Preparation of Stable Phase Change Material Emulsions for Thermal Energy Storage and Thermal Management Applications: A Review
Abstract
:1. Introduction
2. Major Characteristics of PCM Emulsions
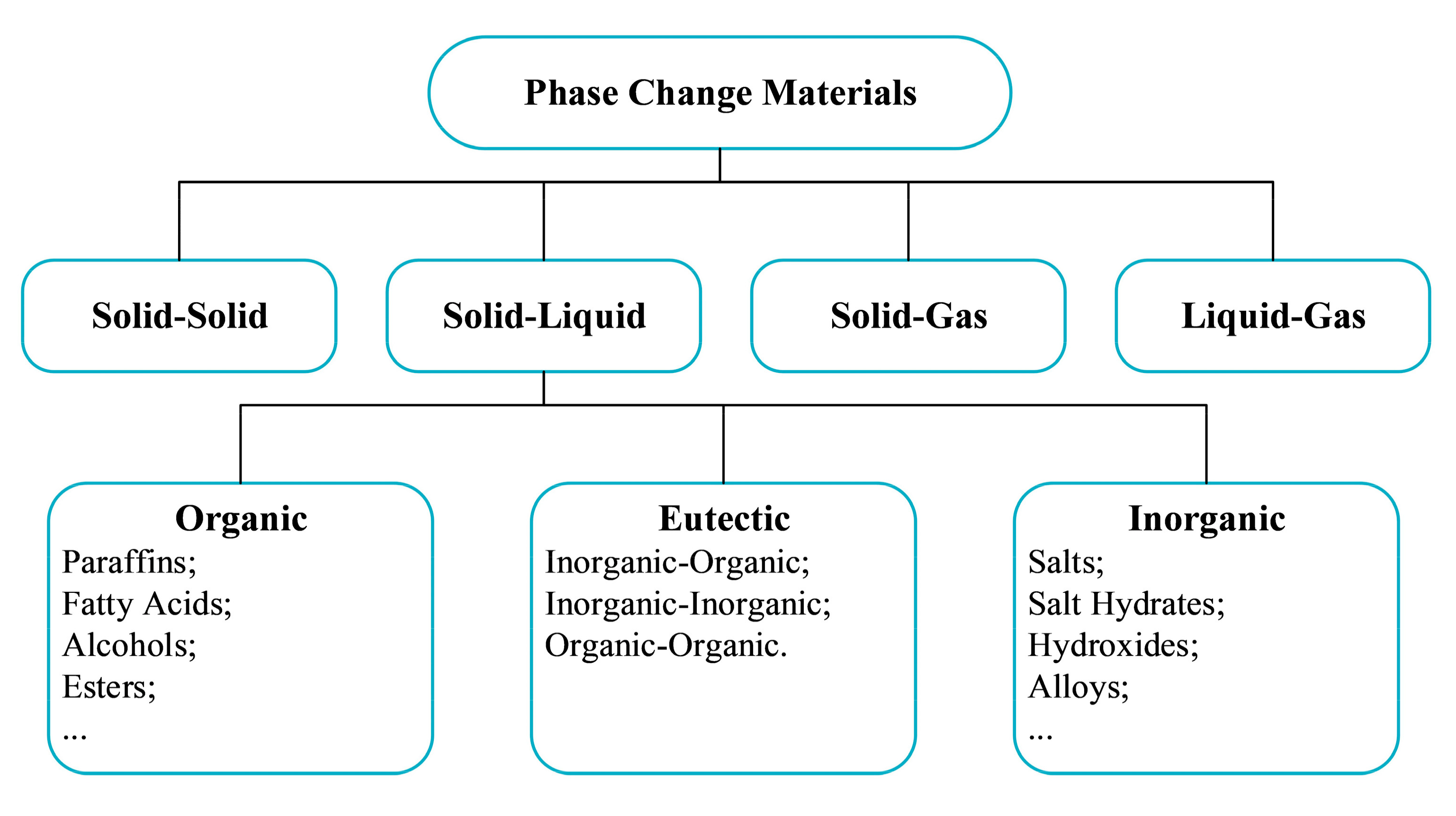
2.1. Classification of PCMs
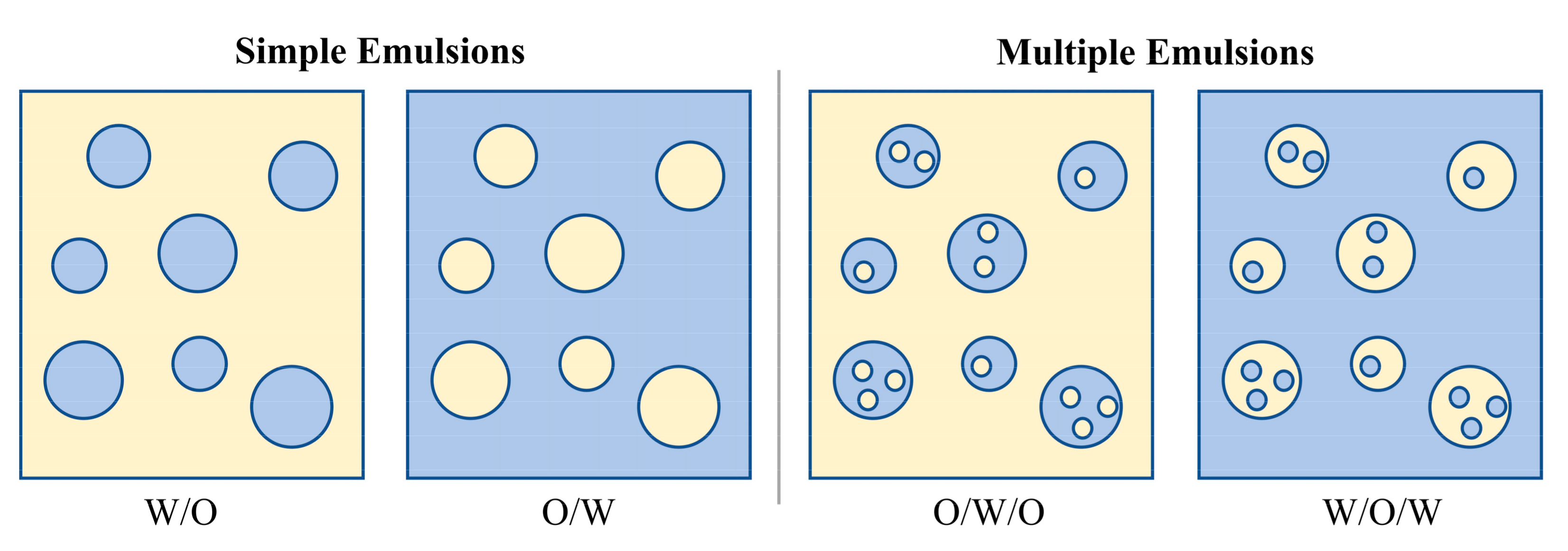
2.2. Classification of PCM Emulsions
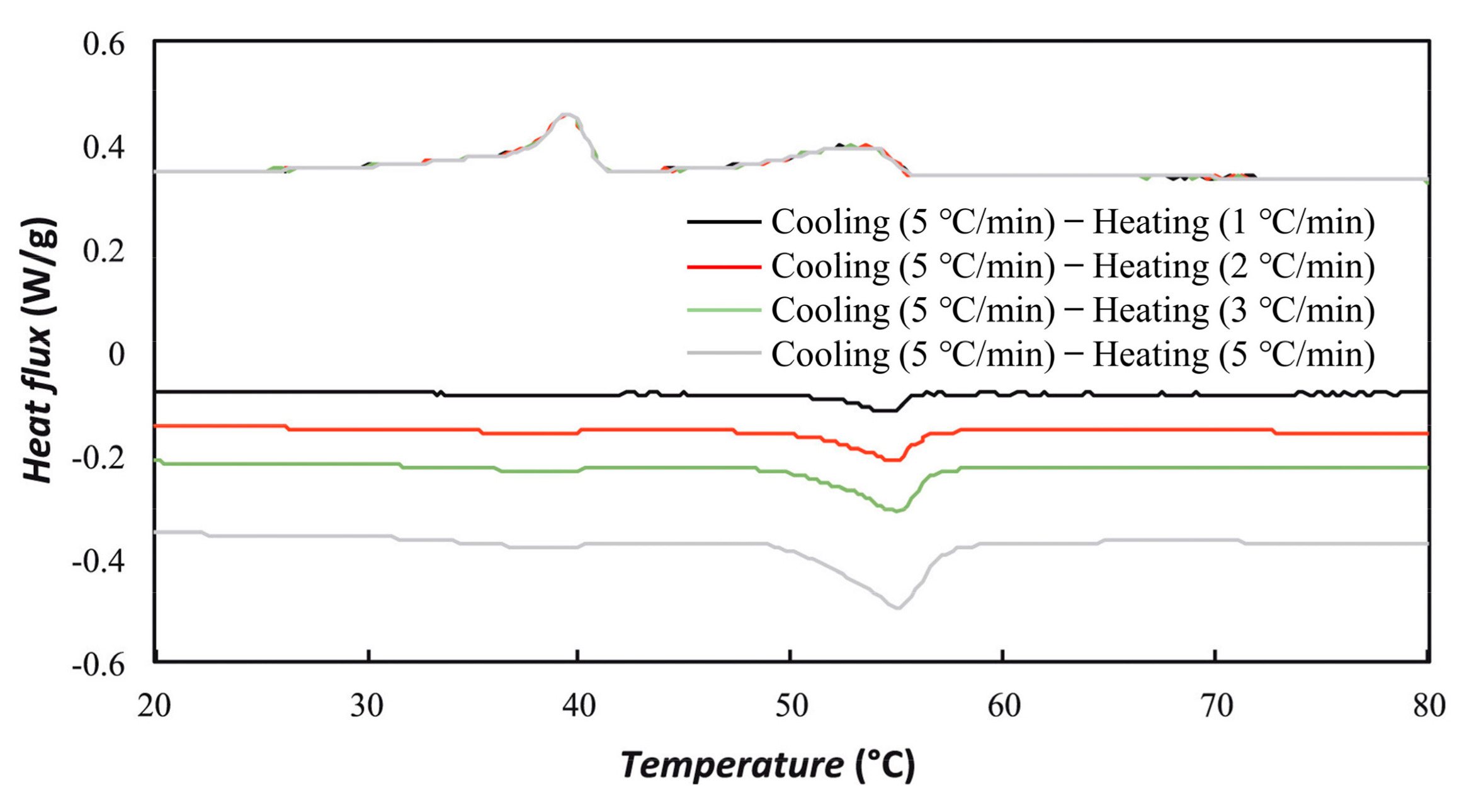
2.3. Supercooling in PCM Emulsions
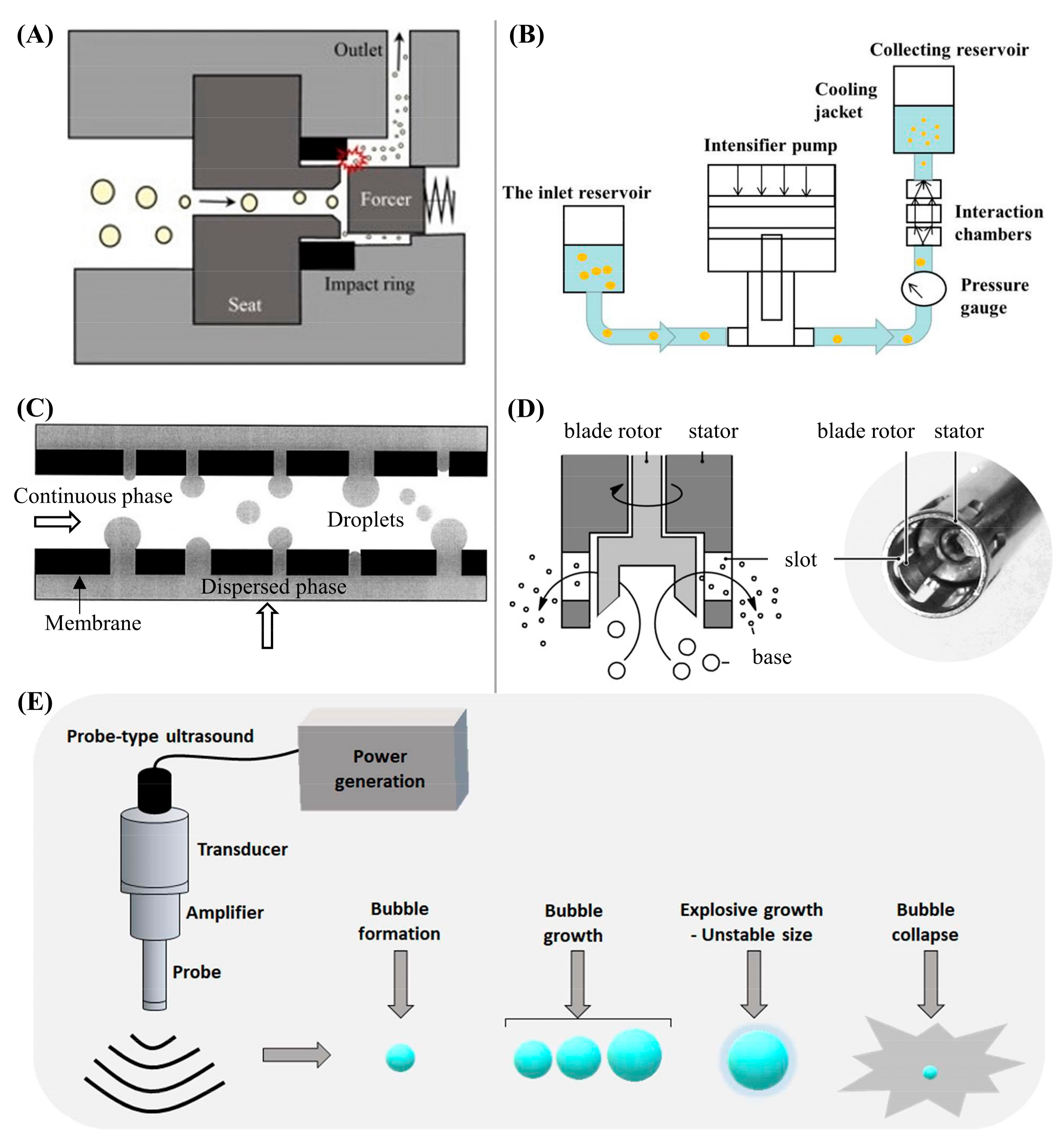
3. Preparation of PCM Emulsions by High-Energy Methods
3.1. General Principles
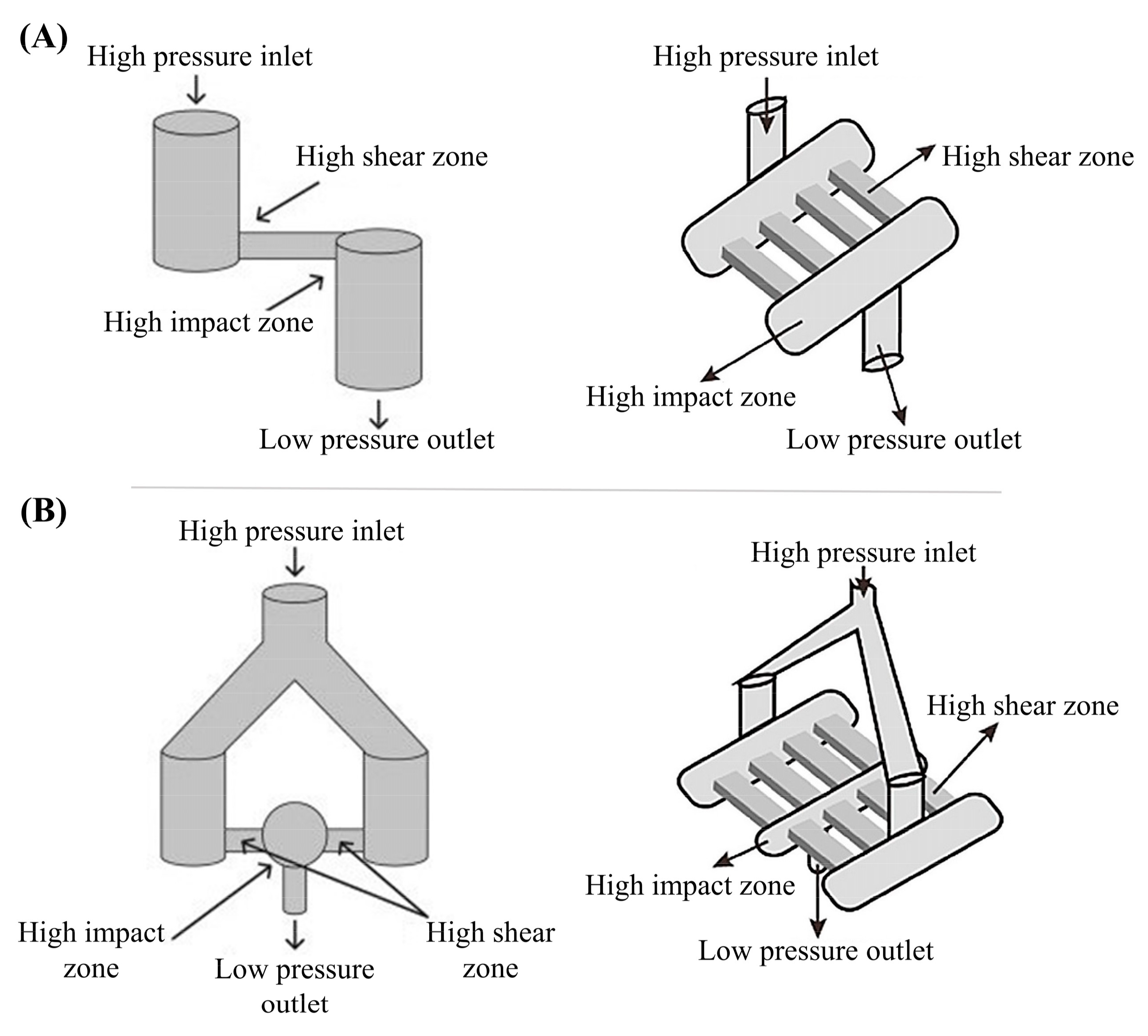
3.1.1. High-Pressure Techniques
3.1.2. Membrane Emulsification
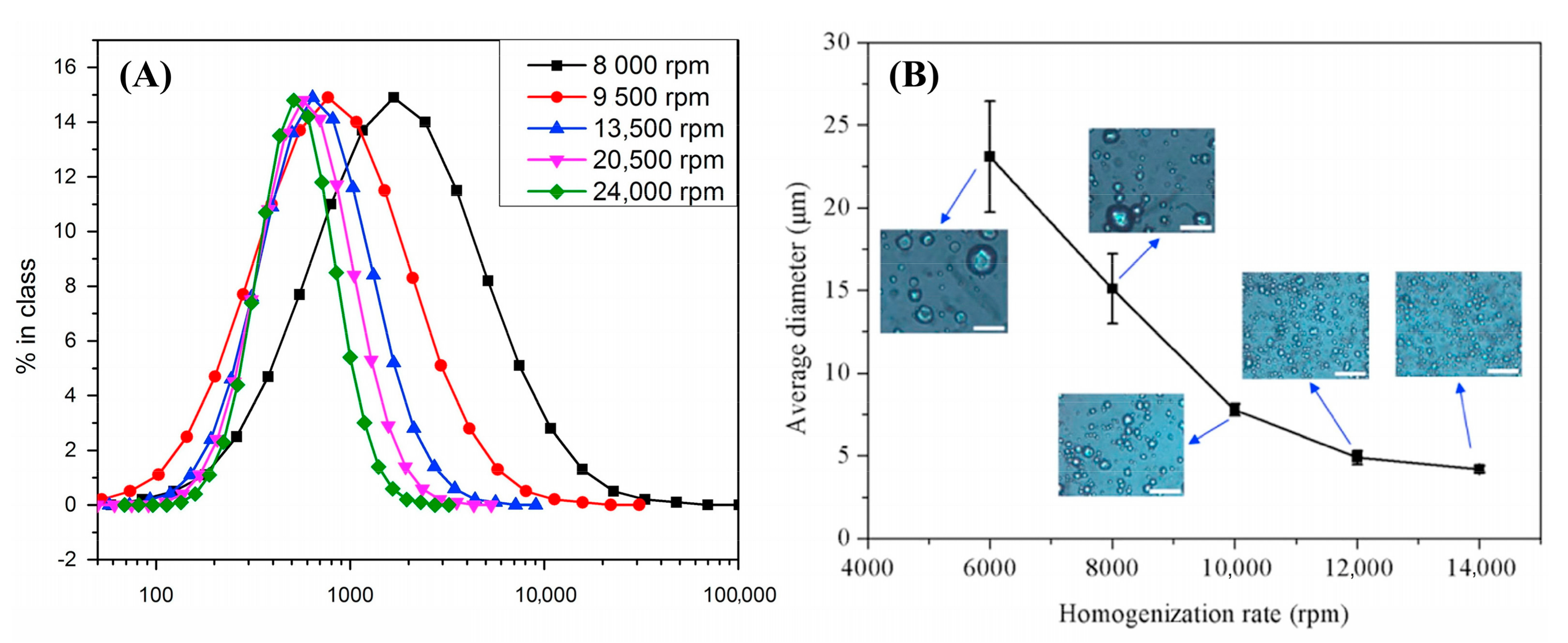
3.1.3. Rotor-Stator Homogenizer
3.1.4. Ultrasonic Emulsification
3.2. Studies of PCM Emulsions
3.2.1. Formulation by Rotor-Stator Homogenizer
3.2.2. Formulation by Sonicator
| PCM | Tm | Surfactants | O/S Ratio | Size | Conditions | NAs | ΔT | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rotor-Stator Homogenizer | ||||||||
| Tetradecane | 4–6 °C | SDS, Tween 40 | - | 3–5 μm | 24,000 rpm | - | 9.7 °C | [79] |
| Tetradecane | 4–6 °C | Triton X/Span60 (1:2) | 25:3 | ~1 μm | 5000 rpm (5 min) | - | 0 °C | [88] |
| RT10 | 10 °C | Brij52/Tween 60 (2:3) | 25:3.75 | ~3 μm | 500 rpm (15 min); 7200 rpm (45 min) | - | - | [75] |
| OP10E | 10 °C | Span80/Tween 80 (57:43) | 6:1 | 3–4 μm | 8000 rpm (3 min) | Graphite | 0 °C | [76] |
| Hexadecane | 18 °C | SDS, Tween 40 | - | 2–5 μm | 24,000 rpm | Paraffin | 2.1 °C | [79] |
| Hexadecane | 18 °C | SDS, Tween20, Tween80 | - | 2.3–4.5 μm | 8000 rpm (10 min) | MWCNTs | 0 °C | [78] |
| Hexadecane | 18 °C | Span80/Tween80 (1:1) | 6:1 | 0.5–1.2 μm | 8000–24,000 rpm (10 min) | SiO2 | 1.2 °C | [74] |
| RT20 | 20 °C | SDS, Tween40 | - | 3–5 μm | 24,000 rpm | - | 9.2 °C | [79] |
| RT25HC | 25 °C | Ethoxylated alcohols | 5:1 | >1 μm | 20,000 s−1 (5 min) | Myristic acid | 0 °C | [80] |
