Hyaluronic Acid-Silver Nanocomposites and Their Biomedical Applications: A Review
Abstract
:1. Introduction
2. Synthesis
3. Physicochemical Properties of HA/AgNPs Biopolymers
4. Mechanism of Action
5. Applications
6. Cytotoxicity
7. Examples of Usage
8. Challenges and Opportunities
9. Conclusions
- ∎
- Hyaluronic acid-nanosilver composites exhibit antimicrobial, anti-angiogenic, anti-inflammatory and anticancer properties, accompanied by a high biocompatibility.
- ∎
- There are various techniques of synthesis of nanocomposites affecting their physicochemical properties, but the crucial points of the majority of the procedures include obtainment of the AgNPs and incorporation of them into biopolymer structure.
- ∎
- Their potential applications include wound dressing production, oncological, vascular and inflammatory diseases treatment, as well as medical devices and implant fabrication.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate Polymer-Based Silver Nanocomposites: Recent Progress in the Antimicrobial Wound Dressings. Carbohydr. Polym. 2020, 231, 115696. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Baek, J.-B. Nanocomposites Derived from Polymers and Inorganic Nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef] [Green Version]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic–Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of Nanotechnology in Targeted Drug Delivery and Imaging: A Concise Review. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 193–212. [Google Scholar] [CrossRef]
- Liao, H.; Nehl, C.L.; Hafner, J.H. Biomedical Applications of Plasmon Resonant Metal Nanoparticles. Nanomedicine 2006, 1, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Ramontja, J. Sodium Alginate Stabilized Silver Nanoparticles–Silica Nanohybrid and Their Antibacterial Characteristics. Int. J. Biol. Macromol. 2016, 93, 712–723. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Brzychczy-Włoch, M.; Gosiewski, T.; Jany, B.; Lishchynskyi, O.; Shymborska, Y.; Nastyshyn, S.; Bernasik, A.; et al. “Command” Surfaces with Thermo-Switchable Antibacterial Activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109806. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Zaboli, M.; Barani, M.; Baino, F.; Bilal, M.; Sanchooli, E. Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies. Nanomaterials 2021, 11, 817. [Google Scholar] [CrossRef]
- Khachatryan, K.; Khachatryan, G.; Fiedorowicz, M.; Para, A.; Tomasik, P. Formation of Nanometal Particles in the Dialdehyde Starch Matrix. Carbohydr. Polym. 2013, 98, 568–573. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Maheriya, P.M. Hyaluronic Acid as Potential Carrier in Biomedical and Drug Delivery Applications. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–265. ISBN 9780081025550. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymer 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y.; Okamoto, A.; Nishinari, K. Viscoelasticity of Hyaluronic Acid with Different Molecular Weights. Biorheology 1994, 31, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.H.; Hussain, Z.; Abourehab, M.A.S.; Hasan, H.; Afzal, S.; Thu, H.E. New Developments and Clinical Transition of Hyaluronic Acid-Based Nanotherapeutics for Treatment of Cancer: Reversing Multidrug Resistance, Tumour-Specific Targetability and Improved Anticancer Efficacy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Guss, J.M.; Hukins, D.W.L.; Smith, P.J.C.; Winter, W.T.; Arnott, S.; Moorhouse, R.; Rees, D.A. Hyaluronic Acid: Molecular Conformations and Interactions in Two Sodium Salts. J. Mol. Biol. 1975, 95, 359–364. