Wetting Behavior of LBE on Corroded Candidate LFR Structural Materials of 316L, T91 and CLAM
Abstract
:1. Introduction
- Typical stainless steel such as 316L and T91 are neither wetting to LBE nor Pb. The differences are mainly induced by the different surface tension of LBE and Pb droplets or the reactivity of liquid metal (LM) on various steel at a certain temperature.
- The high temperature will increase the wettability of stainless steel to LBE. High roughness will make the wetting surfaces more wet and non-wetting surfaces less wet.
- The decrease in surface wettability of LBE on structural materials reduces the elements’ interdiffusion and reaction, which is beneficial to inhibit the occurrence of LME during service.
2. Materials and Methods
2.1. Materials
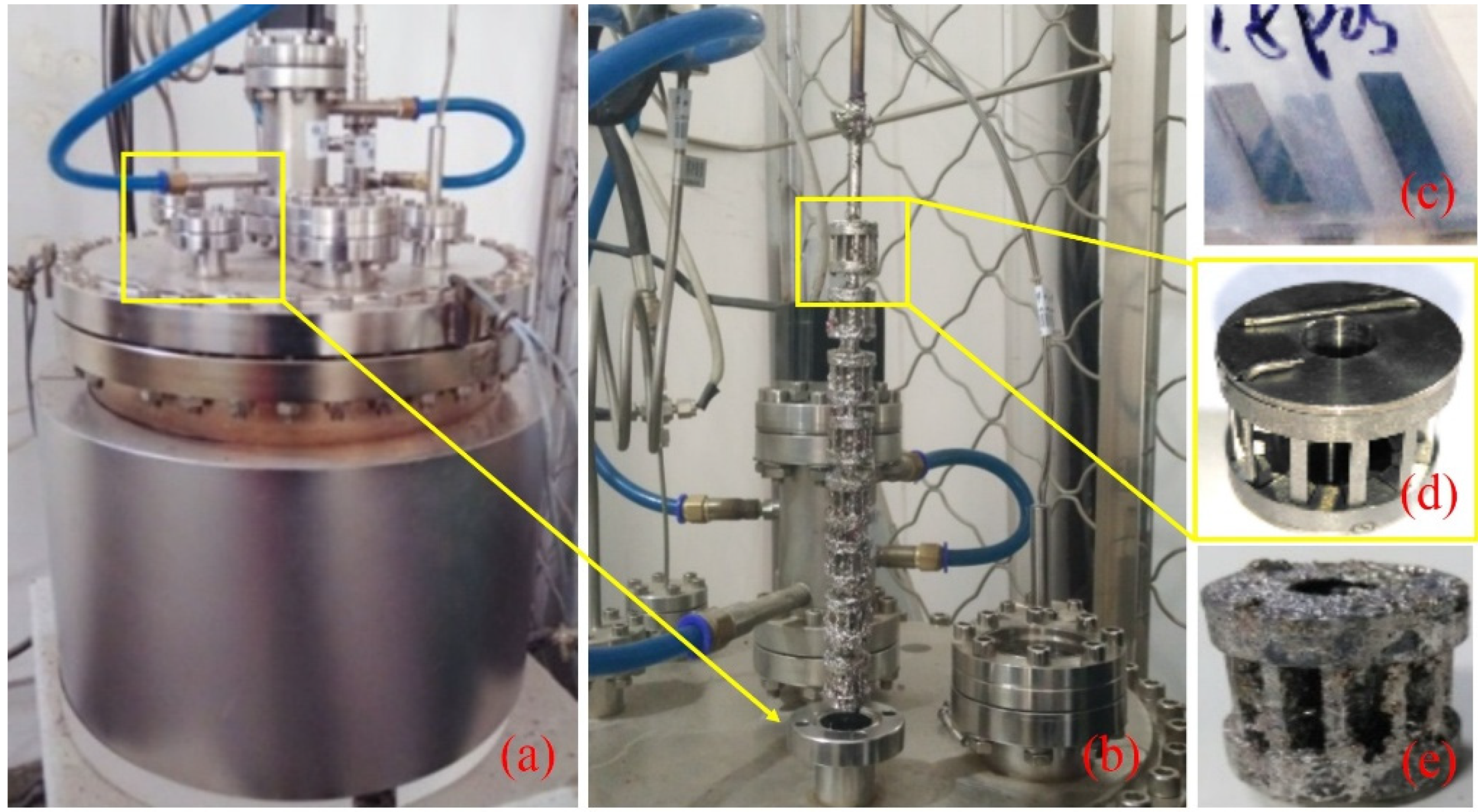
2.2. Corrosion Experiments
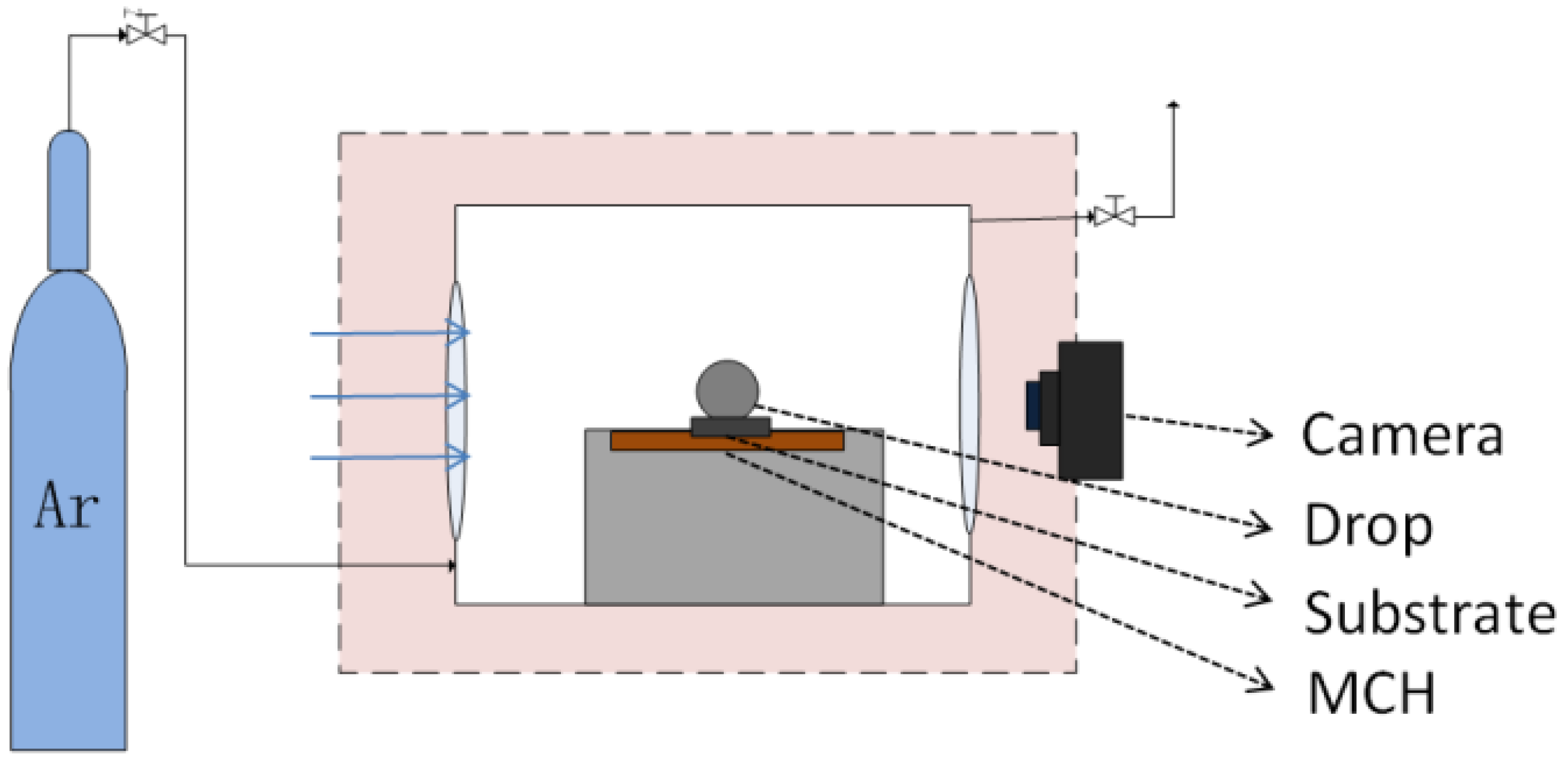
2.3. Wetting Test
3. Results
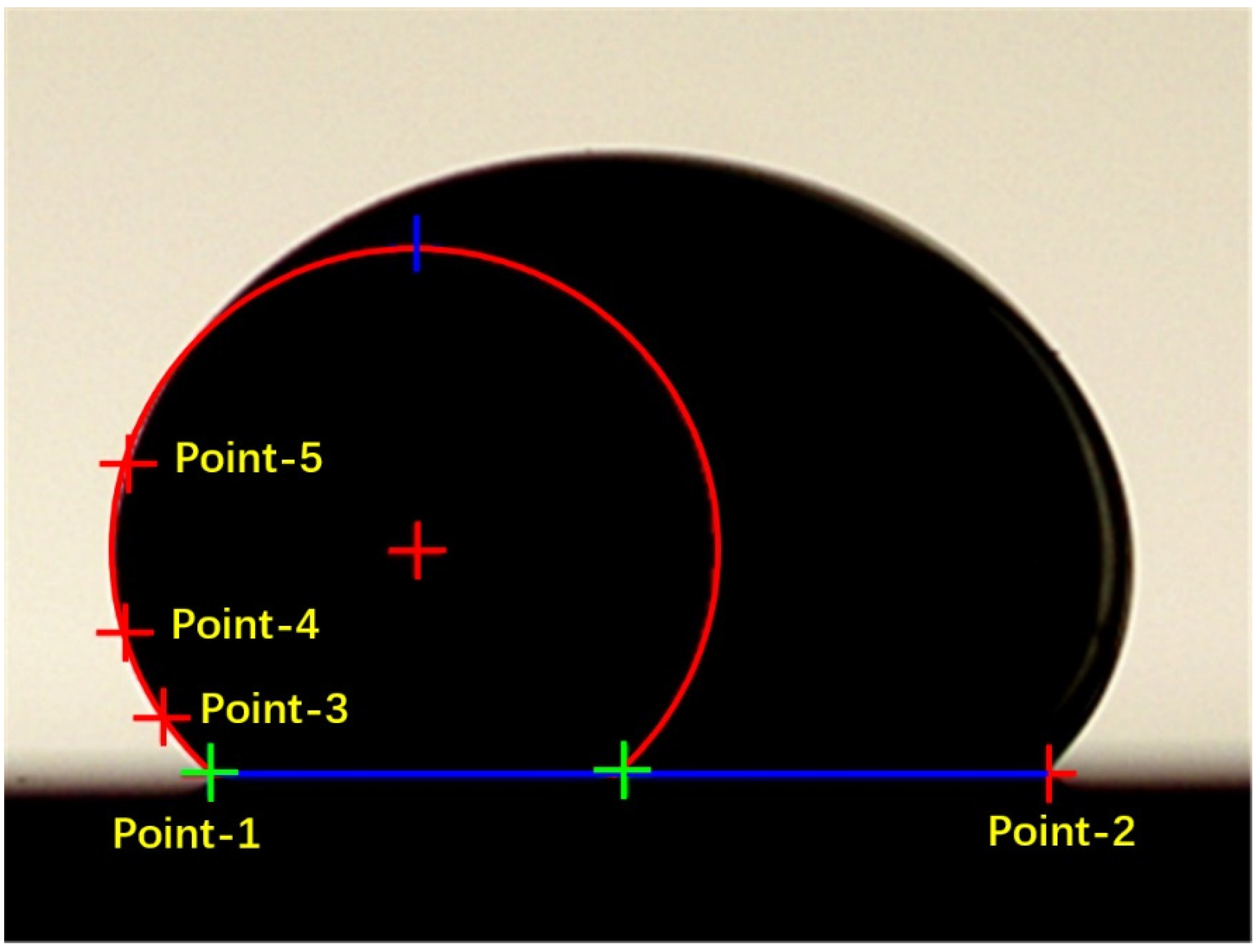
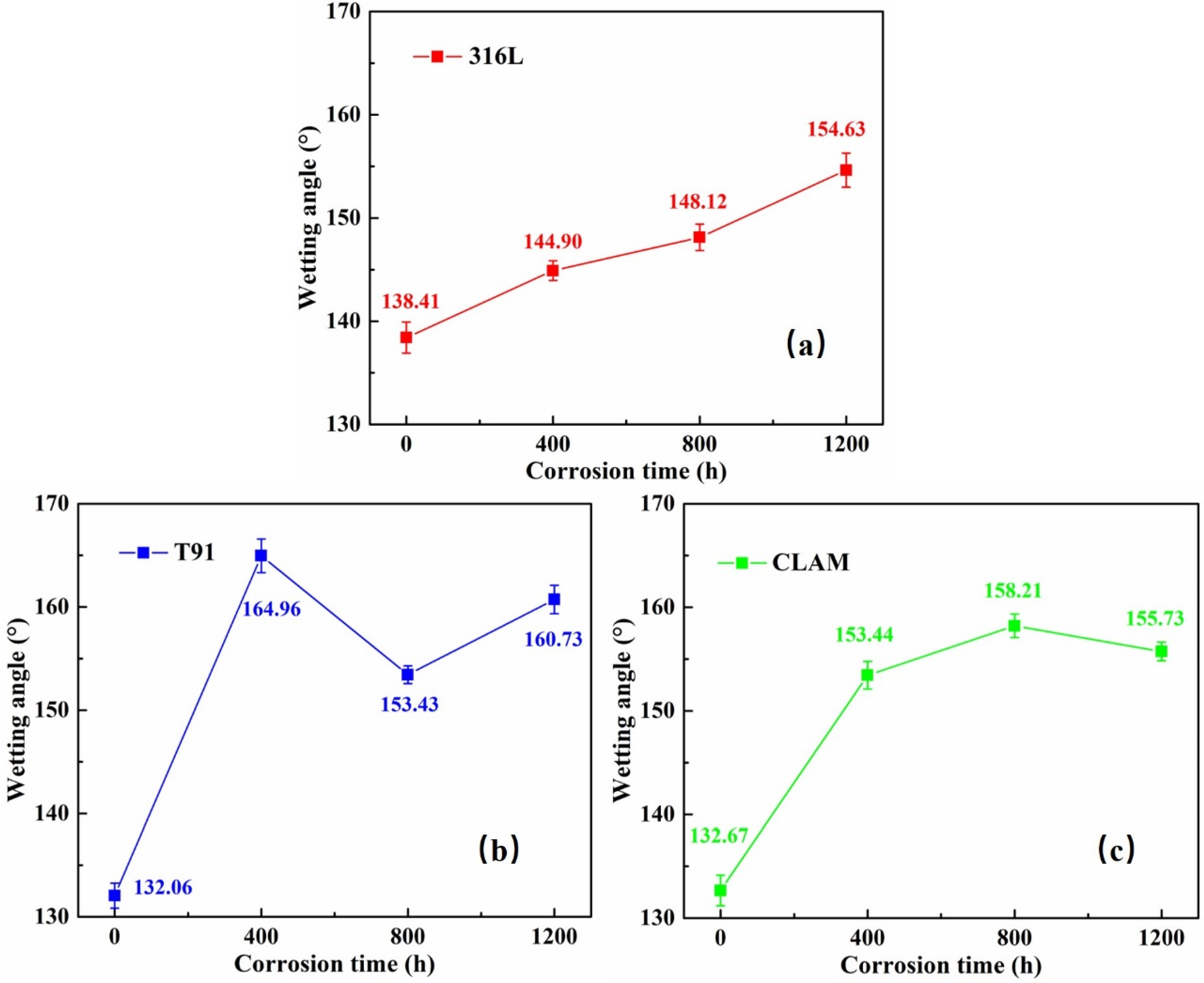
3.1. Contact Angle
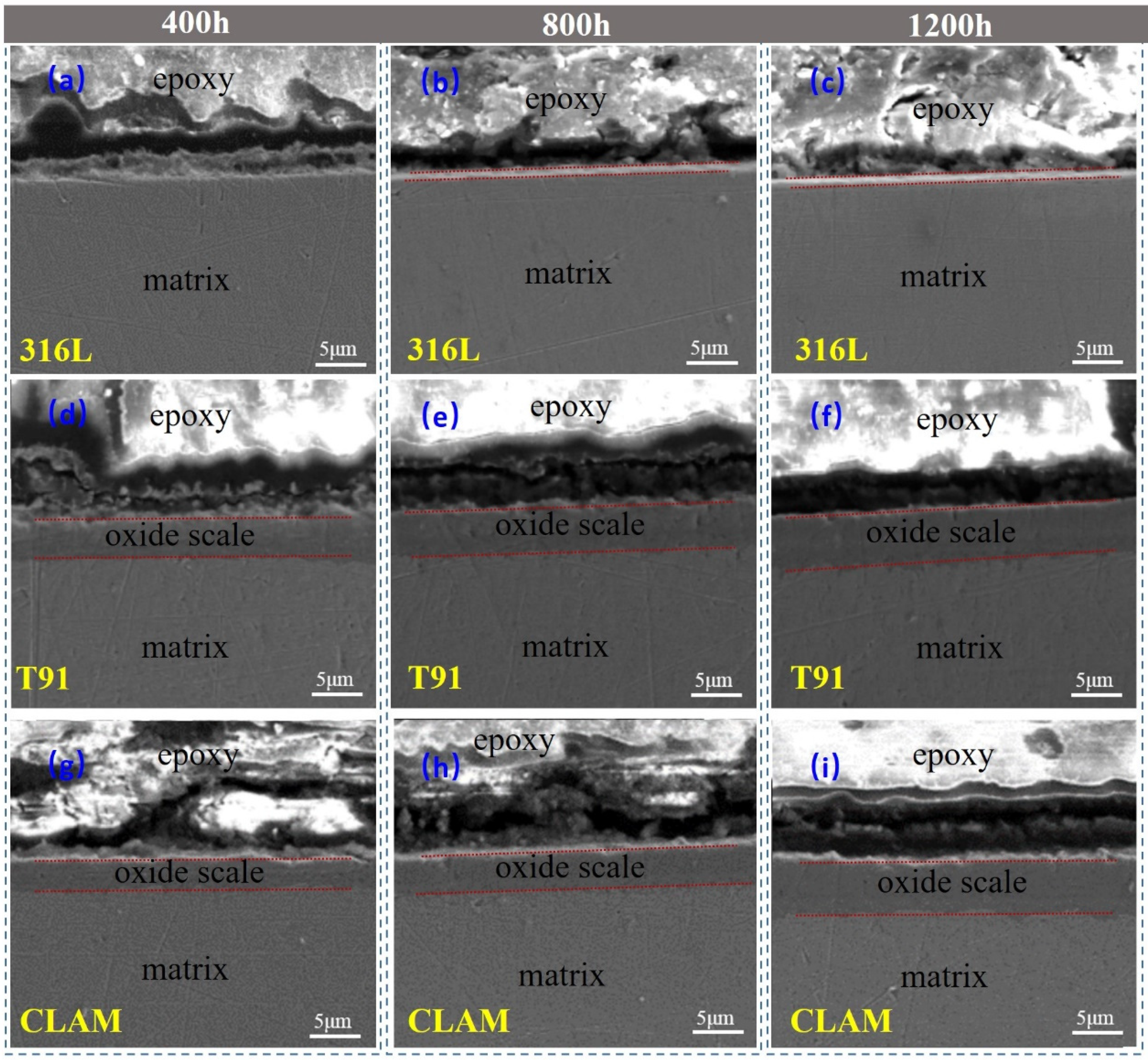
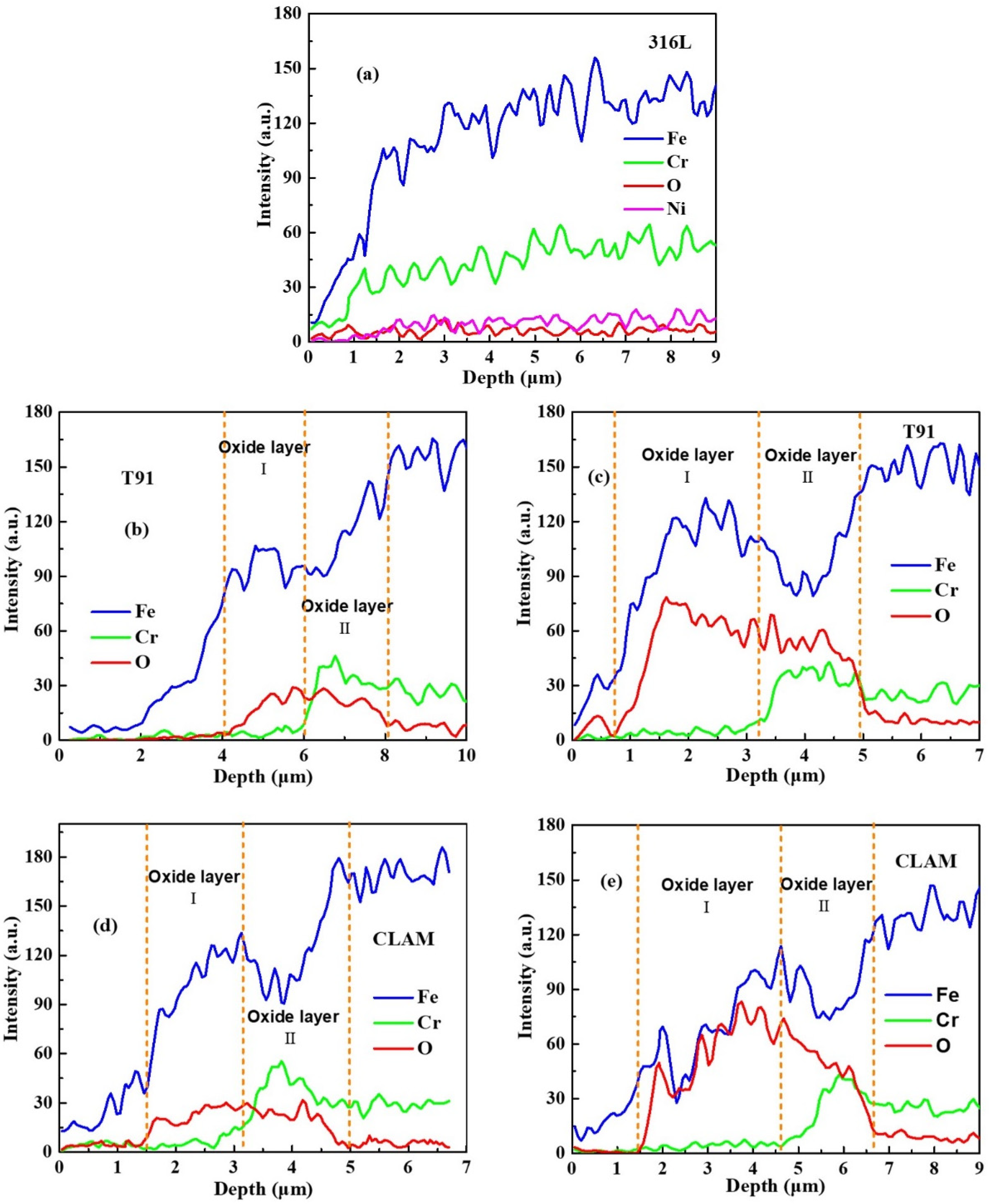
3.2. Cross-Section of the Corrosion Layer
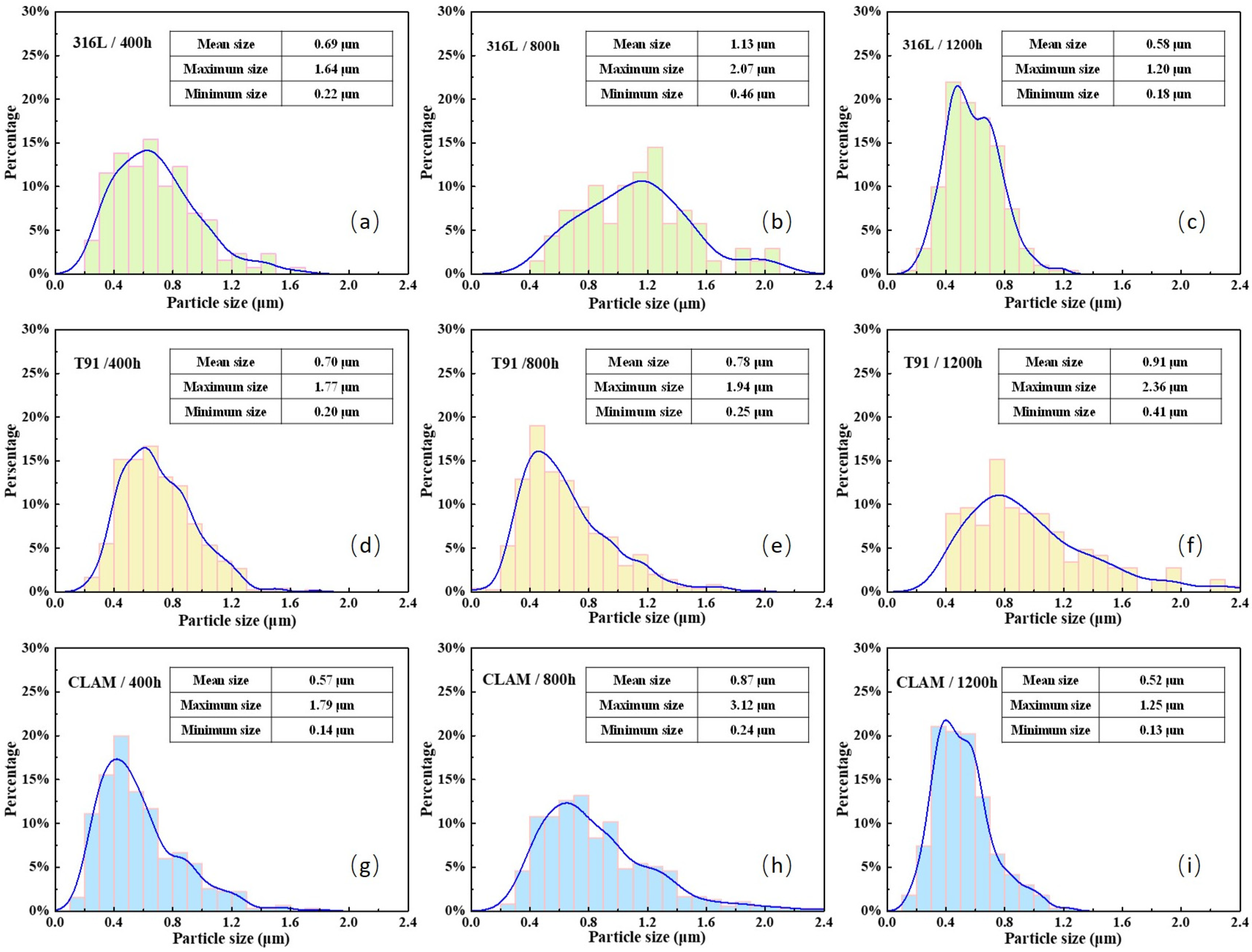
3.3. Surface of Corroded Samples
4. Discussion
4.1. Factors Affecting Wettability
4.2. Wettability and Corrosion
4.3. Wettability and LME
5. Conclusions
- In addition to surface roughness, the increase in surface disorder caused by the growth of corrosion products is also an important factor leading to the decrease in wettability of the materials to LBE.