| Paraffin wax | 40–44 °C | DAS/Span80 | - | 10–20 μm | 10,000–19,000 rpm (3 min) | - | - | [89] |
| Paraffin wax | 42–44 °C | PEG-600/PVA/SMA with GO nanoparticle | - | ~8 μm | 19,000 rpm (5 min) | - | - | [90] |
| Crodatherm−47/53 | 50 °C | Steareth-2/100 (1:3) | 4:1 | 0.5–5 μm | 20,000 s−1 (1–60 min) 50,000 s−1 (1–150 min) | Boronitride Stearic acid | 0 °C | [71] |
| Paraffin wax | 54 °C | PVA/Eumulgin O10 with SiO2@Al2O3 | - | 0.4–0.5 μm | 3000 rpm | - | - | [91] |
| Paraffin wax | 58–60 °C | PVA/PEG-600 (76:24) | 20:2.8 | 4–6 μm | 12,000 rpm (5 min) | - | 0 °C | [92] |
| Paraffin wax | 58–60 °C | Span80/Tween80 (47:53) | 5:2 | ~0.5 μm | 8000 rpm (10 min) | Graphene | 0.3 °C | [77] |
| Paraffin wax | 59 °C | Span60/Tween60 (1:3) with resin as stabilizer | 2:1 | 0.1–9 μm | 500–1500 rpm (10–45 min) | - | - | [70] |
| Octacosane | 60 °C | Span20/Tween80 (1:1) | 4:1 | 0.5–1.5 μm | 8000–24,000 rpm (10 min) | SiO2 | ~1 °C | [72] |
| Paraffin wax | 62–64 °C | PVA/PEG-600 (1:1) | 5:1 | 3–10 μm | 6000–14,000 rpm (5 min) | - | 0 °C | [73] |
| Ultrasonic Emulsification | ||||||||
| RT21HC | 21 °C | SDS | 10:0.3 | 130–160 nm | 65 W for 10 min; 95 W for 20 min | GO, rGO | ~12 °C | [87] |
| RT21HC | 21 °C | SDS | 20:2.5 | 90–150 nm | 65 W (20 kHz) | Paraffin | ~9 °C | [85] |
| Octadecane | 28–30 °C | SDS | (10–40):2 10:1 | 100–200 nm | 70% Amplitude for 10 min 70% Amplitude for 30 min (650 W, 20–25 kHz) | Octadecanol MWCNTs | ~11 °C | [83,84] |
| Eicosane Tricosane | 36–38 °C 45–47 °C | SLS | 4:1 | 150–170 nm | 40% Amplitude for 10 h 20% Amplitude for 24 h (700 W, 20 kHz) | - | ~14 °C | [81,82] |
| RT55 | 55 °C | SDS | 10:0.3 | 200 nm | 65 W for 10 min; 95 W for 20 min | GO, rGO | 1 °C | [87] |
| RT55 | 55 °C | SDS | 8:1 | 173 nm | 65 W for 10 min (20 kHz) | Paraffin | 2.2 °C | [86] |