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Porrelli, D.; Travan, A.; Turco, G.; Crosera, M.; Borgogna, M.; Donati, I.; Paoletti, S.; Adami, G.; Marsich, E. Antibacterial-Nanocomposite Bone Filler Based on Silver Nanoparticles and Polysaccharides. J. Tissue Eng. Regen. Med 2018, 12, e747–e759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Hao, Y.; Ding, Y.; Yuan, Z.; Liu, Y.; Cai, K. Fabrication of Enzyme-Responsive Composite Coating for the Design of Antibacterial Surface. J. Mater. Sci. Mater. Med. 2018, 29, 160. [Google Scholar] [CrossRef] [PubMed]
- Tarusha, L.; Paoletti, S.; Travan, A.; Marsich, E. Alginate Membranes Loaded with Hyaluronic Acid and Silver Nanoparticles to Foster Tissue Healing and to Control Bacterial Contamination of Non-Healing Wounds. J. Mater. Sci. Mater. Med. 2018, 29, 22. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.; Huang, J.; Xu, X.; Yu, C.; Ning, X.; Fan, S.; Zhang, Z. Hyaluronic Acid-Modified Au-Ag Alloy Nanoparticles for Radiation/Nanozyme/Ag+Multimodal Synergistically Enhanced Cancer Therapy. Bioconjugate Chem. 2020, 31, 1756–1765. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aly, A.A.; Abou-Okeil, A. A Non-Woven Fabric Wound Dressing Containing Layer-by-Layer Deposited Hyaluronic Acid and Chitosan. Int. J. Biol. Macromol. 2018, 114, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymer 2019, 11, 1207. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Wang, J.; Li, W.; Xiao, J.; Li, J.; Lin, X.; Xie, Z.; You, L.; Zhang, Q. Silver Nanoparticles-Coated Monolithic Column for in-Tube Solid-Phase Microextraction of Monounsaturated Fatty Acid Methyl Esters. J. Chromatogr. A 2019, 1585, 19–26. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Grzyb, J.; Fiedorowicz, M. Formation and Properties of Hyaluronan/Nano Ag and Hyaluronan-Lecithin/Nano Ag Films. Carbohydr. Polym. 2016, 151, 452–457. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and Characterization of Antibacterial Thermosensitive Hydrogels Based on Corn Silk Extract, Hyaluronic Acid and Nanosilver for Potential Wound Healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Pavliňák, D.; Čileková, M.; Lepcio, P.; Abdel-Rahman, R.M.; Jančář, J. Electrospinning of Hyaluronan/Polyvinyl Alcohol in Presence of in-Situ Silver Nanoparticles: Preparation and Characterization. Int. J. Biol. Macromol. 2019, 139, 730–739. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound Healing of Nanofiber Comprising Polygalacturonic/Hyaluronic Acid Embedded Silver Nanoparticles: In-Vitro and in-Vivo Studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Sheu, C.; Chen, C.H.; Chen, S.H.; Jose, G.; Kuo, C.Y.; Chen, J.P. Multi-Functional Electrospun Antibacterial Core-Shell Nanofibrous Membranes for Prolonged Prevention of Post-Surgical Tendon Adhesion and Inflammation. Acta Biomater. 2018, 72, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.C.; Pereira, P.; Barbosa, A.M.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial Free-Standing Polysaccharide Composite Films Inspired by the Sea. Int. J. Biol. Macromol. 2019, 133, 933–944. [Google Scholar] [CrossRef]
- Francesko, A.; Ivanova, K.; Hoyo, J.; Pérezpérez-Rafael, S.; Petkova, P.; Fernandes, M.M.; Heinze, T.; Mendoza, E.; Tzanov, T. Bottom-up Layer-by-Layer Assembling of Antibacterial Freestanding Nanobiocomposite Films. Biomacromolecules 2018, 19, 3628–3636. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, Y.; Xie, Q.; Fan, C.; Hilborn, J.; Dai, J.; Ossipov, D.A. Moldable Hyaluronan Hydrogel Enabled by Dynamic Metal–Bisphosphonate Coordination Chemistry for Wound Healing. Adv. Healthc. Mater. 2018, 7, 1700973. [Google Scholar] [CrossRef]