- The oxidation structure formed on the surface of the corroded material reduces the wettability of the material surface to LBE and prevents direct contact between the material matrix and LBE, which is beneficial to decrease the corrosion rate and LME tendency of the material.
- The wettability of the material surface can be used as an important basis for predicting the LME tendency of different materials under certain corrosive environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cinotti, L.; Smith, C.F.; Artioli, C.; Grasso, G.; Corsini, G. Volume 23: Lead-Cooled Fast Reactor (LFR) Design: Safety, Neutronics, Thermal Hydraulics, Structural Mechanics, Fuel, Core, and Plant Design. In Handbook of Nuclear Engineering; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2752–2753. ISBN 978-0-387-98130-7. [Google Scholar]
- Waltar, A.E.; Todd, D.R.; Tsvetkov, P.V. Part I: Overview, Fast Spectrum Reactors; Springer: Berlin/Heidelberg, Germany, 2012; pp. 513–532. ISBN 978-1-4419-9571-1. [Google Scholar]
- Auger, T.; Lorang, G.; Guérin, S.; Pastol, J.-L.; Gorse, D. Effect of contact conditions on embrittlement of T91 steel by lead–bismuth. J. Nucl. Mater. 2004, 335, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Hojna, A.; Di Gabriele, F. On the kinetics of LME for the ferritic–martensitic steel T91 immersed in liquid PbBi eutectic. J. Nucl. Mater. 2011, 413, 21–29. [Google Scholar] [CrossRef]