| Paraffin wax | 58–60 °C | Pluronics P-123 | 20:1 | ~600 nm | 20% Amplitude for 60 min (700 W, 20 kHz) | - | 3–4 °C | [93] |
| RT70HC | 70 °C | SDS | 8:1 | 166 nm | 65 W for 10 min (20 kHz) | SWCNHs | 1.8 °C | [86] |
4. Preparation of PCM Emulsions by Low-Energy Methods
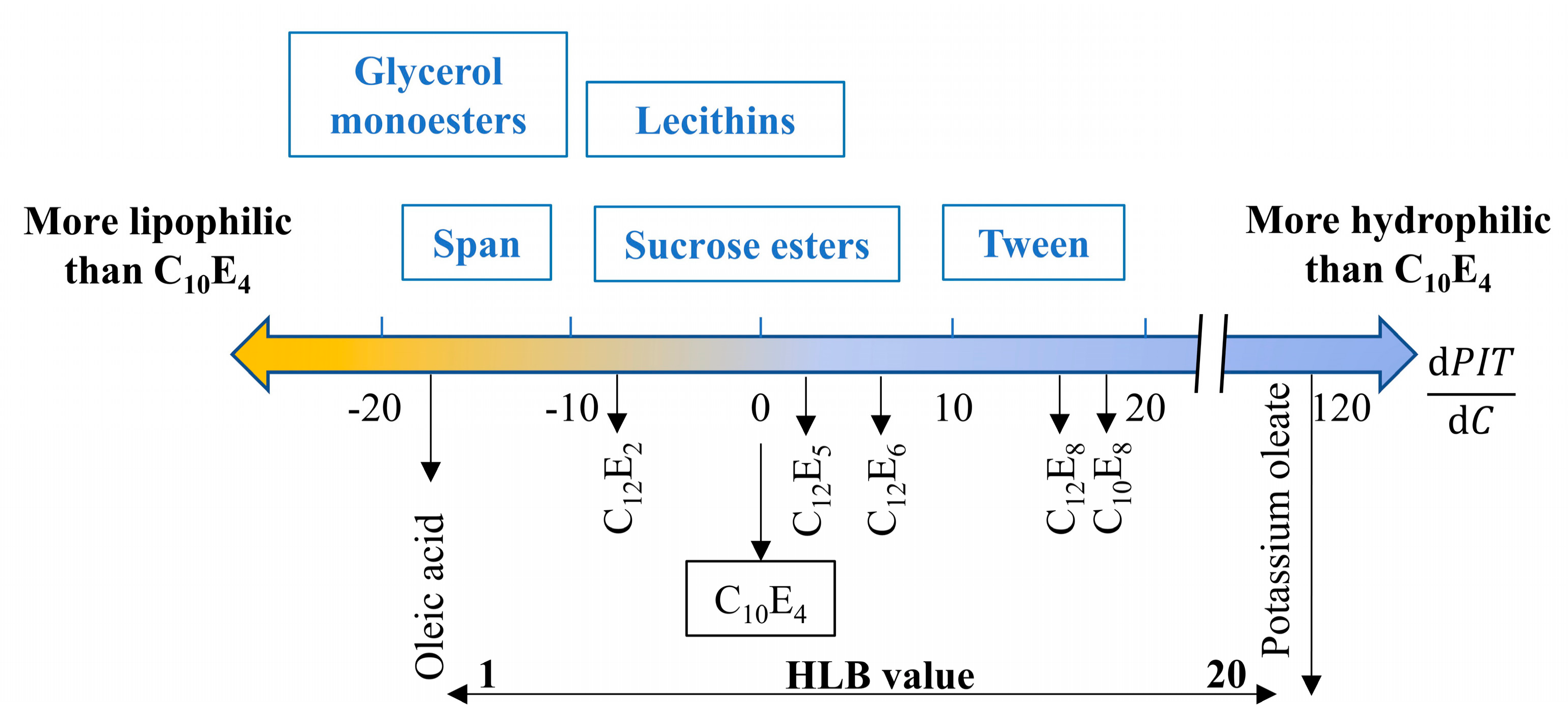
4.1. The Basic Mechanisms
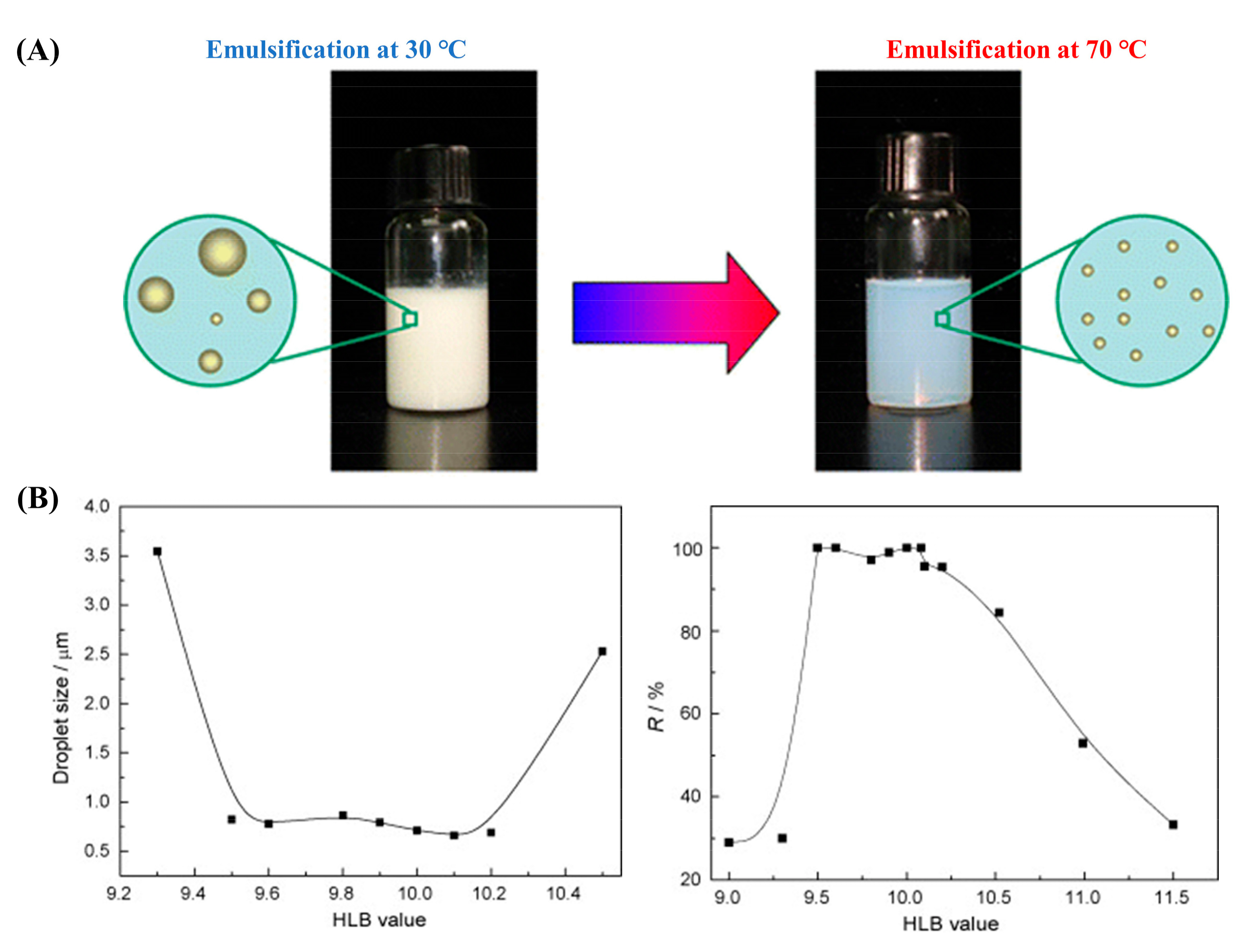
4.2. Preparation and Characterization of PCM Emulsions
4.2.1. PIC Method
| PCM | Tm | Surfactants | O/S Ratio | Size | Methods | NAs | ΔT | Ref. |
|---|---|---|---|---|---|---|---|---|
| Paraffin oil (ρ = 0.83 g/cm3) | - | Span80/Tween80 (HLB = 12–13) | 1:1 | ~50 nm | PIC | - | - | [101] |
| Paraffin oil (ρ = 0.85 g/cm3) | - | Span20/Tween20 (HLB = 12.75) with ionic surfactants or polymers | 2:1 | 80–200 nm | PIC | - | - | [104,105] |
| Paraffin oil (ρ = 0.85 g/cm3) | - | Span80/Tween80 (HLB = 10.3) with CTAB and NaBr | - | 100–200 nm | PIT | - | - | [107] |
| Paraffin oil (ρ = 0.86 g/cm3) | - | Span80/Tween80 (HLB = 11.6) | Varies | 95–170 nm | PIC | - | - | [100] |
| Paraffin oil (ρ = 0.86 g/cm3) | - | Span80/Tween80 (HLB = 10–11) | 1:1 | ~50 nm | PIC | - | - | [103] |
| Tetradecane | 4–6 °C | Span60/Tween60 (1:2) | 10:3 | 200–220 nm | PIT/PIC | - | ~14 °C | [108] |
| Tetradecane | 4–6 °C | Span80/Tween80 (HLB = 11.7) | 5:2 | ~500 nm | UE/PIT | Polymer | ~3 °C | [109] |
| Tetradecane | 4–6 °C | Self-synthesized POP surfactants | - | 40–90 nm | PIT | - | - | [110] |
| RT10 | 10 °C | Span60/Tween60 (1:2) | 10:3 | 157 nm | PIT | - | ~4 °C | [111] |
| Parafol 15-90 | 15 °C | Span60/Tween60 (1:2) | 10:3 | 145 nm | PIT | - | ~12 °C | [111] |
| Hexadecane | 18 | Span60/Tween60 (1:2) | 10:3 | 145 nm | PIT | - | ~11 °C | [111] |
| Hexadecane | 18 °C | Brij L4 | 30:11 30:13 | 60 nm 126 nm | PIT PIC | - SiO2 | ~9℃ ~8 °C | [97] |
| Hexadecane | 18 °C | Brij L4/Tween60 (3:2) Brij L4/Polymer (4:1) | 20:11 | ~60 nm | PIT | Paraffin - | 2–3 °C 5 °C | [112] |
