C-A-S-H Gel and Pore Structure Characteristics of Alkali-Activated Red Mud–Iron Tailings Cementitious Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AACM
2.3. Testing and Characterization
3. Results and Discussion
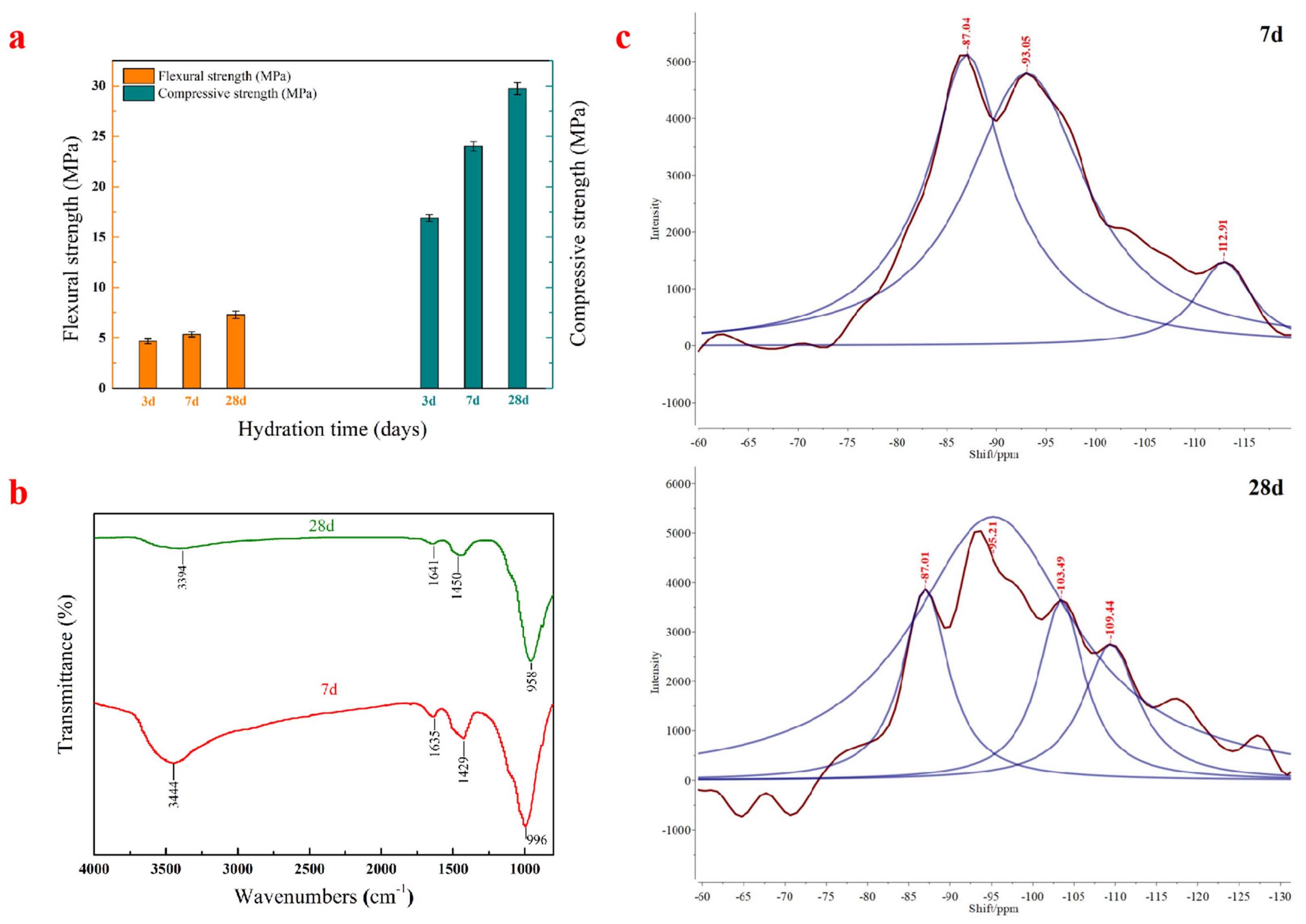
3.1. Strength Development of the AACM
3.2. C-A-S-H Gels Formation in the AACM
3.2.1. FT-IR Analysis
3.2.2. 29Si NMR Analysis
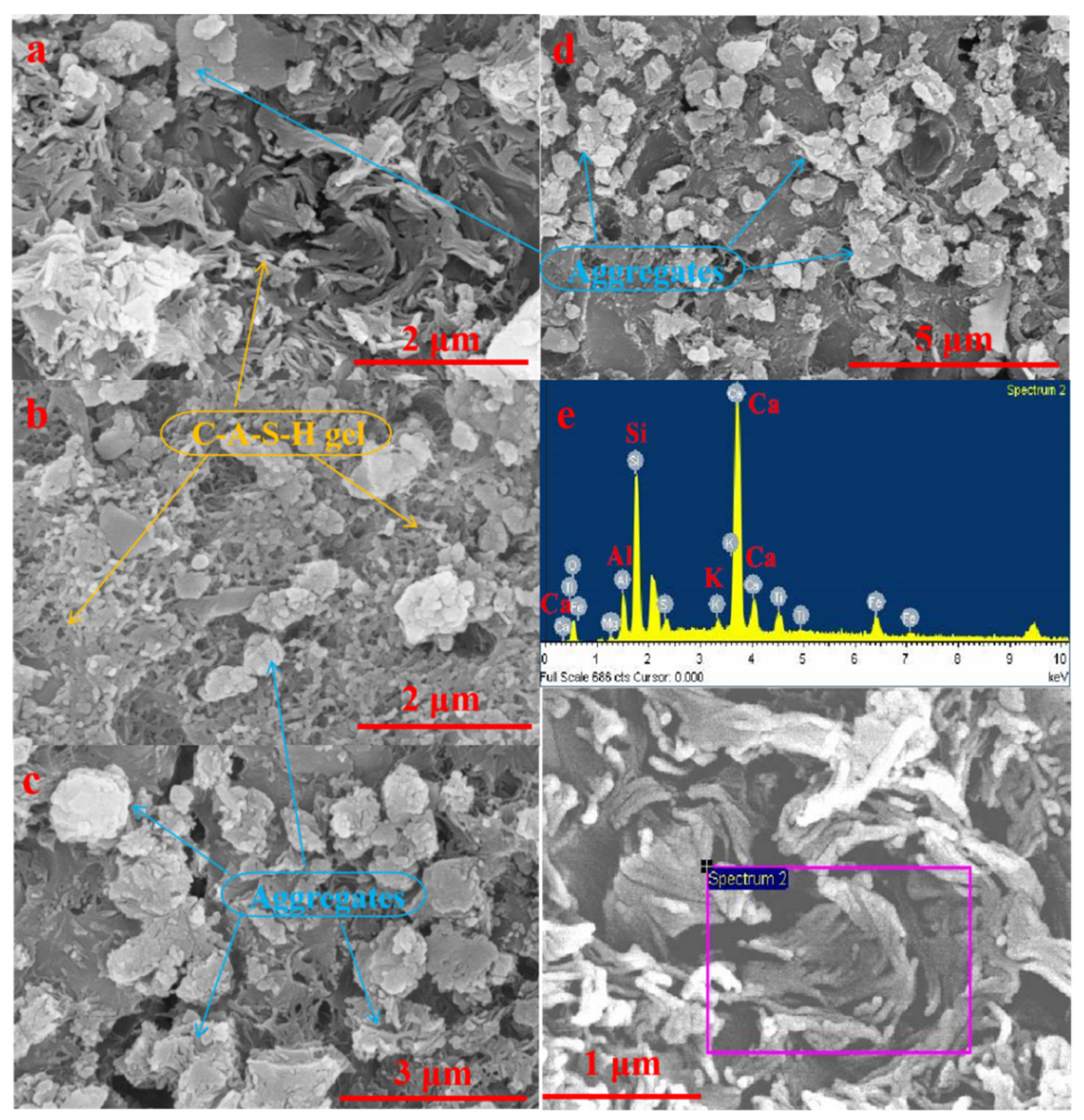
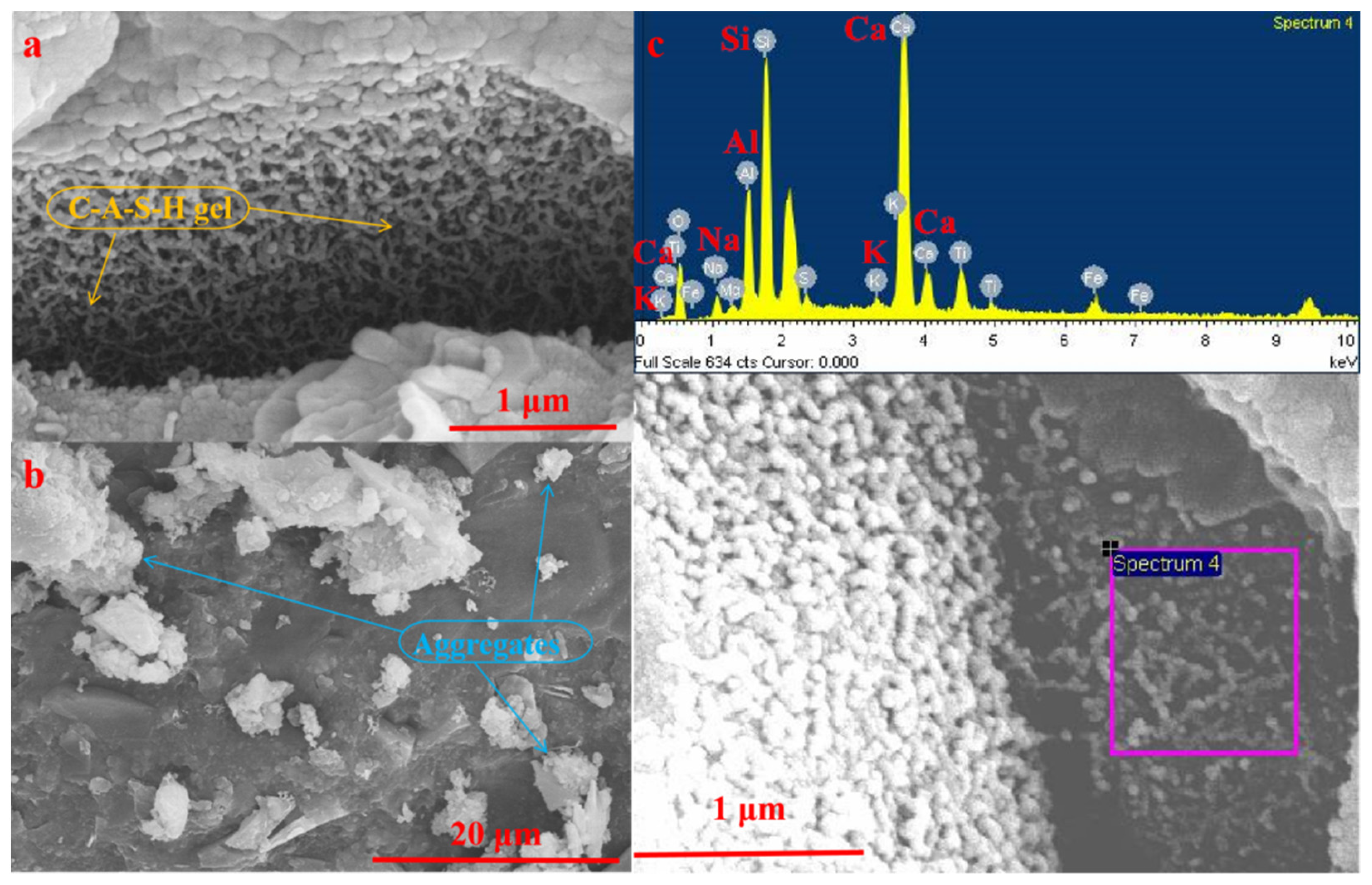
3.2.3. SEM-EDS Analysis
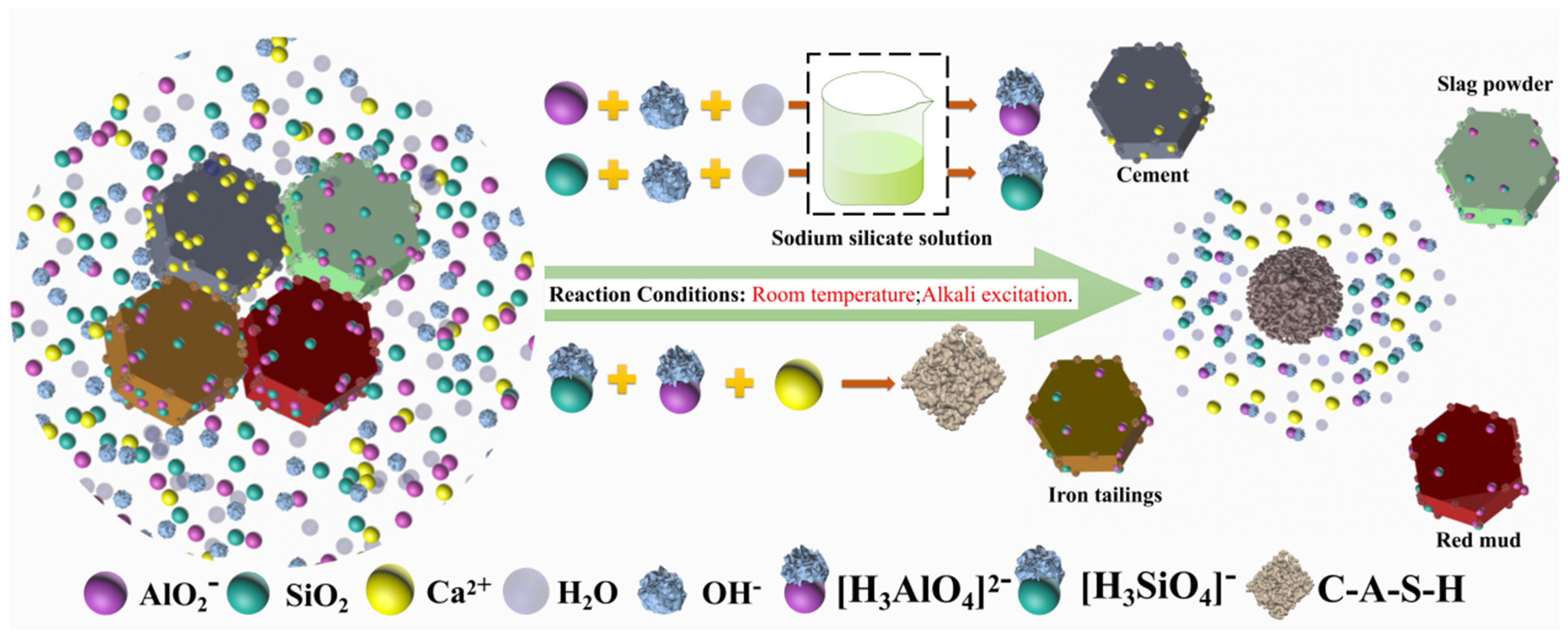
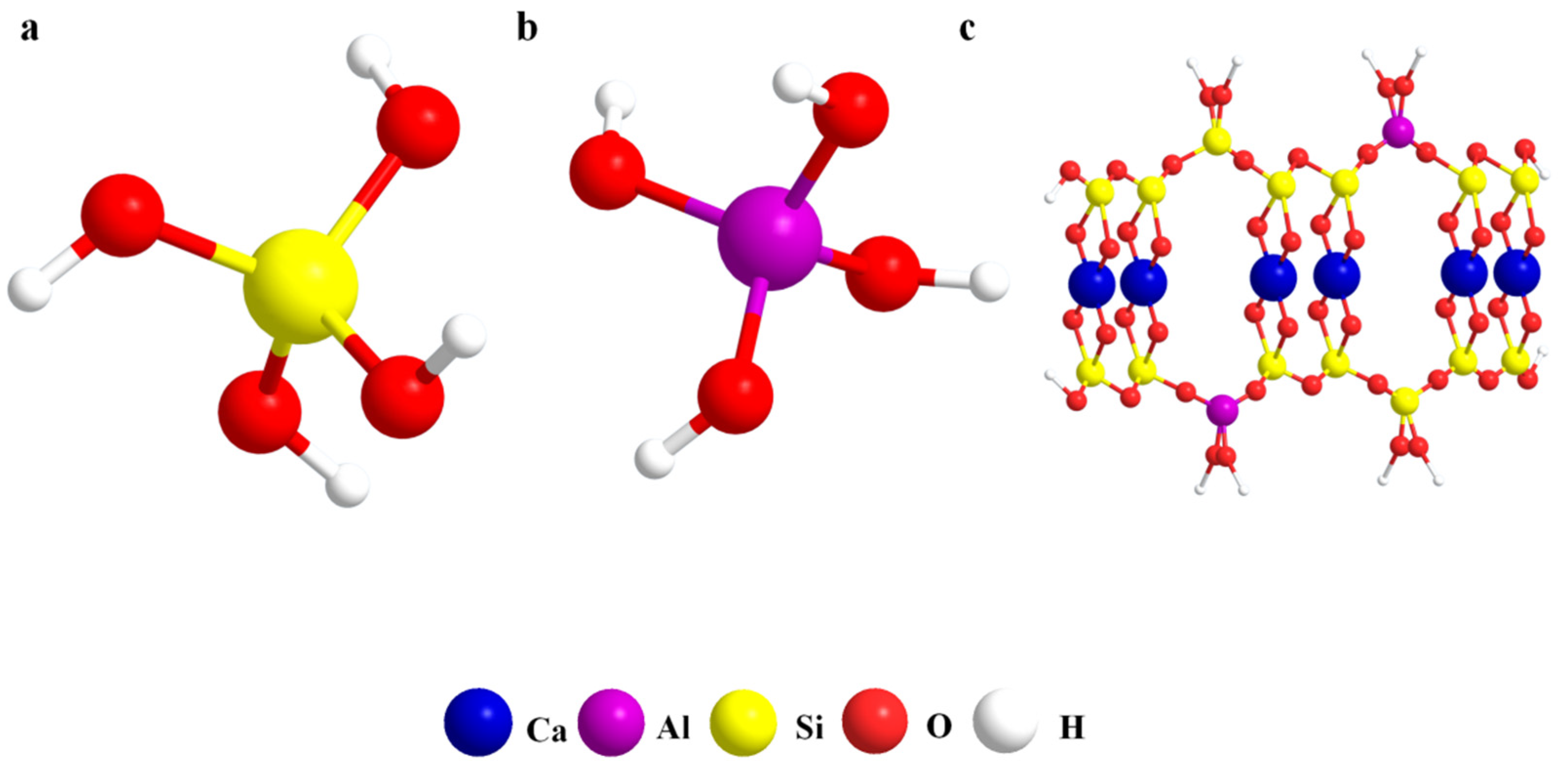
3.2.4. Formation Mechanism of C-A-S-H Gel in the AACM
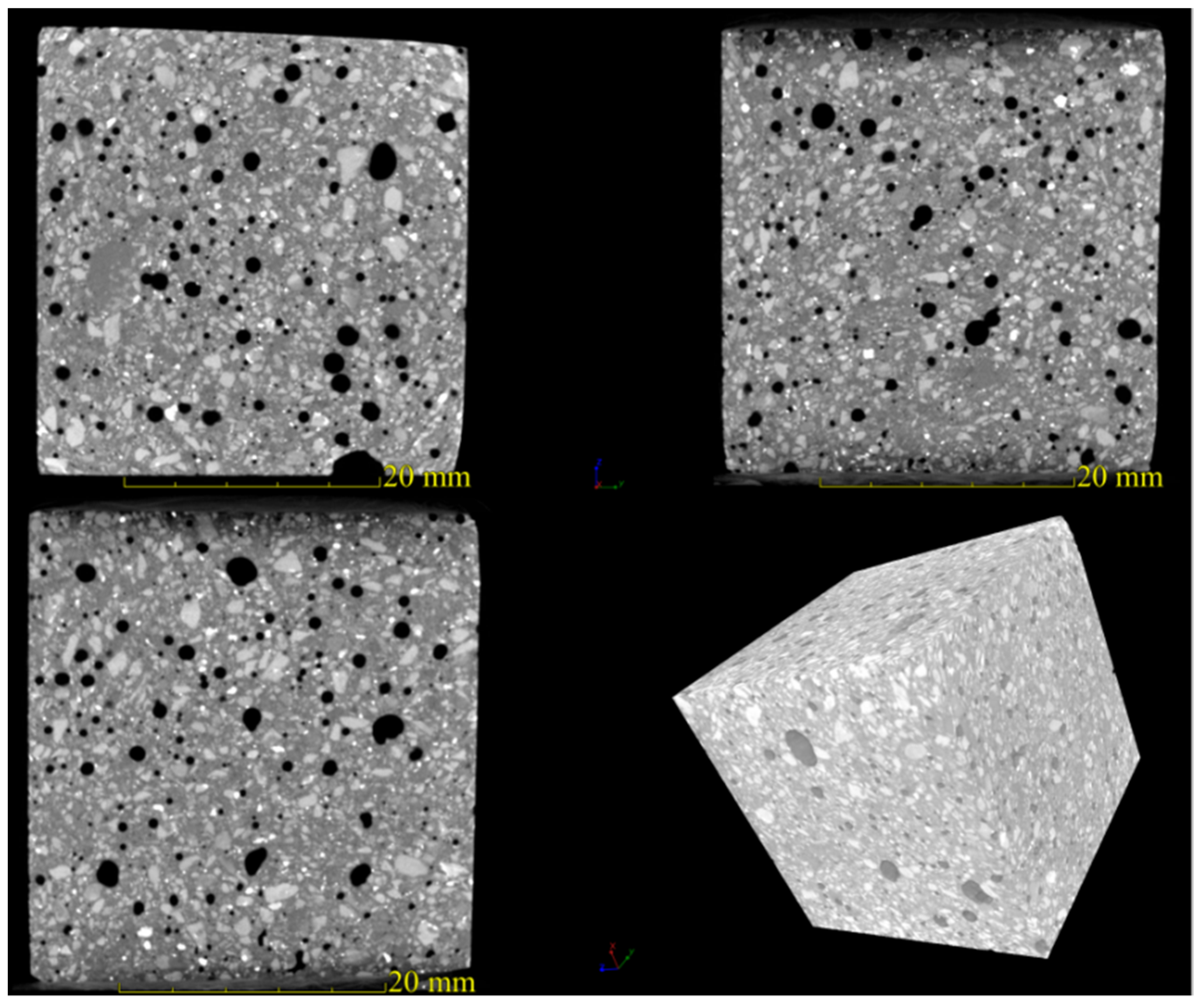
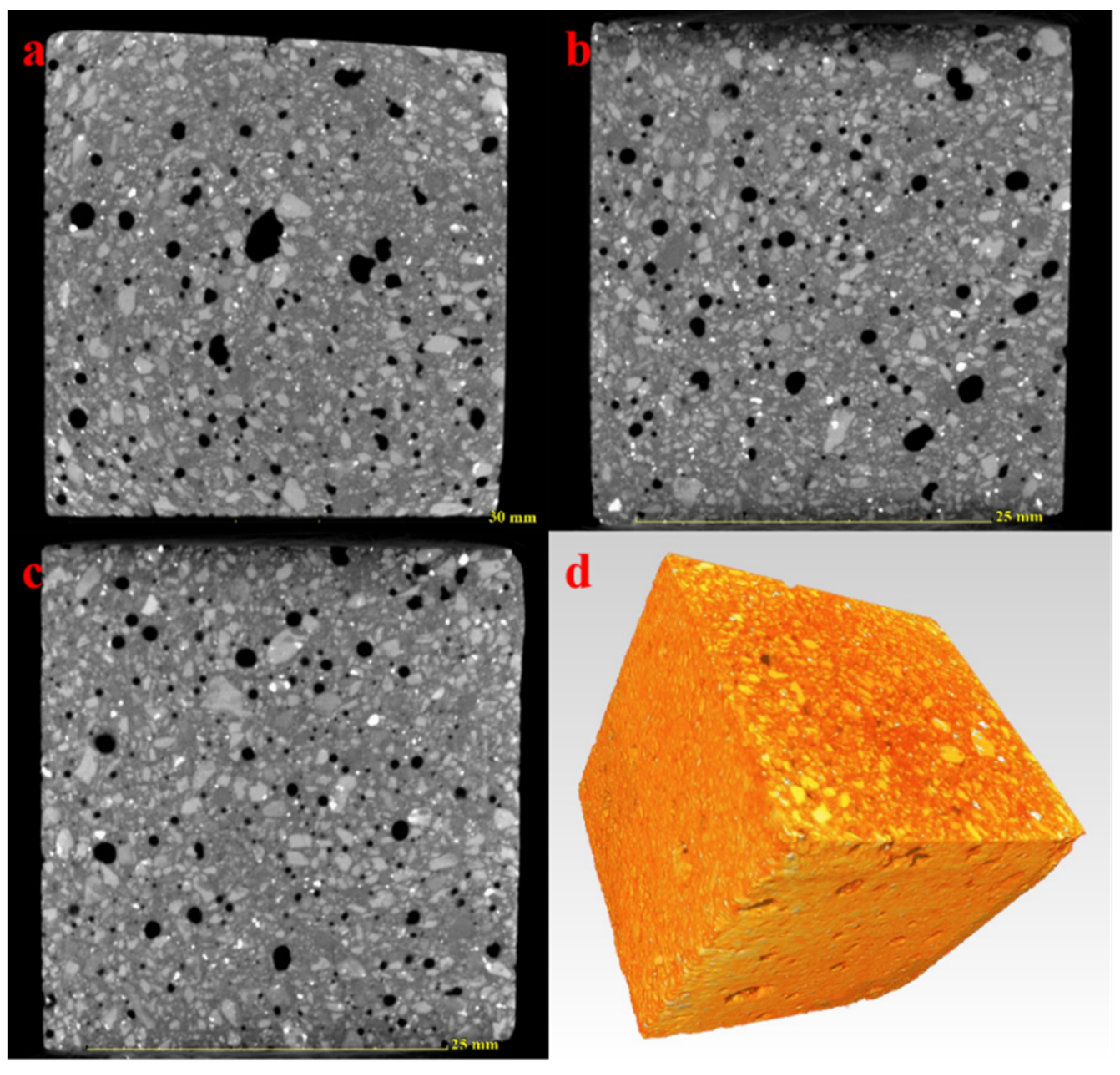
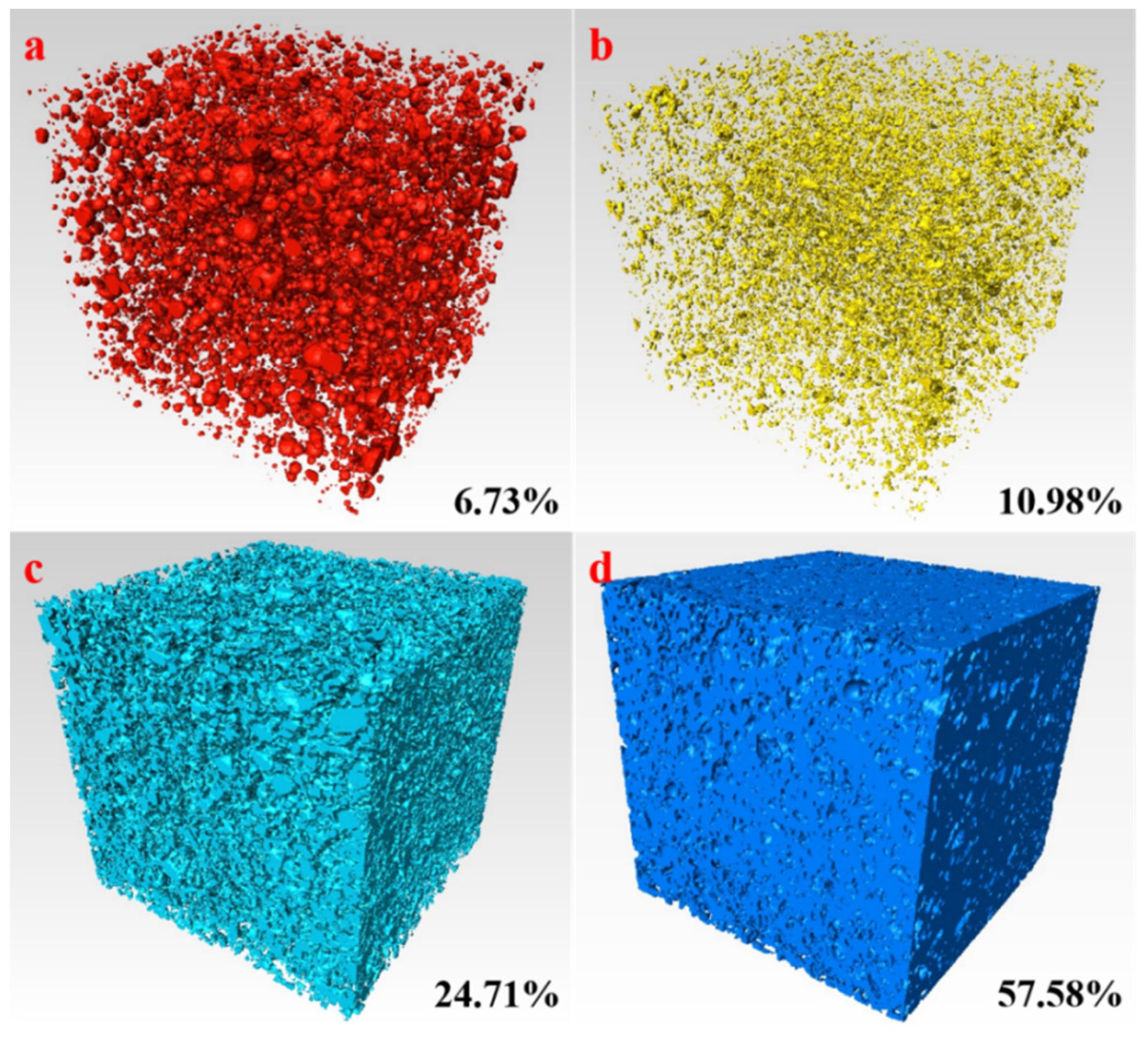