- Serre, I.P.; Vogt, J.B. Liquid metal embritllement sensitivity of the T91 steel in lead, in bismuth and in lead bismuth eutectic. J. Nucl. Mater. 2020, 531, 152021. [Google Scholar] [CrossRef]
- Gong, X.; Marmy, P.; Yin, Y. The role of oxide films in preventing liquid metal embrittlement of T91 steel exposed to liquid lead-bismuth eutectic. J. Nucl. Mater. 2018, 509, 401–407. [Google Scholar] [CrossRef]
- Guerin, S.; Pastol, J.-L.; Leroux, C.; Gorse, D. Synergy effect of LBE and hydrogenated helium on resistance to LME of T91 steel grade. J. Nucl. Mater. 2003, 318, 339–347. [Google Scholar] [CrossRef]
- Halodová, P.; Lorincík, J.; Hojná, A. Microstructural Investigation of LME Crack Initiated in Ferritic/Martensitic Steel T91 Loaded in Liquid Lead-Bismuth Eutectic at 300 °C. Materials 2018, 38, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croll, S. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
- Shi, L.; Hao, T.; Ni, X.; Zhang, L.; Zhang, L. A method by calculation of wetting angle for designing of the corrosion-resistant materials in hot-dip galvanizing. Solid State Commun. 2020, 323, 114102. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, F.; Zhu, W.; Yang, L.; Zhou, Y. Effect of Al2O3 modification on the properties of YSZ: Corrosion resistant, wetting and thermal-mechanical properties. Surf. Coat. Technol. 2018, 357, 161–171. [Google Scholar] [CrossRef]
