High-Entropy Pyrochlore A2B2O7 with Both Heavy and Light Rare-Earth Elements at the A Site
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Synthesis of High Entropy Pyrochlore Oxides
2.2. Characterization
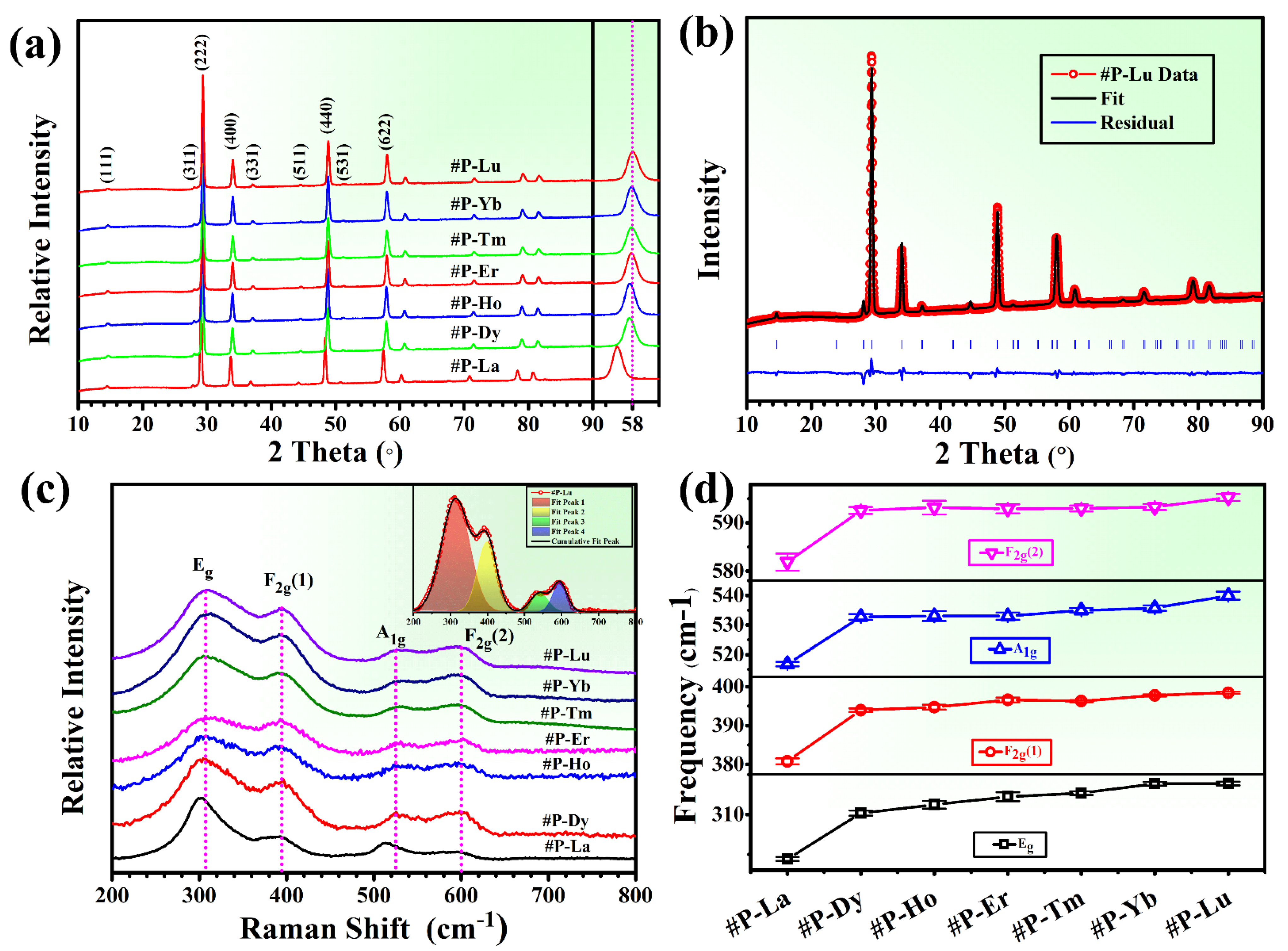
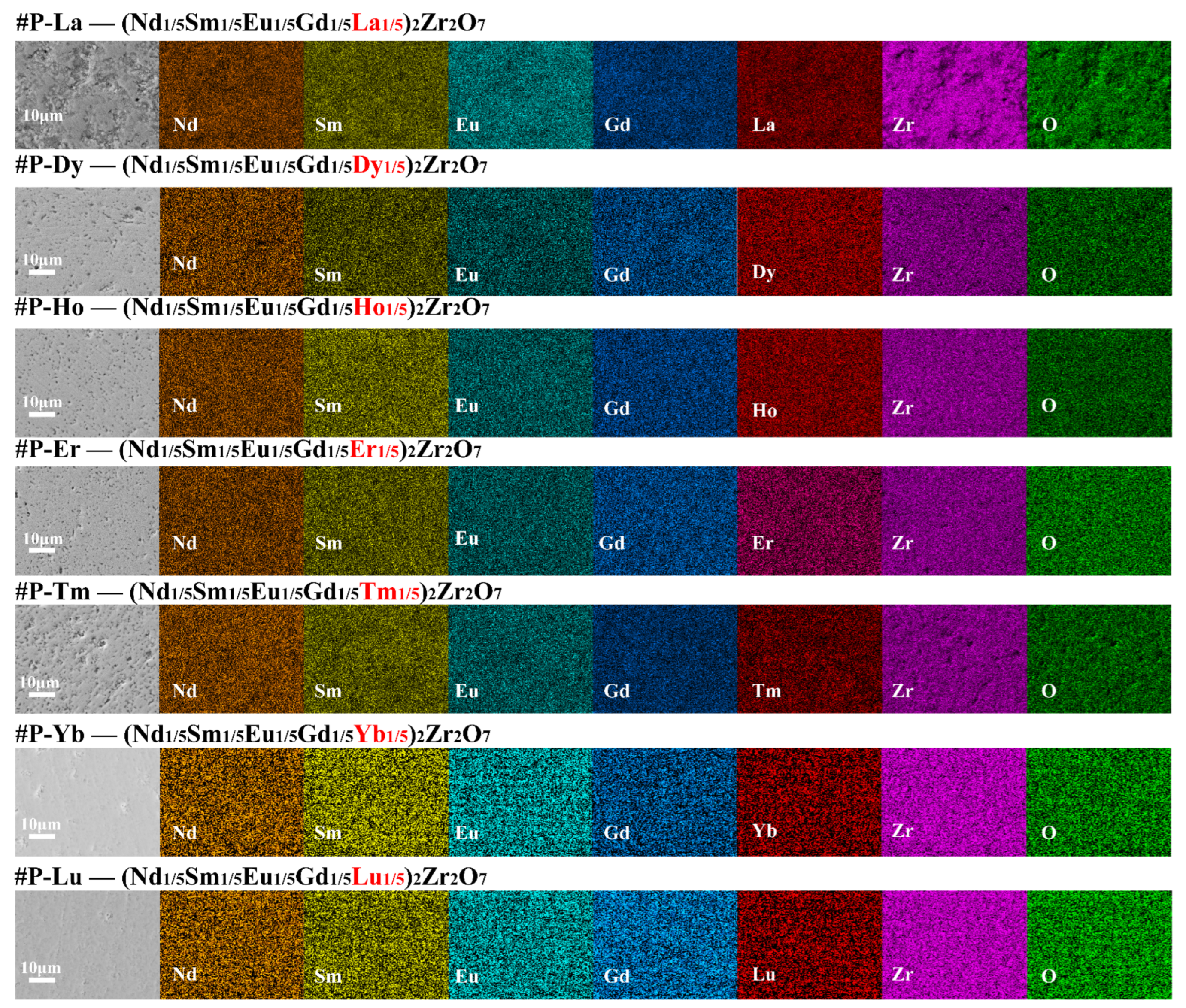
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [Green Version]
- Sarker, P.; Harrington, T.; Toher, C.; Oses, C.; Samiee, M.; Maria, J.P.; Brenner, D.W.; Vecchio, K.S.; Curtarolo, S. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun. 2018, 9, 4980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co, Cr, Fe, Mn, Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Chen, K.; Pei, X.; Tang, L.; Cheng, H.; Li, Z.; Li, C.; Zhang, X.; An, L. A five-component entropy-stabilized fluorite oxide. J. Eur. Ceram. Soc. 2018, 38, 4161–4164. [Google Scholar] [CrossRef]
- Xiang, H.; Xing, Y.; Dai, F.-z.; Wang, H.; Su, L.; Miao, L.; Zhang, G.; Wang, Y.; Qi, X.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Zhou, L.; Li, F.; Liu, J.-X.; Hu, Q.; Bao, W.; Wu, Y.; Cao, X.; Xu, F.; Zhang, G.-J. High-entropy thermal barrier coating of rare-earth zirconate: A case study on (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 prepared by atmospheric plasma spraying. J. Eur. Ceram. Soc. 2020, 40, 5731–5739. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, F.; Liu, Z.; Chen, H.; Shao, Z.; Zhang, X.; Wang, K.; Xue, L. A novel (La0.2Sm0.2Eu0.2Gd0.2Tm0.2)2Zr2O7 high-entropy ceramic nanofiber with excellent thermal stability. Ceram. Int. 2021, 47, 29379–29385. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Zeng, J.; Deng, T.; Luo, B.; Zhang, H.; Huang, X. Preparation of (La0.2Nd0.2Sm0.2Gd0.2Yb0.2)2Zr2O7 high-entropy transparent ceramic using combustion synthesized nanopowder. J. Alloys Compd. 2020, 817, 153328. [Google Scholar] [CrossRef]