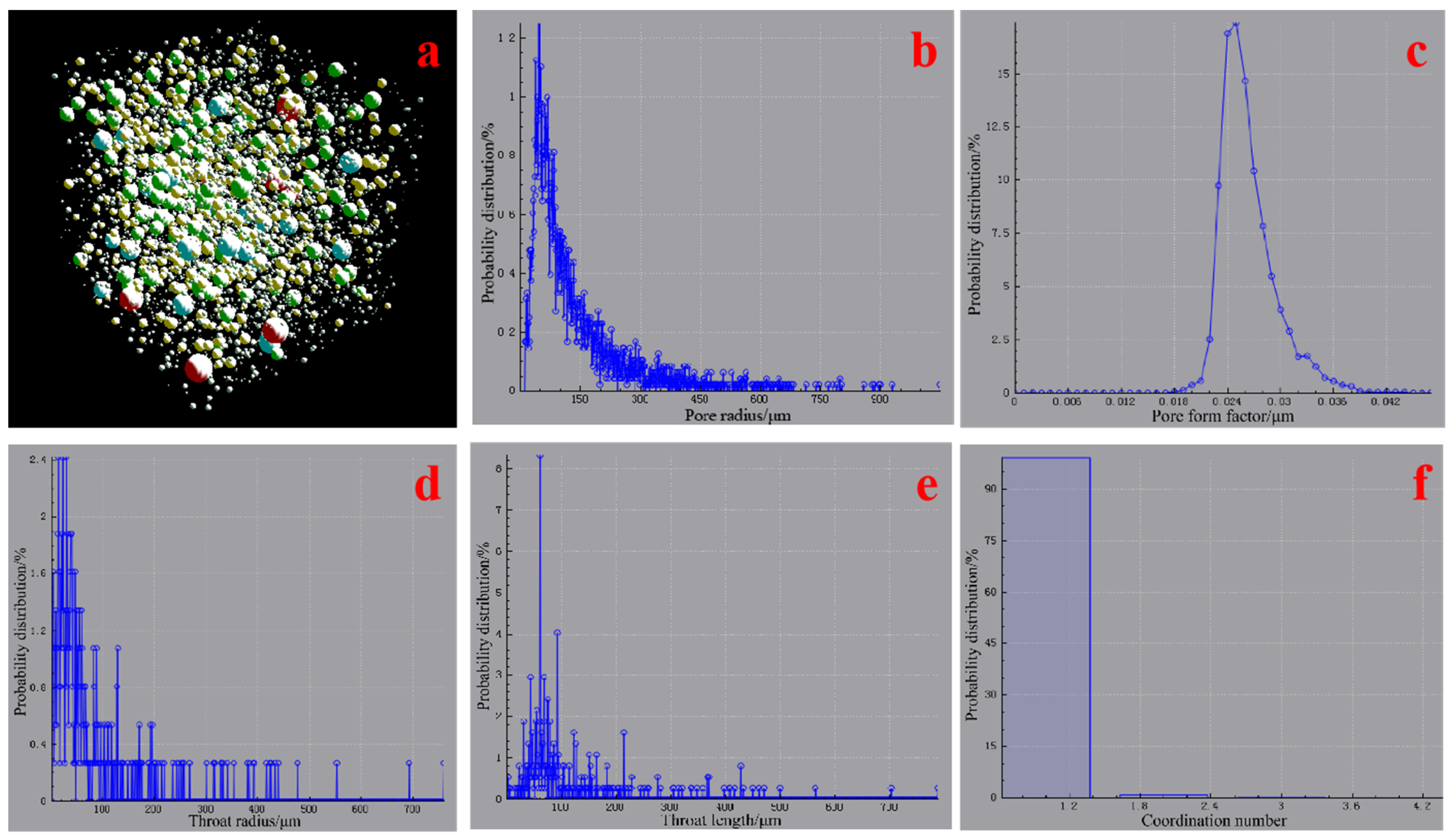
3.3. Pore Structure Characteristics of the AACM
3.4. Environmental Impact and Economic Potential of the AACM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solymar, K.; Sajo, I.; Steiner, J.; Zoldi, J. Characteristics and separability of red mud. Light Met. 1992, 209–223. [Google Scholar]
- Liu, W.C.; Chen, X.Q.; Li, W.X.; Yu, Y.F.; Yan, K. Environmental assessment, management and utilization of red mud in China. J. Clean. Prod. 2014, 84, 606–610. [Google Scholar] [CrossRef]
- Wang, S.H.; Jin, H.X.; Deng, Y.; Xiao, Y.D. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 2–3. [Google Scholar] [CrossRef]