- Lei, X.; Wang, X.; Kong, F.; Chen, Y. The high temperature wetting and corrosion mechanism analysis of Nb by TiAl alloy melt. Corros. Sci. 2021, 186, 109316. [Google Scholar] [CrossRef]
- Lyu, S.; Nguyen, D.C.; Kim, D.; Hwang, W.; Yoon, B. Experimental drag reduction study of super-hydrophobic surface with dual scale structures. Appl. Surf. Sci. 2013, 286, 206–211. [Google Scholar] [CrossRef]
- Rosengarten, G.; Cooper-White, J.; Metcalfe, G. Experimental and analytical study of the effect of contact angle on liquid convective heat transfer in microchannels. Int. J. Heat Mass Transf. 2006, 49, 4161–4170. [Google Scholar] [CrossRef]
- Li, Z.X.; Du, D.X.; Guo, Z.Y. Experimental study on the flow characteristics of liquid in circular microtubes. Microscale Thermophys. Eng. 2003, 7, 253–265. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, P. An experimental study of convective heat transfer in silicon microchannels with different surface conditions. Int. J. Heat Mass Transf. 2003, 46, 2547–2556. [Google Scholar] [CrossRef]
- Zhu, Y.; Granick, S. Limits of the Hydrodynamic No-Slip Boundary Condition. Phys. Rev. Lett. 2002, 88, 106102. [Google Scholar] [CrossRef] [Green Version]
- Giuranno, D.; Gnecco, F.; Ricci, E.; Novakovic, R. Surface tension and wetting behaviour of molten Bi–Pb alloys. Intermetallics 2003, 11, 1313–1317. [Google Scholar] [CrossRef]