- Li, F.; Zhou, L.; Liu, J.-X.; Liang, Y.; Zhang, G.-J. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. J. Adv. Ceram. 2019, 8, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Wang, Q.; Luo, J.; Chen, R. Advanced materials for high-temperature thermal transport. Adv. Funct. Mater. 2019, 30, 1904815. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W. Thermal expansion and conductivity of RE2Sn2O7 (RE = La, Nd, Sm, Gd, Er and Yb) pyrochlores. Scr. Mater. 2013, 69, 401–404. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.-Z.; Peng, Z.; Zhou, Y. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol. 2019, 35, 2647–2651. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, Y.; Feng, X.; Tian, Z.; Song, R. Thermal barrier coatings with high-entropy oxide as a top coat. Ceram. Int. 2022, 48, 1349–1359. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.Y.; Ko, S.T.; Chung, K.M.; Chen, R.K.; Luo, J. Size disorder as a descriptor for predicting reduced thermal conductivity in medium- and high-entropy pyrochlore oxides. Scr. Mater. 2020, 181, 76–81. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. A simple and effective predictor to design novel fluorite-structured High Entropy Oxides (HEOs). Acta Mater. 2021, 202, 181–189. [Google Scholar] [CrossRef]
- Zhou, L.; Li, F.; Liu, J.X.; Sun, S.K.; Liang, Y.; Zhang, G.J. High-entropy A2B2O7-type oxide ceramics: A potential immobilising matrix for high-level radioactive waste. J. Hazard. Mater. 2021, 415, 125596. [Google Scholar] [CrossRef]
