T.; Zhou, L.; Cui, Y.; Qi, Z.; Libretto, N.; Xu, B.; Luo, J.; et al. Two-dimensional transition metal carbides as supports for tuning the chemistry of catalytic nanoparticles. Nat. Commun. 2018, 9, 5258. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Y.; Wu, Z.; Milligan, C.; Zhou, L.; Mitchell, G.; Xu, B.; Shi, E.; Miller, J.T.; Ribeiro, F.H.; et al. Reactive metal–support interactions at moderate temperature in two-dimensional niobium-carbide-supported platinum catalysts. Nat. Catal. 2018, 1, 349–355. [Google Scholar] [CrossRef]
- Chen, J.; Herricks, T.; Xia, Y. Polyol synthesis of platinum nanostructures: Control of morphology through the manipulation of reduction kinetics. Angew. Chem. Int. Ed. 2005, 44, 2589–2592. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, Y.; Zhang, Y.; He, Q.; Xiao, D.; Peng, M.; Zhao, Y.; Zhang, H.; Luo, R.; Gan, T.; et al. Mechanochemical kilogram-scale synthesis of noble metal single-atom catalysts. Cell Rep. Phys. Sci. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Xu, S.L.; Shen, S.C.; Xiong, W.; Zhao, S.; Zuo, L.J.; Wang, L.; Zeng, W.J.; Chu, S.Q.; Chen, P.; Lin, Y.; et al. High-Temperature Synthesis of Small-Sized Pt/Nb Alloy Catalysts on Carbon Supports for Hydrothermal Reactions. Inorg. Chem. 2020, 59, 15953–15961. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Jia, Q.; Bates, M.K.; Li, J.; Xu, C.; Gath, K.; Yang, J.; Waldecker, J.; Che, H.; Liang, W.; et al. Tuning Nb–Pt interactions to facilitate fuel cell electrocatalysis. ACS Catal. 2017, 7, 4936–4946. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Zheng, L.; Yan, Y.; Zhang, Y.; Chen, G.; Sun, S.; Zhang, J. Highly active, stable oxidized platinum clusters as electrocatalysts for the hydrogen evolution reaction. Energy Environ. Sci. 2017, 10, 2450–2458. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, B.; Sun, Z.; Gao, Z.; Qin, Y.; Zhang, C.; Cui, F.; Yang, X. Platinum Nanoparticle-Deposited Ti3C2Tx MXene for Hydrogen Evolution Reaction. Ind. Eng. Chem. Res. 2020, 59, 1822–1828. [Google Scholar]
- Wang, C.K.; Sahu, D.; Wang, S.C.; Huang, J.L. Electrochromic Nb-doped WO3 films: Effects of post annealing. Ceram. Int. 2012, 38, 2829–2833. [Google Scholar]
- Su, T.; Peng, R.; Hood, Z.D.; Naguib, M.; Ivanov, I.N.; Keum, J.K.; Qin, Z.; Guo, Z.; Wu, Z. One-step synthesis of Nb2O5/C/Nb2C (MXene) composites and their use as photocatalysts for hydrogen evolution. ChemSusChem 2018, 11, 688–699. [Google Scholar] [PubMed]
- Huang, K.; Zhao, Z.; Du, H.; Du, P.; Wang, H.; Wang, R.; Lin, S.; Wei, H.; Long, Y.; Lei, M.; et al. Rapid thermal annealing toward high-quality 2D cobalt fluoride oxide as an advanced oxygen evolution electrocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 6905–6913. [Google Scholar]
- Kang, Y.J.; Jung, S.C.; Kim, H.J.; Han, Y.K.; Oh, S.H. Maximum catalytic activity of Pt3M in Li-O2 batteries: M=group V transition metals. Nano Energy 2016, 27, 1–7. [Google Scholar] [CrossRef]
- Yu, F.Y.; Lang, Z.L.; Yin, L.Y.; Feng, K.; Xia, Y.J.; Tan, H.Q.; Zhu, H.T.; Zhong, J.; Kang, Z.H.; Li, Y.J. Pt-O bond as an active site superior to Pt0 in hydrogen evolution reaction. Nat. Commun. 2020, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Amiinu, I.S.; Kou, Z.; Li, W.; Mu, S. RuP2-based catalysts with platinum-like activity and higher durability for the hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 2017, 56, 11559–11564. [Google Scholar]
- Ji, J.; Zhang, Y.; Tang, L.; Liu, C.; Gao, X.; Sun, M.; Zhang, J.; Ling, M.; Liang, C.; Lin, Z. Platinum single-atom and cluster anchored on functionalized MWCNTs with ultrahigh mass efficiency for electrocatalytic hydrogen evolution. Nano Energy 2019, 63, 103849. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Kim, H.E.; Cho, A.; Kim, S.; Ye, Y.; Wan, J.W.; Lee, H.; Jiang, J.H.; Lee, J. Investigation of the support effect in atomically dispersed Pt on WO3−x for utilization of Pt in the hydrogen evolution reaction. Angew. Chem. 2019, 131, 16184–16188. [Google Scholar] [CrossRef]
- Xie, C.; Chen, W.; Du, S.; Yan, D.; Zhang, Y.; Chen, J.; Liu, B.; Wang, S. In-situ phase transition of WO3 boosting electron and hydrogen transfer for enhancing hydrogen evolution on Pt. Nano Energy 2020, 71, 104653. [Google Scholar] [CrossRef]
- Li, Z.; Qi, Z.; Wang, S.; Ma, T.; Zhou, L.; Wu, Z.; Luan, X.; Lin, F.Y.; Chen, M.; Miller, J.T. In situ formed Pt3Ti nanoparticles on a two-dimensional transition metal carbide (MXene) used as efficient catalysts for hydrogen evolution reactions. Nano Lett. 2019, 19, 5102–5108. [Google Scholar] [CrossRef]