- Jing, L.; Zhi-Zhong, J.; Shu-Jian, T.; Qun-Ying, H.; Yang-Yang, H. Wetting of T91 by Molten Pb and PbBi alloy. At. Energy Sci. Technol. 2015, 49, 194–199. [Google Scholar]
- Protsenko, P.; Eustathopoulos, N. Surface and grain boundary wetting of Fe based solids by molten Pb and Pb-Bi eutectic. J. Mater. Sci. 2005, 40, 2383–2387. [Google Scholar] [CrossRef]
- Qi, Z.; Liao, L.; Wang, R.-Y.; Zhang, Y.-G.; Yuan, Z.-F. Roughness-dependent wetting and surface tension of molten lead on alumina. Trans. Nonferrous Met. Soc. China 2021, 31, 2511–2521. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhao, X.; Liu, Y. Lead-bismuth eutectic (LBE) corrosion behavior of AlTiN coatings at 550 and 600 °C. J. Nucl. Mater. 2020, 539, 152280. [Google Scholar] [CrossRef]
- Hamouche-Hadjem, Z.; Auger, T.; Guillot, I.; Gorse, D. Susceptibility to LME of 316L and T91 steels by LBE: Effect of strain rate. J. Nucl. Mater. 2008, 376, 317–321. [Google Scholar] [CrossRef]
- Du, X.; Niu, F.; Zhu, H.; Ma, T.; Zhao, Y.; Xiong, W.; Zhang, H. Influence of oxide scale on the wettability of LBE on T91 steel. Fusion Eng. Des. 2017, 125, 378–383. [Google Scholar] [CrossRef]
- Neyrand, V.; Bergheau, J.-M.; Benayoun, S.; Valette, S. Numerical simulation of wetting on a chemically textured surface with a large intrinsic contact angle ratio by the Lattice Boltzmann Method. Exp. Comput. Multiph. Flow 2021, 1–10. [Google Scholar] [CrossRef]