- Jiang, B.; Bridges, C.A.; Unocic, R.R.; Pitike, K.C.; Cooper, V.R.; Zhang, Y.; Lin, D.Y.; Page, K. Probing the Local Site Disorder and Distortion in Pyrochlore High-Entropy Oxides. J. Am. Chem. Soc. 2021, 143, 4193–4204. [Google Scholar] [CrossRef]
- Mao, H.-R.; Guo, R.-F.; Cao, Y.; Jin, S.-B.; Qiu, X.-M.; Shen, P. Ultrafast densification of high-entropy oxide (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 by reactive flash sintering. J. Eur. Ceram. Soc. 2021, 41, 2855–2860. [Google Scholar] [CrossRef]
- Anand, G.; Wynn, A.P.; Handley, C.M.; Freeman, C.L. Phase stability and distortion in high-entropy oxides. Acta Mater. 2018, 146, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Troparevsky, M.C.; Morris, J.R.; Kent, P.R.C.; Lupini, A.R.; Stocks, G.M. Criteria for Predicting the Formation of Single-Phase High-Entropy Alloys. Phys. Rev. Lett. 2015, 5, 011041. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, M.A.; Ravamudan, G.A.; Rao, G.V.S. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar] [CrossRef]
- Teng, Z.; Tan, Y.; Zeng, S.; Meng, Y.; Chen, C.; Han, X.; Zhang, H. Preparation and phase evolution of high-entropy oxides A2B2O7 with multiple elements at A and B sites. J. Eur. Ceram. Soc. 2021, 41, 3614–3620. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. The Reflection of X-rays crystals. R. Soc. Lond. Proc. 1913, 89, 246–248. [Google Scholar] [CrossRef]
- Wan, C.; Qu, Z.; Du, A.; Pan, W. Influence of B site substituent Ti on the structure and thermophysical properties of A2B2O7-type pyrochlore Gd2Zr2O7. Acta Mater. 2009, 57, 4782–4789. [Google Scholar] [CrossRef]