- China Industry Information. 2019. Available online: https://www.chyxx.com/industry/201906/750518.html (accessed on 23 November 2020).
- Xue, Y.; Liu, X.M. Detoxification, solidification and recycling of municipal solid waste incineration fly ash: A review. Chem. Eng. J. 2021, 420, 2–9. [Google Scholar] [CrossRef]
- Song, S.Q.; Zhou, Y.Z. Mining wasteland and its ecological restoration and reconstruction. Conserv. Util. Miner. Resour. 2001, 5, 44–45. [Google Scholar]
- Han, F.H.; Li, L.; Song, S.M.; Liu, J.H. Early-age hydration characteristics of composites binder containing iron tailing powder. Powder Technol. 2017, 315, 322–331. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, B.W.; Liu, X.M. Cementitious activity of iron tailing and its utilization in cementitious materials, bricks and concrete. Constr. Build. Mater. 2021, 288, 123022. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, X.; Liu, X.; Duan, P.; Yang, H.; Jiang, T.; Wang, D.; Liu, R. Current situation and comprehensive utilization of iron ore tailing resources. J. Min. Sci. 2006, 42, 403–408. [Google Scholar] [CrossRef]
- Adamo, N.; Al-Ansari, N.; Sissakian, V.; Laue, J.; Knutsson, S. Dam safety: The question of tailings dams. J. Earth Sci. Geotech. Eng. 2021, 11, 1–26. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers-Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Liu, J.P.; Li, X.Y.; Lu, Y.S.; Bai, X.H. Effects of Na/Al ratio on mechanical properties and microstructure of red mud-coal metakaolin geopolymer. Constr. Build. Mater. 