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. 1805, 95, 65–87. [Google Scholar]
- Heinzel, A.; Weisenburger, A.; Müller, G. Corrosion behavior of austenitic steels in liquid lead bismuth containing 10-6 wt% and 10-8 wt% oxygen at 400–500 °C. J. Nucl. Mater. Mater. Asp. Fission Fusion 2014, 448, 163–171. [Google Scholar]
- Ye, Z.; Wang, P.; Dong, H.; Li, D.; Zhang, Y.; Li, Y. Oxidation mechanism of T91 steel in liquid lead-bismuth eutectic: With consideration of internal oxidation. Sci. Rep. 2016, 6, 35268. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Chen, Y.; Rusanov, A. Corrosion behaviors of US steels in flowing lead–bismuth eutectic (LBE). J. Nucl. Mater. 2005, 336, 1–10. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by wate. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Zhang, J. A review of steel corrosion by liquid lead and lead–bismuth. Corros. Sci. 2009, 51, 1207–1227. [Google Scholar] [CrossRef]
- Balbaud, F.; Martinelli, L. Corrosion issues in lead-cooled fast reactor (LFR) and accelerator driven systems (ADS). In Nuclear Corrosion Science and Engineering; Woodhead Publishing: Sawston, UK, 2012; pp. 807–841. [Google Scholar]
- Tsisar, V.; Schroer, C.; Wedemeyer, O.; Skrypnik, A.; Konys, J. Long-term corrosion of austenitic steels in flowing LBE at 400 °C and 10 −7 mass% dissolved oxygen in comparison with 450 and 550 °C. J. Nucl. Mater. 2016, 468, 305–312. [Google Scholar] [CrossRef]