- Allison, D.D.; Grande-Allen, K.J. Review. Hyaluronan: A Powerful Tissue Engineering Tool. Tissue Eng. 2006, 12, 2131–2140. [Google Scholar] [CrossRef]
- Price, Z.K.; Lokman, N.A.; Ricciardelli, C. Differing Roles of Hyaluronan Molecular Weight on Cancer Cell Behavior and Chemotherapy Resistance. Cancers 2018, 10, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karbownik, M.S.; Nowak, J.Z. Hyaluronan: Towards Novel Anti-Cancer Therapeutics. Pharmacol. Rep. 2013, 65, 1056–1074. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular Size-Dependent Signaling and Biological Functions in Inflammation and Cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Kim, K.J.; Lee, D.G. A Novel Mechanism for the Antibacterial Effect of Silver Nanoparticles on Escherichia Coli. BioMetals 2014, 27, 1191–1201. [Google Scholar] [CrossRef]
- Cameron, S.J.; Hosseinian, F.; Willmore, W.G. A Current Overview of the Biological and Cellular Effects of Nanosilver. Int. J. Mol. Sci. 2018, 19, 2030. [Google Scholar] [CrossRef] [Green Version]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver Nano—A Trove for Retinal Therapies. J. Control. Rel. 2010, 145, 76–90. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Nastyshyn, S.; Raczkowska, J.; Stetsyshyn, Y.; Orzechowska, B.; Bernasik, A.; Shymborska, Y.; Brzychczy-Włoch, M.; Gosiewski, T.; Lishchynskyi, O.; Ohar, H.; et al. Non-Cytotoxic, Temperature-Responsive and Antibacterial POEGMA Based Nanocomposite Coatings with Silver Nanoparticles. RSC Adv. 2020, 10, 10155–10166. [Google Scholar] [CrossRef]
- Çulha, M.; Kalay, Ş.; Sevim, E.; Pinarbaş, M.; Baş, Y.; Akpinar, R.; Karaoğlu, Ş.A. Biocidal Properties of Maltose Reduced Silver Nanoparticles against American Foulbrood Diseases Pathogens. Bio. Metals 2017, 30, 893–902. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.I.; Meza-Villezcas, A.; J Fournier, P.G.; Soria-Castro, E.; Juarez-MorenoID, K.; Gallego-Herná ndez, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-SaqueroID, A. Enhancement of Antibiotics Antimicrobial Activity Due to the Silver Nanoparticles Impact on the Cell Membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [Green Version]
- El Sayed, M.T.; El-Sayed, A.S.A. Biocidal Activity of Metal Nanoparticles Synthesized by Fusarium Solani against Multidrug-Resistant Bacteria and Mycotoxigenic Fungi. J. Microbiol. Biotechnol. 2020, 30, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.K.; Ma, Q.H.; Li, S.Y.; Zhang, D.Q.; Cong, L.; Tian, Y.L.; Yang, R.Y. The Antifungal Effect of Silver Nanoparticles on Trichosporon Asahii. J. Microbiol. Immunol. Infect. 2016, 49, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thahira Khatoon, U.; Nageswara Rao, G.V.S.; Mohan, K.M.; Ramanaviciene, A.; Ramanavicius, A. Antibacterial and Antifungal Activity of Silver Nanospheres Synthesized by Tri-Sodium Citrate Assisted Chemical Approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Rao, K.; Aziz, S.; Roome, T.; Razzak, A.; Sikandar, B.; Jamali, K.S.; Imran, M.; Jabri, T.; Shah, M.R. Gum Acacia Stabilized Silver Nanoparticles Based Nano-Cargo for Enhanced Anti-Arthritic Potentials of Hesperidin in Adjuvant Induced Arthritic Rats. Artif. Cells Nanomed. Biotechnol. 2018, 46, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, A.L.; Garcia, C.B.; Gallón, S.N.; Webster, T.J. Novel Silver-Platinum Nanoparticles for Anticancer and Antimicrobial Applications. Int. J. Nanomed. 2020, 15, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, K.J.C.; Mendez, M.D. Ophthalmia Neonatorum; StatPearls Publishing: Treasure Island, UK, 2021. [Google Scholar]