2020, 263, 2–9. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.M.; Wen, Q.B.; Meegoda, J.N. Synthesis and characterization of geopolymers derived from coal gangue, fly ash and red mud. Constr. Build. Mater. 2019, 206, 287–296. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.J.; Zhou, W.; Ren, D.M. Fresh properties, compressive strength and microstructure of fly ash geopolymer paste blended with iron ore tailing under thermal cycle. Constr. Build. Mater. 2016, 118, 76–88. [Google Scholar] [CrossRef]
- Defáveri, K.; Santos, L.; Carvalho, J.; Peixoto, R.F.; Brigolini, G.J. Iron ore tailing-based geopolymer containing glass wool residue: A study of mechanical and microstructural properties. Constr. Build. Mater. 2019, 220, 375–385. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wu, S.Y.; Qi, C.H.; Xiao, H.P.; Xie, Y.B.; Wang, M.C. Formula optimization and mechanism of preparing geopolymers based on iron tailings and metakaolin. Metal Mine 2019, 4, 199–203. [Google Scholar]
- Wang, Y.G.; Liu, X.M.; Zhang, W.; Li, Z.P.; Zhang, Y.L.; Li, Y.; Ren, Y.Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Song, S.; Zhang, N.; Yuan, J.B.; Zhang, Y.H. New attempt to produce red mud-iron tailing based alkali-activated mortar: Performance and microstructural characteristics. J. Build. Eng. 2021, 43, 103222. [Google Scholar] [CrossRef]
- GB/T17671-1999Test Method for Strength of Cement Mortar, Chinese Standard Press: Beijing, China, 1999.