| Hexadecane | 18 °C | Tween 60, Tween 80, Brij O10 | - | 422 nm | D-phase | - | ~17 °C | [113] |
| Hexadecane | 18℃ | Tween 80 | 5:2 | 223 nm | D-phase | - | - | [114] |
| Hexadecane | 18 °C | Span80/Tween80 (HLB = 12) | 5:2 | 290 nm | D-phase | ~15 °C | [115] | |
| Octadecane | 28–30 °C | Tween 80 | 5:2 | 220–240 nm | D-phase | - | - | [114] |
| Octadecane | 28–30 °C | Span80/Tween80 (HLB = 12) | 5:2 | 320 nm | D-phase | - | ~13 °C | [115] |
| Stearic acid Myristic acid | 53 °C | SDS/Span85 (1:1) | 3:2 | 40–80 nm | PIC | - | - | [106] |
| Paraffin wax | 51–54 °C | Span80/Tween80 (HLB = 9.5–10.3) | 4:1 | 600–900 nm | PIC | - | ~15 °C | [102] |
4.2.2. PIT Method
4.2.3. D-Phase Method
5. Applications
6. Conclusions
- (1)
- As the effective nucleation agent for emulsions/nano-emulsions, novel nanoparticles with smaller sizes (e.g., quantum dots) or modified interfacial properties could be one of the most promising solutions for effective reduction of supercooling in both high-energy and low-energy methods, which would also bring extra benefits such as enhanced stability and thermal conductivity.
- (2)
- Block copolymers or comb-like polymers are meaningful to be screened, which could work as surfactants and nucleating agents like PE-b-PEG.
- (3)
- The rarely investigated high-pressure homogenizer or microfluidizer and less investigated probe-type sonicator should be conducted, and effects of their processing parameters on properties of PCM emulsions need to be evaluated comprehensively.
- (4)
- Due to the instability characteristic of PCM emulsions, the concept of life cycle assessment should be established and evaluated by continuously monitoring property changes of PCM emulsions against operation time in lab-scale case studies, which would offer useful guidance in future large-scale systems.