- Schluesener, J.K.; Schluesener, H.J. Nanosilver: Application and Novel Aspects of Toxicology. Arch. Toxicol. 2013, 87, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Clement, D.; Ajayan, P.; Mousa, S.; Linhardt, R.J. Hyaluronan- and Heparin-Reduced Silver Nanoparticles with Antimicrobial Properties. Nanomedicine 2009, 4, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Hoseini Alfatemi, S.; Sharifi Rad, M.; Iriti, M.; Sharifi-Rad, J. Antimicrobial Synergic Effect of Allicin and Silver Nanoparticles on Skin Infection Caused by Methicillin-Resistant Staphylococcus aureus spp. Ann. Med. Health Sci. Res. 2014, 4, 863–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.D.; Luo, L.J.; Lai, J.Y. Toward Understanding the Purely Geometric Effects of Silver Nanoparticles on Potential Application as Ocular Therapeutics via Treatment of Bacterial Keratitis. Mater. Sci. Eng. C 2021, 119, 111497. [Google Scholar] [CrossRef]
- Nadhe, S.B.; Singh, R.; Wadhwani, S.A.; Chopade, B.A. Acinetobacter Sp. Mediated Synthesis of AgNPs, Its Optimization, Characterization and Synergistic Antifungal Activity against C. Albicans. J. Appl. Microbiol. 2019, 127, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Artunduaga Bonilla, J.J.; Honorato, L.; Cordeiro de Oliveira, D.F.; Araújo Gonçalves, R.; Guimarães, A.; Miranda, K.; Nimrichter, L. Silver Chitosan Nanocomposites as a Potential Treatment for Superficial Candidiasis. Med. Mycol. 2021, 59, 993–1005. [Google Scholar] [CrossRef]
- Kiseleva, I.v.; Farroukh, M.a.; Skomorokhova, E.A.; Rekstin, A.R.; Bazhenova, E.A.; Magazenkova, D.N.; Orlov, I.A.; Rudenko, L.G.; Broggini, M.; Puchkova, L. v. Anti-Influenza Effect of Nanosilver in a Mouse Model. Vaccines 2020, 8, 679. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental Rsv Infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral Potential of Green Synthesized Silver Nanoparticles of Lampranthus Coccineus and Malephora Lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaid, A.; Zahra, D.; Asim, A.; Javaid, N.; Ashfaq, U.A. Recent Updates on the Role of Nanoparticles in the Treatment of Viral Diseases. Crit. Rev. Ther. Drug Carr. Syst. 2021, 38, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Homayouni-Tabrizi, M.; Soltani, M.; Karimi, E.; Namvar, F.; Pouresmaeil, V.; Es-Haghi, A. Putative Mechanism for Anticancer Properties of Ag-PP (NPs) Extract. IET Nanobiotechnol. 2019, 13, 617–620. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, Y.; Zhang, Y.; Wang, Q.; Huang, Z.; Ding, Q.; Guo, Z.; Zhou, X.; Zhu, L.; Gu, N. The Cellular Uptake and Cytotoxic Effect of Silver Nanoparticles on Chronic Myeloid Leukemia Cells. J. Biomed. Nanotechnol. 2014, 10, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Bagherifam, S.; Bekhradnia, S.; Nyström, B. Anti-Cancerous Effect of Albumin Coated Silver Nanoparticles on MDA-MB 231 Human Breast Cancer Cell Line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative Cytotoxicity of Nanosilver in Human Liver HepG2 and Colon Caco2 Cells in Culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Yu, D.; Zhao, D. AgNPs and Ag/C225 Exert Anticancerous Effects via Cell Cycle Regulation and Cytotoxicity Enhancement. J. Nanomater. 2017, 2017, 7920368. [Google Scholar] [CrossRef] [Green Version]