- Emile, M.; Liu, X.M.; Zhang, L.L.; Zhang, N. Preparation and characterization of a red mud-based road base material: Strength formation mechanism and leaching characteristics. Constr. Build. Mater. 2019, 220, 297–307. [Google Scholar]
- Zhang, N.; Liu, X.M.; Sun, H.H.; Li, L.T. Pozzolanic behavior of compound-activated red mud-coal gangue mixture. Cem. Concr. Res. 2011, 41, 270–278. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.X.; Zhao, Y.Z.; Liu, X.M. Hydration characteristics and environmental friendly performance of a cementitious material composed of calcium silicate slag. J. Hazard. Mater. 2016, 306, 67–76. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhao, X.B.; Yin, H.F.; Chen, J.L.; Zhang, N. Intermediate-calcium based cementitious materials prepared by MSWI fly ash and other solid wastes: Hydration characteristics and heavy metals solidification behavior. J. Hazard. Mater. 2018, 349, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Liu, X.M.; Xu, Y.T.; Tang, B.W.; Wang, Y.G. Synergic effects of electrolytic manganese residue-red mud-carbide slag on the road base strength and durability properties. Constr. Build. Mater. 2019, 220, 364–374. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Incorporation of aluminum in the calcium silicate hydrate (C-S-H) of hydrated portland cements: A High-Field 27Al and 29Si MAS NMR Investigation. Inorg. Chem. 2003, 42, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Puertas, F.; Fernández-Jiménez, A.; Blanco-Varela, M.T. Pore solution in alkali activated slag cement pastes. Relation to the composition and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 139–148. [Google Scholar] [CrossRef]
- Ding, Z.; Hong, X.; Zhu, J.X.; Tian, B.Y.; Fang, Y. Alkali-activated red mud-slag cementitious materials. J. Chin. Electron Microsc. Soc. 2018, 37, 149–150. [Google Scholar]
- Bayat, A.; Hassani, A.; Yousefi, A.A. Effects of red mud on the properties of fresh and hardened alkali-activated slag paste and mortar. Constr. Build. Mater. 2018, 167, 775–790. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Ye, N.; Yang, J.K.; Liang, S.; Hu, Y.; Hu, J.P. Synthesis and strength optimization of one-part geopolymer based on red mud. Constr. Build. Mater. 2016, 111, 317–325. [Google Scholar] [CrossRef]
- Kubicki, J.D.; Apitz, S.; Blake, G. G2 theory calculations on [H3SiO4]−, [H4SiO4], [H3AlO4]2−, [H4AlO4]− and [H5AlO4]: Basis set and electron correlation effects on molecular structures, atomic charges, infrared spectra, and potential energies. Phys. Chem. Miner. 1995, 22, 481–488. [Google Scholar] [CrossRef]
- Wang, Q.; Kang, S.R.; Wu, L.M.; Zhang, Q.; Ding, Z.Y. Structural modeling and molecular dynamics simulation of geopolymers gel. Mater. Rep. 2020, 34, 04056–04058. [Google Scholar]
- Inés, G.; Shane, D.; Ana, F.; Ángel, P. Hydration of hybrid alkaline cement containing a very large proportion of fly ash: A descriptive model. Materials 2016, 9, 5–10. [Google Scholar]
- Tao, W.H.; Fu, X.H.; Sun, F.J.; Yang, Z.X. Studies on properties and mechanism of geopolymer cementitious material. Bull. Chin. Ceram. Soc. 2008, 27, 730–739. [Google Scholar]
- Ye, N.; Chen, Y.; Yang, J.K.; Liang, S.; Hu, Y. Transformation of Na, Al, Si and Fe species in red mud during synthesis of one-part geopolymers. Cem. Concr. Res. 2017, 101, 123–130. [Google Scholar] [CrossRef]