- (5)
- The application fields should be expanded, such as for electronics thermal management. In addition, the current practical applications should be enriched such as cooling performance evaluation for PV modules under outdoor conditions and cooling/heating performance to the room through well-designed heat exchange units.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Niu, J. Two performance indices of TES apparatus: Comparison of MPCM slurry vs. stratified water storage tank. Energy Build. 2016, 127, 512–520. [Google Scholar] [CrossRef]
- Xu, H.; Miao, Y.; Wang, N.; Qu, Z.; Wang, X. Experimental investigations of heat transfer characteristics of MPCM during charging. Appl. Therm. Eng. 2018, 144, 721–725. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W.; Bai, Z.Y.; Ye, J. Rheological and energy transport characteristics of a phase change material slurry. Energy 2016, 106, 63–72. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef] [Green Version]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Ran, F.; Chen, Y.; Cong, R.; Fang, G. Flow and heat transfer characteristics of microencapsulated phase change slurry in thermal energy systems: A review. Renew. Sustain. Energy Rev. 2020, 134, 110101. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Zalba, B. Review on phase change material emulsions and microencapsulated phase change material slurries: Materials, heat transfer studies and applications. Renew. Sustain. Energy Rev. 2012, 16, 253–273. [Google Scholar] [CrossRef]
- Youssef, Z.; Delahaye, A.; Huang, L.; Trinquet, F.; Fournaison, L.; Pollerberg, C.; Doetsch, C. State of the art on phase change material slurries. Energy Convers. Manag. 2013, 65, 120–132. [Google Scholar] [CrossRef]
- Shao, J.; Darkwa, J.; Kokogiannakis, G. Review of phase change emulsions (PCMEs) and their applications in HVAC systems. Energy Build. 2015, 94, 200–217. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Lin, W.; Ling, Z.; Fang, X. A comprehensive review on phase change material emulsions: Fabrication, characteristics, and heat transfer performance. Sol. Energy Mater. Sol. Cells 2019, 191, 218–234. [Google Scholar] [CrossRef]
- O’Neill, P.; Fischer, L.; Revellin, R.; Bonjour, J. Phase change dispersions: A literature review on their thermo-rheological performance for cooling applications. Appl. Therm. Eng. 2021, 192, 116920. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-stabilized phase change materials based on porous supports for thermal energy storage applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.A.; Saidur, R.; Al-Sulaiman, F.A. A review for phase change materials (PCMs) in solar absorption refrigeration systems. Renew. Sustain. Energy Rev. 2017, 76, 105–137. [Google Scholar] [CrossRef]
- Yan, S.-R.; Fazilati, M.A.; Samani, N.; Ghasemi, H.R.; Toghraie, D.; Nguyen, Q.; Karimipour, A. Energy efficiency optimization of the waste heat recovery system with embedded phase change materials in greenhouses: A thermo-economic-environmental study. J. Energy Storage 2020, 30, 101445. [Google Scholar] [CrossRef]
- Prieto, C.; Cabeza, L.F. Thermal energy storage (TES) with phase change materials (PCM) in solar power plants (CSP). Concept and plant performance. Appl. Energy 2019, 254, 113646. [Google Scholar] [CrossRef]
- Fernández, A.G.; Muñoz-Sánchez, B.; Nieto-Maestre, J.; García-Romero, A. High temperature corrosion behavior on molten nitrate salt-based nanofluids for CSP plants. Renew. Energy 2019, 130, 902–909. [Google Scholar] [CrossRef]
- Ibrahim, A.; Peng, H.; Riaz, A.; Abdul Basit, M.; Rashid, U.; Basit, A. Molten salts in the light of corrosion mitigation strategies and embedded with nanoparticles to enhance the thermophysical properties for CSP plants. Sol. Energy Mater. Sol. Cells 2021, 219, 110768. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhang, T.; Zhang, D.; Ji, H. Supercooling suppression and thermal behavior improvement of erythritol as phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 171, 60–71. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Deen, G.R.; Skovgaard, J.; Pedersen, J.S. Chapter 6—Formation and properties of nanoemulsions. In Emulsions; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 193–226. [Google Scholar]
- Komaiko, J.S.; McClements, D.J. Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. A Quantitative kinetic theory of emulsion type, I. Physical chemistry of the emulsifying agent, Gas/Liquid and Liquid/Liquid Interface. Proc. Int. Congr. Surf. Act. 1957, 42, 426–438. [Google Scholar]
- McClements, D.J. Emulsion Design to Improve the Delivery of Functional Lipophilic Components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef]
- Degrand, L.; Michon, C.; Bosc, V. New insights into the study of the destabilization of oil-in-water emulsions with dextran sulfate provided by the use of light scattering methods. Food Hydrocoll. 2016, 52, 848–856. [Google Scholar] [CrossRef]
- Kabong, M.A.; Focke, W.W.; Du Toit, E.L.; Rolfes, H.; Ramjee, S. Breakdown mechanisms of oil-in-water emulsions stabilised with Pluronic F127 and co-surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124101. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Koroleva, M.; Nagovitsina, T.; Yurtov, E. Nanoemulsions stabilized by non-ionic surfactants: Stability and degradation mechanisms. Phys. Chem. Chem. Phys. 2018, 20, 10369–10377. [Google Scholar] [CrossRef]
- Karthik, P.; Ezhilarasi, P.N.; Anandharamakrishnan, C. Challenges associated in stability of food grade nanoemulsions. Crit. Rev. Food Sci. Nutr. 2017, 57, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Xu, W.; Liang, H.; Li, Y.; Liu, S.; Li, B. Chapter 1—Nanoemulsions for food: Properties, production, characterization, and applications. In Emulsions; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–36. [Google Scholar]
- Günther, E.; Schmid, T.; Mehling, H.; Hiebler, S.; Huang, L. Subcooling in hexadecane emulsions. Int. J. Refrig. 2010, 33, 1605–1611. [Google Scholar] [CrossRef]
- Mura, E.; Ding, Y. Nucleation of melt: From fundamentals to dispersed systems. Adv. Colloid Interface Sci. 2021, 289, 102361. [Google Scholar] [CrossRef]
- Cholakova, D.; Denkov, N. Rotator phases in alkane systems: In bulk, surface layers and micro/nano-confinements. Adv. Colloid Interface Sci. 2019, 269, 7–42. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, G.; Xie, B.; Fu, D.; Wang, D. Crystallization Features of Normal Alkanes in Confined Geometry. Acc. Chem. Res. 2014, 47, 192–201. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Håkansson, A.; Rayner, M. Chapter 5—General Principles of Nanoemulsion Formation by High-Energy Mechanical Methods. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 103–139. [Google Scholar]
- Guo, X.; Chen, M.; Li, Y.; Dai, T.; Shuai, X.; Chen, J.; Liu, C. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends Food Sci. Technol. 2020, 100, 223–234. [Google Scholar] [CrossRef]