- Zhang, J.; Ning, L. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Li, C.; Fang, X.; Wang, Q.; Shen, M.; Wang, H.; Zeng, X.; Liu, Y.; Meng, G. A synergy of different corrosion failure modes pertaining to T91 steel impacted by extreme lead–bismuth eutectic flow pattern. Corros. Sci. 2020, 180, 109214. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Zhang, F.; Fang, X.; Liu, Z. Erosion-corrosion of 304N austenitic steels in liquid Pb-Bi flow perpendicular to steel surface. Mater. Charact. 2021, 175, 111054. [Google Scholar] [CrossRef]
- Jing, L. Stress Corrosion Behavior of T91 and 316L Steels in Liquid Lead-Bismuth Eutectic. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2015. [Google Scholar]


| Materials | Fe | Cr | C | Si | Ni | V | Mn | N | P | S | Mo | Nb | W | Ta | Al | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 316L | Bal. | 16 | 0.022 | 0.51 | 10.1 | - | 1.58 | - | 0.029 | 0.016 | 2.2 | - | - | - | - | - |
| T91 | Bal. | 8.2 | 0.09 | 0.31 | 0.06 | 0.2 | 0.39 | 0.44 | 0.1 | 0.02 | 0.93 | 0.07 | - | 0.05 | 0.02 | |
| CLAM | Bal. | 8.91 | 0.12 | 0.066 | 0.043 | 0.2 | 0.35 | 0.0084 | 0.003 | 0.002 | - | - | 1.47 | 0.14 | 0.027 | - |
| Materials | Corrosion Time (h) | Corrosion Product Structure | Density (cm−2) | Roughness (μm) | Oxide Layer Thickness (μm) |
|---|---|---|---|---|---|
| 316L | 0 | - | - | 0.024 (±0.008) | - |
| 400 | Clusters | 1.166 (±0.157) × 108 | 0.451 (±0.251) | <1 | |