- Nandi, S.; Jana, Y.M.; Sarkar, S.; Jana, R.; Mukherjee, G.D.; Gupta, H.C. Synthesis, structure, UV-Vis-NIR, infrared and Raman spectroscopy, and force-field investigation for A2GaSbO7 (A3+ = Y, Dy, Gd) pyrochlores. J. Alloys Compd. 2019, 771, 88–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]

| Abbreviation | Composition |
|---|---|
| #P-La | (Nd1/5Sm1/5Eu1/5Gd1/5La1/5)2Zr2O7 |
| #P-Dy | (Nd1/5Sm1/5Eu1/5Gd1/5Dy1/5)2Zr2O7 |
| #P-Ho | (Nd1/5Sm1/5Eu1/5Gd1/5Ho1/5)2Zr2O7 |
| #P-Er | (Nd1/5Sm1/5Eu1/5Gd1/5Er1/5)2Zr2O7 |
| #P-Tm | (Nd1/5Sm1/5Eu1/5Gd1/5Tm1/5)2Zr2O7 |
| #P-Yb | (Nd1/5Sm1/5Eu1/5Gd1/5Yb1/5)2Zr2O7 |
| #P-Lu | (Nd1/5Sm1/5Eu1/5Gd1/5Lu1/5)2Zr2O7 |
| Abbreviation | Lattice Parameter (Å) | x-Parameter | rA/rB | |
|---|---|---|---|---|
| #P-La | 10.64364(26) | 0.34416(79) | 1.5158 | 3.18% |
| #P-Dy | 10.57011(32) | 0.35109(93) | 1.4817 | 2.55% |
| #P-Ho | 10.55579(30) | 0.35412(95) | 1.4783 | 2.90% |
| #P-Er | 10.56713(28) | 0.35504(94) | 1.4753 | 3.25% |
| #P-Tm | 10.55427(35) | 0.35599(99) | 1.4725 | 3.58% |
| #P-Yb | 10.55395(32) | 0.35444(92) | 1.4700 | 3.89% |
| #P-Lu | 10.54532(30) | 0.35170(79) | 1.4686 | 4.06% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Z.; Tan, Y.; Zhang, H. High-Entropy Pyrochlore A2B2O7 with Both Heavy and Light Rare-Earth Elements at the A Site. Materials 2022, 15, 129. https://doi.org/10.3390/ma15010129
Teng Z, Tan Y, Zhang H. High-Entropy Pyrochlore A2B2O7 with Both Heavy and Light Rare-Earth Elements at the A Site. Materials. 2022; 15(1):129. https://doi.org/10.3390/ma15010129
Chicago/Turabian StyleTeng, Zhen, Yongqiang Tan, and Haibin Zhang. 2022. "High-Entropy Pyrochlore A2B2O7 with Both Heavy and Light Rare-Earth Elements at the A Site" Materials 15, no. 1: 129. https://doi.org/10.3390/ma15010129