- Charcosset, C.; Limayem, I.; Fessi, H. The membrane emulsification process—A review. J. Chem. Technol. Biotechnol. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Kano, T.; Aota, Y.; Maruoka, K. Rate Acceleration of Solid-Liquid Phase-Transfer Catalysis by Rotor-Stator Homogenizer. Adv. Synth. Catal. 2016, 358, 2996–2999. [Google Scholar] [CrossRef]
- Costa, J.M.; Almeida Neto, A.F.D. Ultrasound-assisted electrodeposition and synthesis of alloys and composite materials: A review. Ultrason. Sonochem. 2020, 68, 105193. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and Applications Beyond Microbial Inactivation. Food Eng. Rev. 2021, 13, 490–508. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Yan, B.; Balasubramaniam, V.M. Engineering Process Characterization of High-Pressure Homogenization—from Laboratory to Industrial Scale. Food Eng. Rev. 2017, 9, 143–169. [Google Scholar] [CrossRef]
- Vinchhi, P.; Patel, J.K.; Patel, M.M. High-Pressure Homogenization Techniques for Nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Patel, J.K., Pathak, Y.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 263–285. [Google Scholar]
- Schultz, S.; Wagner, G.; Urban, K.; Ulrich, J. High-Pressure Homogenization as a Process for Emulsion Formation. Chem. Eng. Technol. 2004, 27, 361–368. [Google Scholar] [CrossRef]
- Walstra, P. Principles of emulsion formation. Chem. Eng. Sci. 1993, 48, 333–349. [Google Scholar] [CrossRef]
- Slatter, W.; Powell, R. A study of the cavitation effect in the homogenization of dairy products. J. Dairy Sci. 1950, 33, 692–702. [Google Scholar]
- Jafari, S.M.; He, Y.; Bhandari, B. Optimization of nano-emulsions production by microfluidization. Eur. Food Res. Technol. 2007, 225, 733–741. [Google Scholar] [CrossRef]
- Villalobos-Castillejos, F.; Granillo-Guerrero, V.G.; Leyva-Daniel, D.E.; Alamilla-Beltrán, L.; Gutiérrez-López, G.F.; Monroy-Villagrana, A.; Jafari, S.M. Chapter 8—Fabrication of Nanoemulsions by Microfluidization. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 207–232. [Google Scholar]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, E.; Drioli, E.; Giorno, L. Membrane emulsification technology: Twenty-five years of inventions and research through patent survey. J. Membr. Sci. 2014, 468, 410–422. [Google Scholar] [CrossRef]
- Nakashima, T.; Shimizu, M.; Kukizaki, M. Membrane Emulsification by Microporous Glass. Key Eng. Mater. 1992, 61–62, 513–516. [Google Scholar] [CrossRef]
- Fuchigami, T.; Toki, M.; Nakanishi, K. Membrane Emulsification Using Sol-Gel Derived Macroporous Silica Glass. J. Sol-Gel Sci. Technol. 2000, 19, 337–341. [Google Scholar] [CrossRef]
- Suzuki, K.; Fujiki, I.; Hagura, Y. Preparation of Corn Oil/Water and Water/Corn Oil Emulsions Using PTFE Membranes. Food Sci. Technol. Int. Tokyo 1998, 4, 164–167. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Liang, C.; Wang, W. Measurement and analysis of bimodal drop size distribution in a rotor–stator homogenizer. Chem. Eng. Sci. 2013, 102, 622–631. [Google Scholar] [CrossRef]
- Gallassi, M.; Gonçalves, G.F.N.; Botti, T.C.; Moura, M.J.B.; Carneiro, J.N.E.; Carvalho, M.S. Numerical and experimental evaluation of droplet breakage of O/W emulsions in rotor-stator mixers. Chem. Eng. Sci. 2019, 204, 270–286. [Google Scholar] [CrossRef]
- Håkansson, A. Rotor-Stator Mixers: From Batch to Continuous Mode of Operation—A Review. Processes 2018, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Rueger, P.E.; Calabrese, R.V. Dispersion of water into oil in a rotor–stator mixer. Part 2: Effect of phase fraction. Chem. Eng. Res. Des. 2013, 91, 2134–2141. [Google Scholar] [CrossRef]
- Leng, D.E.; Calabrese, R.V. Immiscible liquid–liquid systems. Handb. Ind. Mix. Sci. Pract. 2004, 3952, 639–753. [Google Scholar]
- Li, W.; Leong, T.S.H.; Ashokkumar, M.; Martin, G.J.O. A study of the effectiveness and energy efficiency of ultrasonic emulsification. Phys. Chem. Chem. Phys. 2018, 20, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Delmas, H.; Barthe, L. Chapter 25—Ultrasonic mixing, homogenization, and emulsification in food processing and other applications. In Power Ultrasonics; Gallego-Juárez, J.A., Graff, K.F., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 757–791. [Google Scholar]
- Modarres-Gheisari, S.M.M.; Gavagsaz-Ghoachani, R.; Malaki, M.; Safarpour, P.; Zandi, M. Ultrasonic nano-emulsification—A review. Ultrason. Sonochem. 2019, 52, 88–105. [Google Scholar] [CrossRef]
- Kumar, A.; Gogate, P.R.; Pandit, A.B. Mapping of Acoustic Streaming in Sonochemical Reactors. Ind. Eng. Chem. Res. 2007, 46, 4368–4373. [Google Scholar] [CrossRef]
- Peshkovsky, S.L.; Peshkovsky, A.S. Shock-wave model of acoustic cavitation. Ultrason. Sonochem. 2008, 15, 618–628. [Google Scholar] [CrossRef]
- Vilasau, J.; Solans, C.; Gómez, M.J.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Stability of oil-in-water paraffin emulsions prepared in a mixed ionic/nonionic surfactant system. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 222–229. [Google Scholar] [CrossRef]
- Hagelstein, G.; Gschwander, S. Reduction of supercooling in paraffin phase change slurry by polyvinyl alcohol. Int. J. Refrig. 2017, 84, 67–75. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.P.; Liao, K.J. The Preparation of Wax Emulsions Stabilized by C5 Petroleum Resin. Pet. Sci. Technol. 2013, 31, 284–292. [Google Scholar] [CrossRef]
- Fischer, L.; Mura, E.; O’Neill, P.; von Arx, S.; Worlitschek, J.; Qiao, G.; Li, Q.; Ding, Y. Thermophysical properties of a phase change dispersion for cooling around 50 °C. Int. J. Refrig. 2020, 119, 410–419. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.-Y.; Niu, J. PCM-in-water emulsion for solar thermal applications: The effects of emulsifiers and emulsification conditions on thermal performance, stability and rheology characteristics. Sol. Energy Mater. Sol. Cells 2016, 147, 211–224. [Google Scholar] [CrossRef]
- Wang, F.; Fang, X.; Zhang, Z. Preparation of phase change material emulsions with good stability and little supercooling by using a mixed polymeric emulsifier for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 381–390. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Wu, J.-Y. Development and characterization of novel and stable silicon nanoparticles-embedded PCM-in-water emulsions for thermal energy storage. Appl. Energy 2019, 238, 1407–1416. [Google Scholar] [CrossRef]
- Shao, J.; Darkwa, J.; Kokogiannakis, G. Development of a novel phase change material emulsion for cooling systems. Renew. Energy 2016, 87, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, C.; Liu, J.; Fang, X.; Zhang, Z. Highly stable graphite nanoparticle-dispersed phase change emulsions with little supercooling and high thermal conductivity for cold energy storage. Appl. Energy 2017, 188, 97–106. [Google Scholar] [CrossRef]
- Xiang, N.; Yuan, Y.; Sun, L.; Cao, X.; Zhao, J. Simultaneous decrease in supercooling and enhancement of thermal conductivity of paraffin emulsion in medium temperature range with graphene as additive. Thermochim. Acta 2018, 664, 16–25. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Zhang, S.; Wu, J.-Y. PCM in Water Emulsions: Supercooling Reduction Effects of Nano-Additives, Viscosity Effects of Surfactants and Stability. Adv. Eng. Mater. 2015, 17, 181–188. [Google Scholar] [CrossRef]