| 800 | Clusters | 6.261 (±0.112) × 107 | 0.635 (±0.347) | <1 | |
| 1200 | Needles | 4.117 (±0.214) × 108 | 0.485 (±0.297) | <1 | |
| T91 | 0 | - | - | 0.018 (±0.004) | - |
| 400 | Needles | 4.292 (±0.397) × 108 | 2.583 (±0.854) | 3.95 (±0.15) | |
| 800 | Needles | 4.166 (±0.318) × 108 | 2.712 (±0.426) | 4.65 (±0.20) | |
| 1200 | Rods/Flakes | 1.295 (±0.145) × 108 | 2.657 (±0.757) | 5.28 (±0.23) | |
| CLAM | 0 | - | - | 0.026 (±0.006) | - |
| 400 | Needles/Flakes | 2.644 (±0.263) × 108 | 2.251 (±0.815) | 3.26 (±0.18) | |
| 800 | Needles | 3.183 (±0.309) × 108 | 2.483 (±0.765) | 4.06 (±0.27) | |
| 1200 | Needles/blocks | 3.064 (±0.246) × 108 | 3.095 (±0. 631) | 5.39 (±0.19) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Du, X.; Liu, X.; Yan, T.; Li, X.; Wang, Y.; Qi, M.; Tu, X. Wetting Behavior of LBE on Corroded Candidate LFR Structural Materials of 316L, T91 and CLAM. Materials 2022, 15, 102. https://doi.org/10.3390/ma15010102
Zhu H, Du X, Liu X, Yan T, Li X, Wang Y, Qi M, Tu X. Wetting Behavior of LBE on Corroded Candidate LFR Structural Materials of 316L, T91 and CLAM. Materials. 2022; 15(1):102. https://doi.org/10.3390/ma15010102
Chicago/Turabian StyleZhu, Huiping, Xiaochao Du, Xudong Liu, Tingxu Yan, Xiaobo Li, Yifeng Wang, Muran Qi, and Xu Tu. 2022. "Wetting Behavior of LBE on Corroded Candidate LFR Structural Materials of 316L, T91 and CLAM" Materials 15, no. 1: 102. https://doi.org/10.3390/ma15010102