- Arshad, R.; Barani, M.; Rahdar, A.; Sargazi, S.; Cucchiarini, M.; Pandey, S.; Kang, M. Multi-Functionalized Nanomaterials and Nanoparticles for Diagnosis and Treatment of Retinoblastoma. Biosensors 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Patel, M.M. Threatening Cancer with Nanoparticle Aided Combination Oncotherapy. J. Control. Release 2019, 301, 76–109. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, K.; Khachatryan, L.; Krzan, M.; Krystyjan, M.; Krzemińska-Fiedorowicz, L.; Lenart-Boroń, A.; Koronowicz, A.; Drozdowska, M.; Khachatryan, G. Formation and Investigation of Physicochemical, Biological and Bacteriostatic Properties of Nanocomposite Foils Containing Silver Nanoparticles and Graphene Oxide in Hyaluronic Acid Matrix. Materials 2021, 14, 3377. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, X.; Wang, J.; Zhang, P.; Huang, X.; Zhang, Z.; Guo, D.S.; Yang, X. Ag@: S -Nitrosothiol Core-Shell Nanoparticles for Chemo and Photothermal Synergistic Tumor Targeted Therapy. J. Mater. Chem. B 2020, 8, 5483–5490. [Google Scholar] [CrossRef]
- Li, W.; Ma, C.; Song, Y.; Hong, C.; Qiao, X.; Yin, B. Sensitive Detection of Carcinoembryonic Antigen (CEA) by a Sandwich-Type Electrochemical Immunosensor Using MOF-Ce@HA/Ag-HRP-Ab 2 as a Nanoprobe. Nanotechnology 2020, 31, 185605. [Google Scholar] [CrossRef]
- Bi, Y.; Hao, F.; Yan, G.; Teng, L.; Lee, R.J.; Xie, J. Actively Targeted Nanoparticles for Drug Delivery to Tumor. Curr. Drug Metab. 2016, 17, 763–782. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Hyaluronic Acid-Based Biopharmaceutical Delivery and Tumor-Targeted Drug Delivery System. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Przysiecka, Ł.; Peplińska, B.; Jarek, M.; Coy, E.; Jurga, S. Gel with Silver and Ultrasmall Iron Oxide Nanoparticles Produced with Amanita Muscaria Extract: Physicochemical Characterization, Microstructure Analysis and Anticancer Properties. Sci. Rep. 2018, 8, 13260. [Google Scholar] [CrossRef]
- Lallana, E.; Rios De La Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef]
- Liu, T.; Han, M.; Tian, F.; Cun, D.; Rantanen, J.; Yang, M. Budesonide Nanocrystal-Loaded Hyaluronic Acid Microparticles for Inhalation: In Vitro and in Vivo Evaluation. Carbohydr. Polym. 2018, 181, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Elkhooly, T.A.; Elsherbiny, S.M.; Reicha, F.M.; Shokeir, A.A. Facile Coating of Urinary Catheter with Bio–Inspired Antibacterial Coating. Heliyon 2019, 5, e02986. [Google Scholar] [CrossRef] [Green Version]
- Koc, H.; Kilicay, E.; Karahaliloglu, Z.; Hazer, B.; Denkbas, E.B. Prevention of Urinary Infection through the Incorporation of Silver-Ricinoleic Acid-Polystyrene Nanoparticles on the Catheter Surface. J. Biomater. Appl. 2021, 36, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ji, X.; Wei, D.; Deng, J. Antibacterial Properties and Application of Nanosilver Material in the Tracheal Tube. J. Nanosci. Nanotechnol. 2020, 20, 6542–6546. [Google Scholar] [CrossRef]
- Wekwejt, M.; Michno, A.; Truchan, K.; Pałubicka, A.; Świeczko-żurek, B.; Osyczka, A.M.; Zieliński, A. Antibacterial Activity and Cytocompatibility of Bone Cement Enriched with Antibiotic, Nanosilver, and Nanocopper for Bone Regeneration. Nanomater. 2019, 9, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wekwejt, M.; Chen, S.; Kaczmarek-Szczepańska, B.; Nadolska, M.; Łukowicz, K.; Pałubicka, A.; Michno, A.; Osyczka, A.M.; Michálek, M.; Zieliński, A. Nanosilver-Loaded PMMA Bone Cement Doped with Different Bioactive Glasses-Evaluation of Cytocompatibility, Antibacterial Activity, and Mechanical Properties. Biomater. Sci. 2021, 9, 3112–3126. [Google Scholar] [CrossRef]