- Han, J.; Meng, X.; Lu, L.; Wang, Z.L.; Sun, C. Triboelectric nanogenerators powered electrodepositing tri-functional electrocatalysts for water splitting and rechargeable zinc-air battery: A case of Pt nanoclusters on NiFe-LDH nanosheets. Nano Energy 2020, 72, 104669. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.L.; Liu, R.S.; Han, C.P.; Li, Y. Gogotsi, Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Huang, K.; Wang, R.; Wu, H.; Wang, H.; He, X.; Wei, H.; Wang, S.; Zhang, R.; Lei, M.; Guo, W. Direct immobilization of an atomically dispersed Pt catalyst by suppressing heterogeneous nucleation at −40 °C. J. Mater. Chem. A 2019, 7, 25779–25784. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Zhou, L.; Chen, C.; Han, Z.; Zhang, B.; Wu, Q.; Yang, L.; Du, L.; Bu, Y.; et al. The simplest construction of single-site catalysts by the synergism of micropore trapping and nitrogen anchoring. Nat. Commun. 2019, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ye, R.; Wang, Q.; Liu, X.; Fang, P.; Hu, J. Facile synthesis of coral-like Pt nanoparticles/MXene (Ti3C2Tx) with efficient hydrogen evolution reaction activity. Ionics 2021, 27, 1221–1231. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Handoko, A.D.; Johnson, L.R.; Meng, X.; Lin, M.; Subramanian, G.S.; Anasori, B.; Gogotsi, Y.; Vojvodic, A.; She, Z.W. 2H-MoS2 on Mo2CTx MXene Nanohybrid for Efficient and Durable Electrocatalytic Hydrogen Evolution. ACS Nano 2020, 14, 16140–16155. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, B.; Wu, S.; Yu, J. A general approach to the synthesis of transition metal phosphide nanoarrays on MXene nanosheets for pH-universal hydrogen evolution and alkaline overall water splitting. J. Mater. Chem. A 2020, 8, 14234–14242. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Liu, Q.; Sun, P.; Wang, Y.; Chou, S.; Hu, Z.; Zhang, Z. Single-atom Ru anchored in nitrogen-doped MXene (Ti3C2Tx) as an efficient catalyst for the hydrogen evolution reaction at all pH values. J. Mater. Chem. A 2020, 8, 24710–24717. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, S.; Mi, Y.; Han, L.; Liu, X.; Qi, D.; Sun, J.; Liu, Y.; Bao, H.; Zhuo, L. Trifunctional Single-Atomic Ru Sites Enable Efficient Overall Water Splitting and Oxygen Reduction in Acidic Media. Small 2020, 16, 2002888. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Y.; Liu, S.; Li, J.; Bu, L.; Chen, J.; Xiao, X.; Choi, J.H.; Gao, L.; Lee, J.M. Confined growth of pyridinic N-Mo2C sites on MXenes for hydrogen evolution. J. Mater. Chem. A 2020, 8, 7109–7116. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, J.; Song, Y.; Zhang, Y. Single molybdenum atom anchored on 2D Ti2NO2 MXene as a promising electrocatalyst for N2 fixation. Nanoscale 2019, 11, 18132–18141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhuo, H.; Li, S.; Bao, Z.; Deng, S.; Zhuang, G.; Zhong, X.; Wei, Z.; Yao, Z.; Wang, J.G. Effects of surface functionalization of MXene-based nanocatalysts on hydrogen evolution reaction performance. Catal. Today 2020, 168, 187–195. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, S.; Wang, Z.; Liu, J.; Pei, W.; Yang, P.; Zhao, J.; Qiu, J. Engineering multifunctional collaborative catalytic interface enabling efficient hydrogen evolution in all pH range and seawater. Adv. Energy Mater. 2019, 9, 1901333. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Du, P.; Ma, X.; Wang, R.; Ma, J.; Wang, Y.; Fan, D.; Long, Y.; Deng, B.; Huang, K.; et al. Mechanochemical Synthesis of Pt/Nb2CTx MXene Composites for Enhanced Electrocatalytic Hydrogen Evolution. Materials 2021, 14, 2426. https://doi.org/10.3390/ma14092426
Fan X, Du P, Ma X, Wang R, Ma J, Wang Y, Fan D, Long Y, Deng B, Huang K, et al. Mechanochemical Synthesis of Pt/Nb2CTx MXene Composites for Enhanced Electrocatalytic Hydrogen Evolution. Materials. 2021; 14(9):2426. https://doi.org/10.3390/ma14092426
Chicago/Turabian StyleFan, Xiaoyuan, Peng Du, Xiaoxuan Ma, Ruyue Wang, Jingteng Ma, Yonggang Wang, Dongyu Fan, Yuanzheng Long, Bohan Deng, Kai Huang, and et al. 2021. "Mechanochemical Synthesis of Pt/Nb2CTx MXene Composites for Enhanced Electrocatalytic Hydrogen Evolution" Materials 14, no. 9: 2426. https://doi.org/10.3390/ma14092426
APA StyleFan, X., Du, P., Ma, X., Wang, R., Ma, J., Wang, Y., Fan, D., Long, Y., Deng, B., Huang, K., & Wu, H. (2021). Mechanochemical Synthesis of Pt/Nb2CTx MXene Composites for Enhanced Electrocatalytic Hydrogen Evolution. Materials, 14(9), 2426. https://doi.org/10.3390/ma14092426