- Tang, B.W.; Gao, S.; Wang, Y.G.; Liu, X.M.; Zhang, N. Pore structure analysis of electrolytic manganese residue based permeable brick by using industrial CT. Constr. Build. Mater. 2019, 208, 697–709. [Google Scholar] [CrossRef]
- Wan, J.B.; Sun, B.D.; Chen, S.J.; Du, H.H.; Zhang, L. Digital core technology research and its applications. Well Logging Technol. 2012, 36, 154–159. [Google Scholar]
- Arand, F.; Hesser, J. Accurate and efficient maximal ball algorithm for pore network extraction. Comput. Geosci. 2017, 101, 28–37. [Google Scholar] [CrossRef]
- Yu, J.; Mishra, D.K.; Hu, C.L.; Leung, K.Y.; Shah, S.P. Mechanical, environmental and economic performance of sustainable Grade 45 concrete with ultrahigh-volume Limestone-Calcined Clay (LCC). Resour. Conserv. Recycl. 2021, 175, 105846. [Google Scholar] [CrossRef]
- Hammond, G.; Jones, C. Inventory of Carbon and Energy, 3rd ed.; University of Bath: Bath, UK, 2019. [Google Scholar]
- GB 50010-2010Code for Design of Concrete Structures, Chinese Standard Press: Beijing, China, 2010.

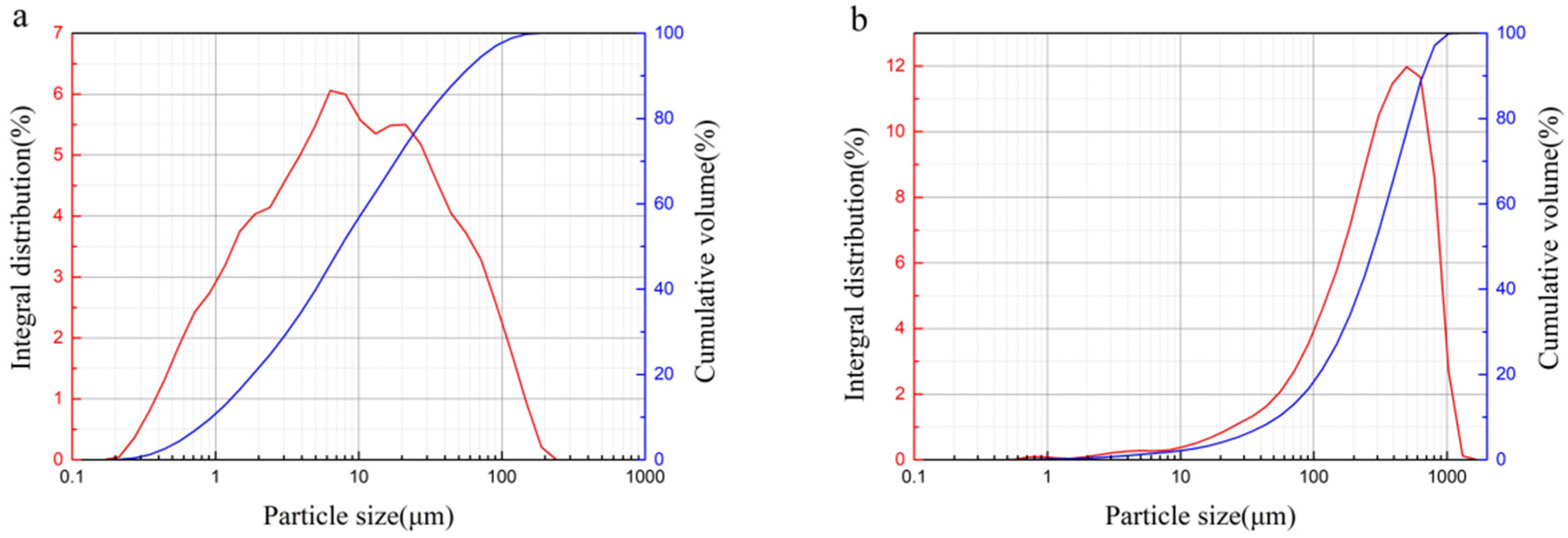
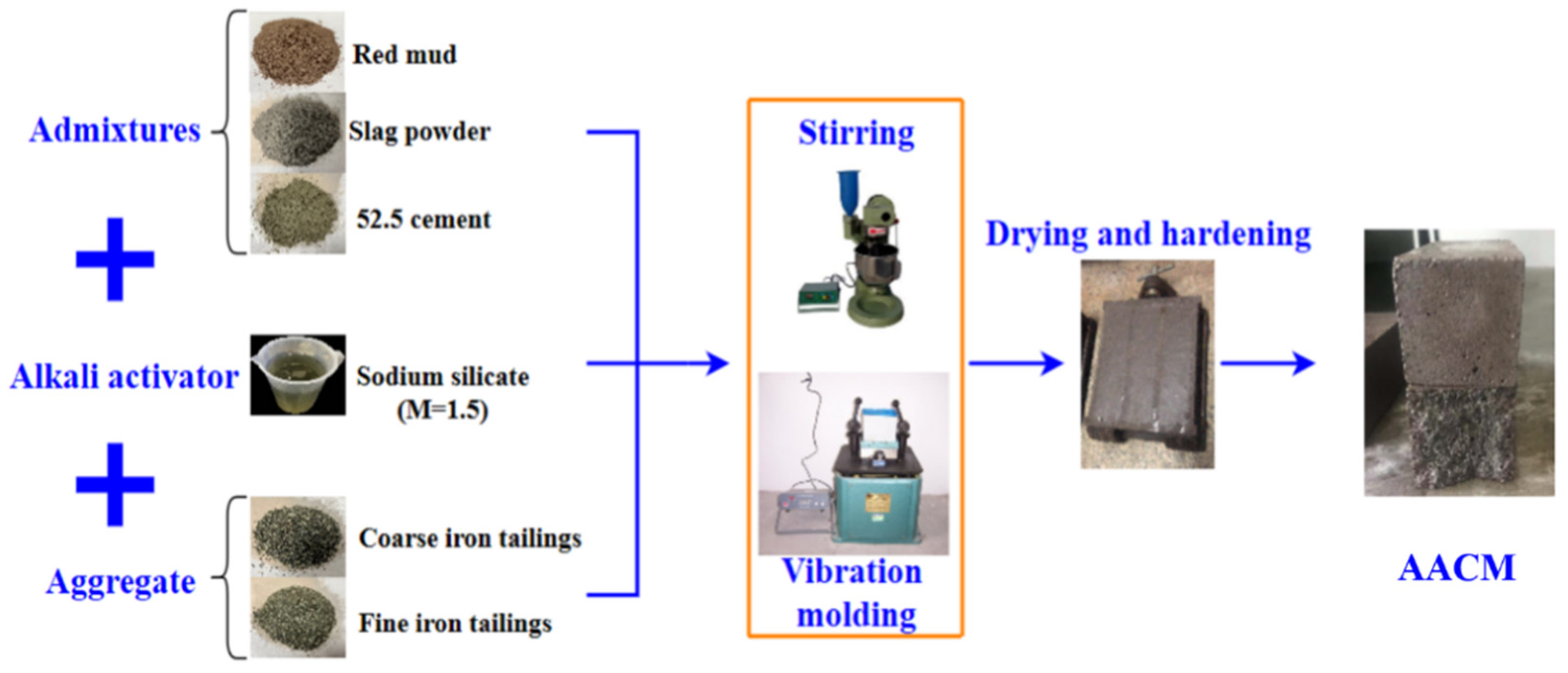

| Chemical Constitution/% | Red Mud | Iron Tailings | Slag Powder | 52.5 Portland Cement |
|---|---|---|---|---|
| Al2O3 | 24.66 | 17.87 | 17.3 | 6.05 |
| SiO2 | 20.57 | 36.42 | 32.64 | 21.68 |
| CaO | 20.86 | 14.21 | 37.66 | 62.86 |
| Na2O | 16.16 | 0.94 | 0.5 | 0.2 |
| Fe2O3 | 7.67 | 16.36 | 0.66 | 3.26 |
| TiO2 | 6.17 | 2.42 | 0.72 | 0.32 |
| SO3 | 1.08 | - | 1.63 | 2.61 |
| K2O | 0.62 | 1.08 | 0.76 | 1.09 |
| MgO | 0.39 | 6.41 | 7.42 | 1.51 |
| Place of origin | Hejin, Shanxi Province of China | Laiyuan, Hebei Province of China | Hejin, Shanxi Province of China | Conch Cement Co. Ltd., Anhui Province of China |
| Sample | Resolution/μm | Voltage/kV | Current Flow/μA | Time of Exposure/s | Scanning Time/min |
|---|---|---|---|---|---|
| AACM | 30.53 | 190 | 130 | 0.65 | 90 |
| Structural Unit | 7 d | 28 d | ||
|---|---|---|---|---|
| Chemical Shift (ppm) | Relative Area | Chemical Shift (ppm) | Relative Area | |
| SiQ2 | −87.0 | 79.4 | −87.0 | 20.33 |
| SiQ3 | −93.0 | 100 | −95.2 | 100 |
| SiQ4 | −112.9 | 14.4 | −103.5 | 19.13 |
| −109.4 | 17.58 | |||
| RBO | 66.59% | 77.61% | ||
| Chemical Element | 7 d | 28 d |
|---|---|---|
| O | 41.30 | 51.35 |
| Al | 4.44 | 6.86 |
| Si | 17.71 | 15.17 |
| Ca | 26.85 | 17.85 |
| Na+K | 1.04 | 2.88 |
| Material | Embodied Carbon (kg CO2/kg) | Market Price (CNY/Metric ton) |
|---|---|---|
| Iron tailings | 0.00747 | 100 |
| Red mud | <0.1 | 250 |
| 52.5 cement | 0.912 | 630 |
| Blast furnace slag | 0.0416 | 300 |
| Sodium silicate | 2.28 | 850 |
| Water | 0.00034 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhang, N.; Zhang, J.; Song, S.; Zhang, Y. C-A-S-H Gel and Pore Structure Characteristics of Alkali-Activated Red Mud–Iron Tailings Cementitious Mortar. Materials 2022, 15, 112. https://doi.org/10.3390/ma15010112
Li C, Zhang N, Zhang J, Song S, Zhang Y. C-A-S-H Gel and Pore Structure Characteristics of Alkali-Activated Red Mud–Iron Tailings Cementitious Mortar. Materials. 2022; 15(1):112. https://doi.org/10.3390/ma15010112
Chicago/Turabian StyleLi, Chao, Na Zhang, Jiancong Zhang, Shuai Song, and Yihe Zhang. 2022. "C-A-S-H Gel and Pore Structure Characteristics of Alkali-Activated Red Mud–Iron Tailings Cementitious Mortar" Materials 15, no. 1: 112. https://doi.org/10.3390/ma15010112