- Huang, L.; Günther, E.; Doetsch, C.; Mehling, H. Subcooling in PCM emulsions—Part 1: Experimental. Thermochim. Acta 2010, 509, 93–99. [Google Scholar] [CrossRef]
- Fischer, L.J.; von Arx, S.; Wechsler, U.; Züst, S.; Worlitschek, J. Phase change dispersion properties, modeling apparent heat capacity. Int. J. Refrig. 2017, 74, 240–253. [Google Scholar] [CrossRef]
- Ho, C.J.; Jheng, S.-R.; Yang, T.-F.; Pourfattah, F.; Yan, W.-M. Thermophysical properties of water-based nano-emulsion of tricosane—An Experimental investigation. Case Stud. Therm. Eng. 2021, 24, 100849. [Google Scholar] [CrossRef]
- Ho, C.J.; Lin, K.-H.; Rashidi, S.; Toghraie, D.; Yan, W.-M. Experimental study on thermophysical properties of water-based nanoemulsion of n-eicosane PCM. J. Mol. Liq. 2021, 321, 114760. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhao, C.Y. Synthesis and characterization of a narrow size distribution nano phase change material emulsion for thermal energy storage. Sol. Energy 2017, 147, 406–413. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Z.; Cui, G.; Dou, B.; Lu, W.; Yan, X. Fabrication of a novel nano phase change material emulsion with low supercooling and enhanced thermal conductivity. Renew. Energy 2020, 151, 542–550. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Agresti, F.; Barison, S.; Marcos, M.A.; Prado, J.I.; Rossi, S.; Bobbo, S.; Fedele, L. Development of paraffinic phase change material nanoemulsions for thermal energy storage and transport in low-temperature applications. Appl. Therm. Eng. 2019, 159, 113868. [Google Scholar] [CrossRef]
- Agresti, F.; Fedele, L.; Rossi, S.; Cabaleiro, D.; Bobbo, S.; Ischia, G.; Barison, S. Nano-encapsulated PCM emulsions prepared by a solvent-assisted method for solar applications. Sol. Energy Mater. Sol. Cells 2019, 194, 268–275. [Google Scholar] [CrossRef]
- Barison, S.; Cabaleiro, D.; Rossi, S.; Kovtun, A.; Melucci, M.; Agresti, F. Paraffin–graphene oxide hybrid nano emulsions for thermal management systems. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127132. [Google Scholar] [CrossRef]
- Dao, V.-D.; Choi, H.-S. N-tetradecane/Water Emulsion as a Low-cost Phase Change Material for Efficient Packaging and Shipping of Vaccines. Clean Technol. 2017, 23, 325–330. [Google Scholar]
- Ding, Z.; Zhang, H.; He, F.; Li, Y.; He, R.; Zhang, K.; Fan, J.; Yang, Z.; Yang, W. Novel paraffin/ethylene propylene diene monomer phase change latex with excellent stability and low viscosity. Sol. Energy Mater. Sol. Cells 2019, 200, 109957. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, W.; Zhang, H.; He, F.; Yan, H.; He, R.; Zhang, K.; Fan, J. Graphene oxide Pickering phase change material emulsions with high thermal conductivity and photo-thermal performance for thermal energy management. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 42–49. [Google Scholar] [CrossRef]
- Koroleva, M.Y.; Gorbachevski, O.S.; Yurtov, E.V. Paraffin wax emulsions stabilized with polymers, surfactants, and nanoparticles. Theor. Found. Chem. Eng. 2017, 51, 125–132. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Fang, X.; Zhang, Z. Graphite nanoparticles-dispersed paraffin/water emulsion with enhanced thermal-physical property and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2016, 147, 101–107. [Google Scholar] [CrossRef]
- Sivapalan, B.; Neelesh Chandran, M.; Manikandan, S.; Saranprabhu, M.K.; Pavithra, S.; Rajan, K.S. Paraffin wax–water nanoemulsion: A superior thermal energy storage medium providing higher rate of thermal energy storage per unit heat exchanger volume than water and paraffin wax. Energy Convers. Manag. 2018, 162, 109–117. [Google Scholar] [CrossRef]
- Shinoda, K.; Saito, H. The effect of temperature on the phase equilibria and the types of dispersions of the ternary system composed of water, cyclohexane, and nonionic surfactant. J. Colloid Interface Sci. 1968, 26, 70–74. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Perazzo, A.; Preziosi, V.; Guido, S. Phase inversion emulsification: Current understanding and applications. Adv. Colloid Interface Sci. 2015, 222, 581–599. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Wu, J.-Y. Evaluation and manipulation of the key emulsification factors toward highly stable PCM-water nano-emulsions for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 219, 110820. [Google Scholar] [CrossRef]
- Salager, J.-L.; Marquez, N.; Graciaa, A.; Lachaise, J. Partitioning of Ethoxylated Octylphenol Surfactants in Microemulsion-Oil-Water Systems: Influence of Temperature and Relation between Partitioning Coefficient and Physicochemical Formulation. Langmuir 2000, 16, 5534–5539. [Google Scholar] [CrossRef]
- Ontiveros, J.F.; Pierlot, C.; Catté, M.; Molinier, V.; Salager, J.-L.; Aubry, J.-M. A simple method to assess the hydrophilic lipophilic balance of food and cosmetic surfactants using the phase inversion temperature of C10E4/n-octane/water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 32–39. [Google Scholar] [CrossRef]
- Hessien, M.; Singh, N.; Kim, C.; Prouzet, E. Stability and Tunability of O/W Nanoemulsions Prepared by Phase Inversion Composition. Langmuir 2011, 27, 2299–2307. [Google Scholar] [CrossRef]
- Kim, E.H.; Cho, W.G. Stable liquid paraffin-in-water nanoemulsions prepared by phase inversion composition method. J. Soc. Cosmet. Sci. Korea 2014, 40, 133–139. [Google Scholar]
- Li, C.; Mei, Z.; Liu, Q.; Wang, J.; Xu, J.; Sun, D. Formation and properties of paraffin wax submicron emulsions prepared by the emulsion inversion point method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 356, 71–77. [Google Scholar] [CrossRef]
- Yu, L.; Li, C.; Xu, J.; Hao, J.; Sun, D. Highly Stable Concentrated Nanoemulsions by the Phase Inversion Composition Method at Elevated Temperature. Langmuir 2012, 28, 14547–14552. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhang, H.; Xu, G.; Tan, Y.; Zhang, J.; Lv, X. Influence of CTAB and SDS on the properties of oil-in-water nano-emulsion with paraffin and span 20/Tween 20. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 60–67. [Google Scholar] [CrossRef]
- Yang, Q.; Xin, X.; Wang, L.; Lu, H.; Ren, H.; Tan, Y.; Xu, G. Modification of the stability of oil-in-water nano-emulsions by polymers with different structures. Colloid Polym. Sci. 2014, 292, 1297–1306. [Google Scholar] [CrossRef]
- Puupponen, S.; Seppälä, A.; Vartia, O.; Saari, K.; Ala-Nissilä, T. Preparation of paraffin and fatty acid phase changing nanoemulsions for heat transfer. Thermochim. Acta 2015, 601, 33–38. [Google Scholar] [CrossRef]
- Mei, Z.; Liu, S.; Wang, L.; Jiang, J.; Xu, J.; Sun, D. Preparation of positively charged oil/water nano-emulsions with a sub-PIT method. J. Colloid Interface Sci. 2011, 361, 565–572. [Google Scholar] [CrossRef]
- Schalbart, P.; Kawaji, M.; Fumoto, K. Formation of tetradecane nanoemulsion by low-energy emulsification methods. Int. J. Refrig. 2010, 33, 1612–1624. [Google Scholar] [CrossRef]
- Fumoto, K.; Sato, N.; Kawaji, M.; Kawanami, T.; Inamura, T. Phase Change Characteristics of a Nanoemulsion as a Latent Heat Storage Material. Int. J. Thermophys. 2014, 35, 1922–1932. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Schalbart, P.; Kawaji, M. Comparison of paraffin nanoemulsions prepared by low-energy emulsification method for latent heat storage. Int. J. Therm. Sci. 2013, 67, 113–119. [Google Scholar] [CrossRef]
- Liu, L.; Niu, J.; Wu, J.-Y. Formulation of highly stable PCM nano-emulsions with reduced supercooling for thermal energy storage using surfactant mixtures. Sol. Energy Mater. Sol. Cells 2021, 223, 110983. [Google Scholar] [CrossRef]
- Kawanami, T.; Togashi, K.; Fumoto, K.; Hirano, S.; Zhang, P.; Shirai, K.; Hirasawa, S. Thermophysical properties and thermal characteristics of phase change emulsion for thermal energy storage media. Energy 2016, 117, 562–568. [Google Scholar] [CrossRef]
- Morimoto, T.; Togashi, K.; Kumano, H.; Hong, H. Thermophysical properties of phase change emulsions prepared by D-phase emulsification. Energy Convers. Manag. 2016, 122, 215–222. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P. Preparation and characterization of nano-sized phase change emulsions as thermal energy storage and transport media. Appl. Energy 2017, 190, 868–879. [Google Scholar] [CrossRef]
- Sagitani, H. Formation of o/w emulsions by surfactant phase emulsification and the solution behavior of nonionic surfactant system in the emulsification process. J. Dispers. Sci. Technol. 1988, 9, 115–129. [Google Scholar] [CrossRef]
- Himeur, Y.; Ghanem, K.; Alsalemi, A.; Bensaali, F.; Amira, A. Artificial intelligence based anomaly detection of energy consumption in buildings: A review, current trends and new perspectives. Appl. Energy 2021, 287, 116601. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Peñalosa, C.; Dolado, P.; Zalba, B. Experimental analysis of a low cost phase change material emulsion for its use as thermal storage system. Energy Convers. Manag. 2015, 106, 201–212. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Peñalosa, C.; Marín, J.M.; Zalba, B. Experimental analysis of a coiled stirred tank containing a low cost PCM emulsion as a thermal energy storage system. Energy 2017, 138, 590–601. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Niu, J.; Wu, J.-Y. Evaluation of the energy storage performance of PCM nano-emulsion in a small tubular heat exchanger. Case Stud. Therm. Eng. 2021, 26, 101156. [Google Scholar] [CrossRef]
- Biedenbach, M.; Poetzsch, L.; Gschwander, S. Characterization of an n-octadecane PCS in a 0.5 m3 storage tank test facility. Int. J. Refrig. 2019, 104, 76–83. [Google Scholar] [CrossRef]
- Shabani, B.; Biju, M. Theoretical Modelling Methods for Thermal Management of Batteries. Energies 2015, 8, 10153–10177. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Rajkumar, S.; Valavan, D. A review on the thermal regulation techniques for non integrated flat PV modules mounted on building top. Energy Build. 2015, 86, 692–697. [Google Scholar] [CrossRef]
- Wang, F.; Cao, J.; Ling, Z.; Zhang, Z.; Fang, X. Experimental and simulative investigations on a phase change material nano-emulsion-based liquid cooling thermal management system for a lithium-ion battery pack. Energy 2020, 207, 118215. [Google Scholar] [CrossRef]
- Cao, J.; He, Y.; Feng, J.; Lin, S.; Ling, Z.; Zhang, Z.; Fang, X. Mini-channel cold plate with nano phase change material emulsion for Li-ion battery under high-rate discharge. Appl. Energy 2020, 279, 115808. [Google Scholar] [CrossRef]
- Cao, J.; Feng, J.; Fang, X.; Ling, Z.; Zhang, Z. A delayed cooling system coupling composite phase change material and nano phase change material emulsion. Appl. Therm. Eng. 2021, 191, 116888. [Google Scholar] [CrossRef]
- Feng, J.; Huang, J.; Ling, Z.; Fang, X.; Zhang, Z. Performance enhancement of a photovoltaic module using phase change material nanoemulsion as a novel cooling fluid. Sol. Energy Mater. Sol. Cells 2021, 225, 111060. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, X.; Zhao, X.; Li, P.; Ali, S. Experimental investigation of the energy performance of a novel Micro-encapsulated Phase Change Material (MPCM) slurry based PV/T system. Appl. Energy 2016, 165, 260–271. [Google Scholar] [CrossRef]
- Eisapour, M.; Eisapour, A.H.; Hosseini, M.J.; Talebizadehsardari, P. Exergy and energy analysis of wavy tubes photovoltaic-thermal systems using microencapsulated PCM nano-slurry coolant fluid. Appl. Energy 2020, 266, 114849. [Google Scholar] [CrossRef]
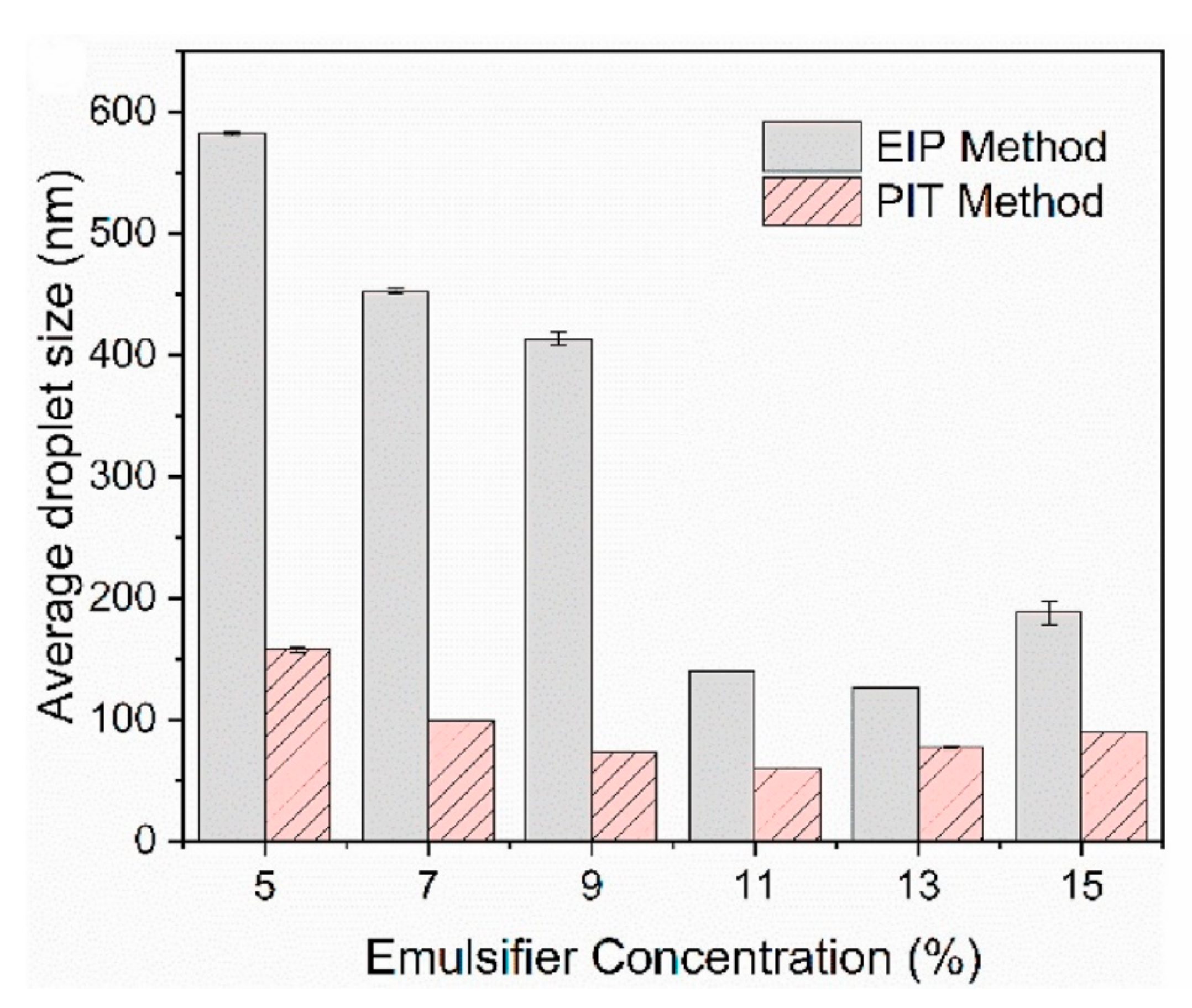
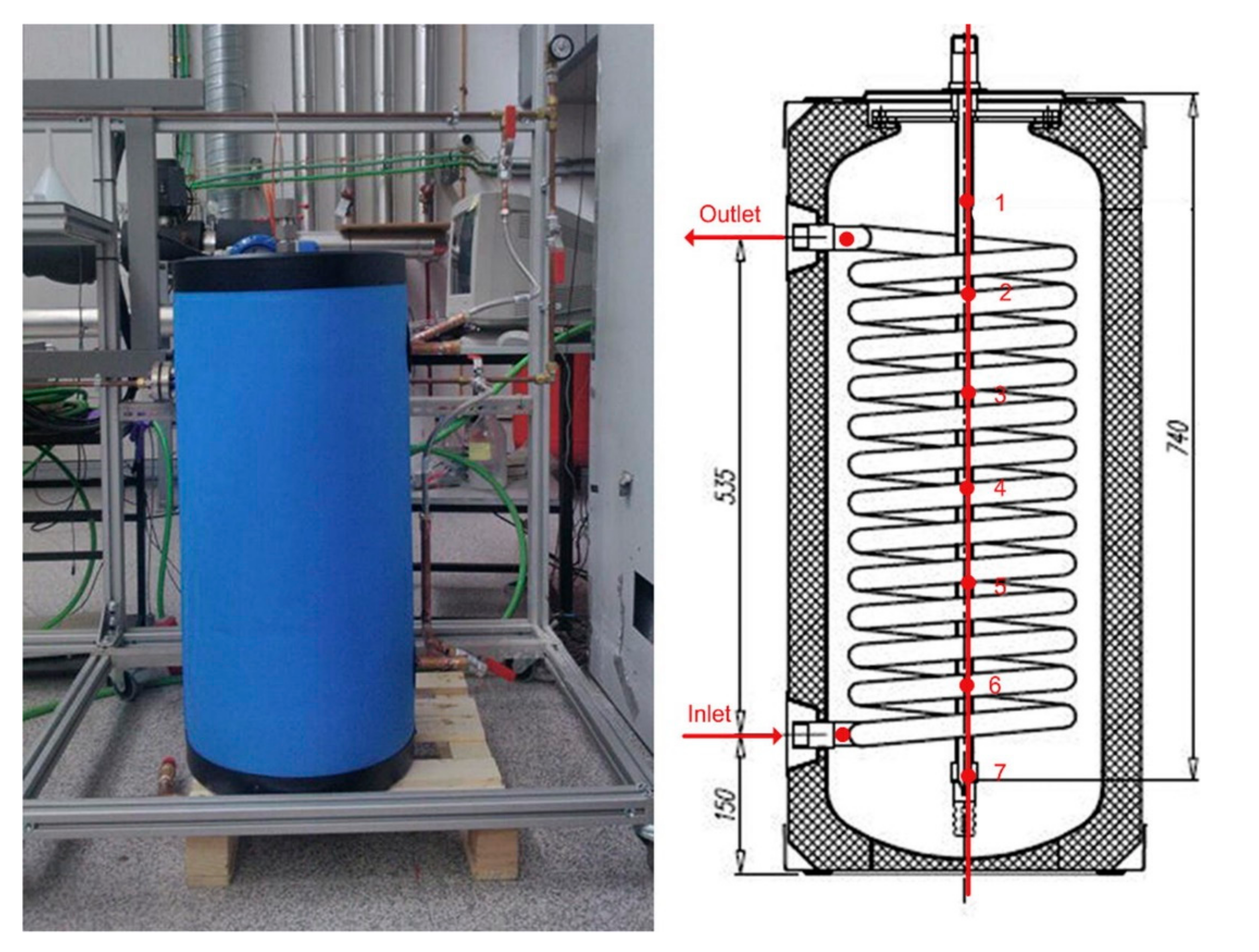
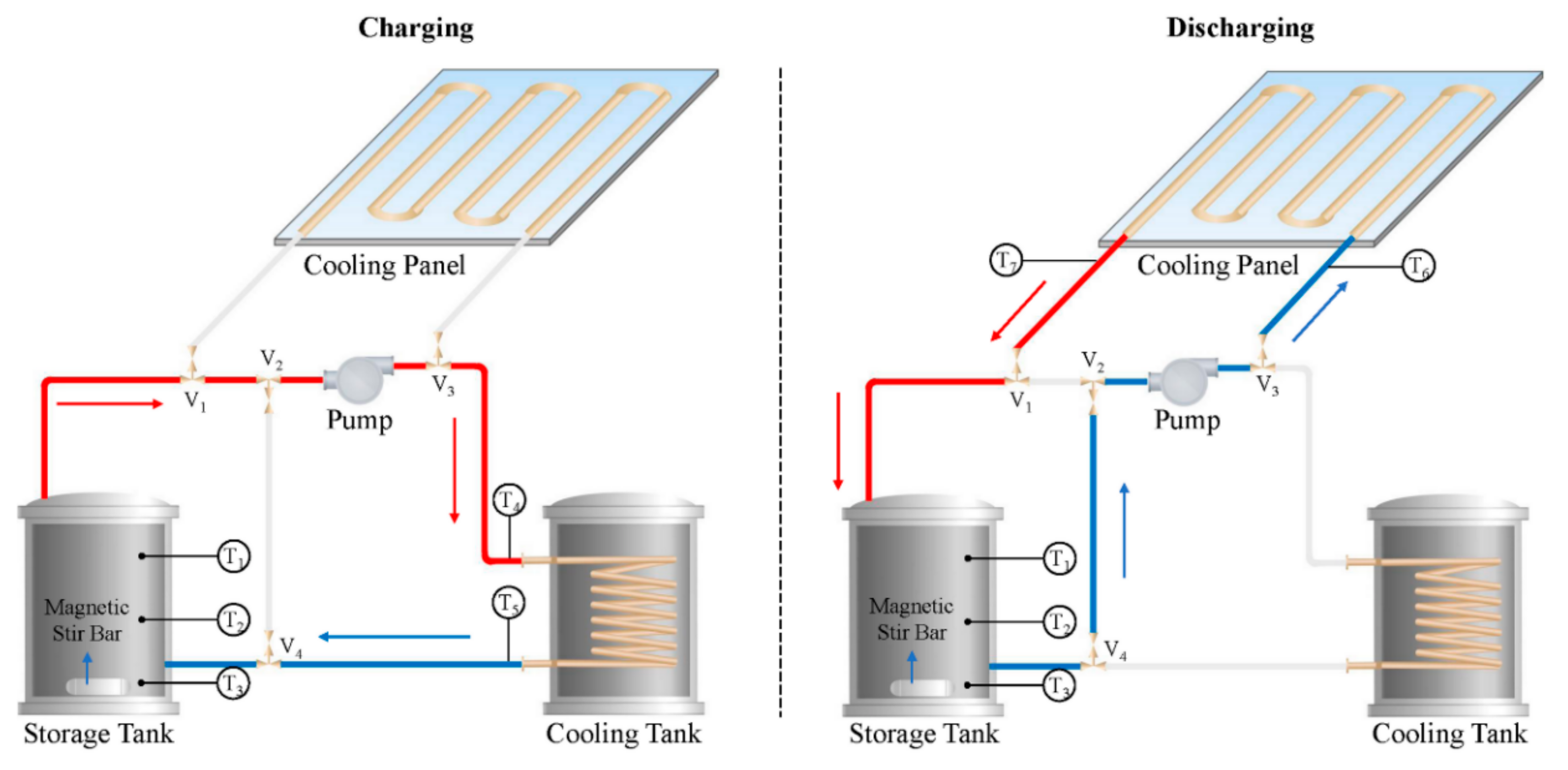


| Topic | Ref. |
|---|---|
| PCM emulsions and microencapsulated PCM material slurries: materials, heat transfer studies and applications | Delgado et al. (2012) [8] |
| State of the art on PCM slurries | Youssef et al. (2013) [9] |
| PCM emulsions and their applications in HVAC systems | Shao et al. (2015) [10] |
| A comprehensive review on PCM emulsions: fabrication, characteristics, and heat transfer performance | Wang et al. (2019) [11] |
| PCM dispersions: thermo-rheological performance for cooling applications | O’Neil et al. (2021) [12] |
| Characteristics | Emulsion | Nano-Emulsion | Micro-Emulsion |
|---|---|---|---|
| Size | >200 nm | 20–200 nm | 5–50 nm |
| Shape | Spherical | Spherical | Spherical, lamellar |
| Stability | Thermodynamically and kinetically unstable | Thermodynamically unstable; kinetically stable | Thermodynamically and kinetically stable |
| Appearance | Milky white | Transparent, translucent, or creamy; | Transparent |
| Fabrication | High-energy methods Low-energy methods | High-energy methods Low-energy methods | Low-energy methods |
| Conditions | HPH | ME | HSP | UE |
|---|---|---|---|---|
| Common devices | Microfluidizer; valves | SPG/PTFE membranes | Rotor-stator homogenizer | Probe-type ultrasonicator |
| Droplet breakup mechanism | Turbulence; shear stress; cavitation | Drop-by-drop | Turbulence; shear stress | Cavitation |
| Minimum size | 0.1 μm | 0.2–0.5 μm | 1 μm | 0.1 μm |
| Residence time | 0.1 < t < 3 ms | - | 0.1 < t < 1 s | - |
| Viscosity range | Low-medium | Low-medium | Low-high | Low |
| Energy density | Medium-high | Low-medium | Low-high | Medium-high |
| Energy efficiency | High | Very high | Low | High |
| Cost | Medium-high | Medium-high | Low | Low-medium |
| Application | Lab/industrial | Lab | Lab/industrial | Lab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Niu, J.; Wu, J.-Y. Preparation of Stable Phase Change Material Emulsions for Thermal Energy Storage and Thermal Management Applications: A Review. Materials 2022, 15, 121. https://doi.org/10.3390/ma15010121
Liu L, Niu J, Wu J-Y. Preparation of Stable Phase Change Material Emulsions for Thermal Energy Storage and Thermal Management Applications: A Review. Materials. 2022; 15(1):121. https://doi.org/10.3390/ma15010121
Chicago/Turabian StyleLiu, Liu, Jianlei Niu, and Jian-Yong Wu. 2022. "Preparation of Stable Phase Change Material Emulsions for Thermal Energy Storage and Thermal Management Applications: A Review" Materials 15, no. 1: 121. https://doi.org/10.3390/ma15010121