- Porenczuk, A.; Grzeczkowicz, A.; Maciejewska, I.; Gołaś, M.; Piskorska, K.; Kolenda, A.; Gozdowski, D.; Kopeć-Swoboda, E.; Granicka, L.; Olczak-Kowalczyk, D. An Initial Evaluation of Cytotoxicity, Genotoxicity and Antibacterial Effectiveness of a Disinfection Liquid Containing Silver Nanoparticles Alone and Combined with a Glass-Ionomer Cement and Dentin Bonding Systems. Adv. Clin. Exp. Med. 2019, 28, 75–83. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver Nanoparticles in Dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; He, X.; Hou, A. Meta-Analysis of the Therapeutic Effect of Nanosilver on Burned Skin. J. Nanosci. Nanotechnol. 2020, 20, 7730–7734. [Google Scholar] [CrossRef]
- Neema, S.; Chatterjee, M. Nano-Silver Dressing in Toxic Epidermal Necrolysis. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 121–124. [Google Scholar] [CrossRef]
- Abedini, R.; Mahmoudi, H.; Kordestani, S.; Habib, F.N.; Abyaneh, M.; Rahemi, H. Comparison of Topical Nanocolloidal Silver Formulation Use with Eosin 2% Solution in Management of Hard-to-Heal Ulcers in Patients with Pemphigus Vulgaris. J. Wound Care 2020, 29, 664–668. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver Nanoparticle Impregnated Chitosan-PEG Hydrogel Enhances Wound Healing in Diabetes Induced Rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of Keratin Biomaterial Containing Silver Nanoparticles as a Potential Wound Dressing in Full-Thickness Skin Wound Model in Diabetic Mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef]
- Hill, W.R.; Pillsbury, P.D. Argyria: The Pharmacology of Silver. Arch Derm Syphilol. 1940, 41, 995–996. [Google Scholar] [CrossRef]
- Li, W.T.; Chang, H.W.; Yang, W.C.; Lo, C.; Wang, L.Y.; Pang, V.F.; Chen, M.H.; Jeng, C.R. Immunotoxicity of Silver Nanoparticles (AgNPs) on the Leukocytes of Common Bottlenose Dolphins (Tursiops Truncatus). Sci. Rep. 2018, 8, 5593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wypij, M.; Jedrzejewski, T.; Ostrowski, M.; Trzcinska, J.; Rai, M.; Golinska, P. Biogenic Silver Nanoparticles: Assessment of Their Cytotoxicity, Genotoxicity and Study of Capping Proteins. Molecules 2020, 25, 3022. [Google Scholar] [CrossRef] [PubMed]
- Karimi, H.; Latifi, N.A.; Mehrjerdi, A.Z.; Jafarnejad, B.; Karimi, A.M. Histopathological Changes of Organs (Lungs, Liver, Kidney, and Brain) after Using Two Types of Agicoat and Acticoat Nanosilver Dressings on Deep Second-Degree Burn in Rat. J. Burn. Care Res. 2020, 41, 141–150. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of Silver Nanoparticles in Biological Systems: Does the Complexity of Biological Systems Matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Du, Y.; Wang, Z.; Wang, H.; Pu, F.; Ren, J.; Qu, X. Hyaluronic Acid-Templated Ag Nanoparticles/Graphene Oxide Composites for Synergistic Therapy of Bacteria Infection. ACS Appl. Mater. Interfaces 2017, 9, 19717–19724. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lu, F.; Zou, Y.; Liu, J.; Rong, B.; Li, Z.; Dai, F.; Wu, D.; Lan, G. In Situ Reduction of Silver Nanoparticles by Chitosan-l-Glutamic Acid/Hyaluronic Acid: Enhancing Antimicrobial and Wound-Healing Activity. Carbohydr. Polym. 2017, 173, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; Docking, R.; Schofield, P.; Boateng, J. Advanced Multi-Targeted Composite Biomaterial Dressing for Pain and Infection Control in Chronic Leg Ulcers. Carbohydr. Polym. 2017, 172, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Jancar, J.; Abdel-Rahman, R.M.; Vojtek, L.; Hyršl, P.; Dušková, M.; Nejezchlebová, H. A Novel in Situ Silver/Hyaluronan Bio-Nanocomposite Fabrics for Wound and Chronic Ulcer Dressing: In Vitro and in Vivo Evaluations. Int. J. Pharm. 2017, 520, 241–253. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Hsu, I.-L.; Cheng, T.-Y.; Wu, W.-J.; Lee, C.-W.; Li, T.-J.; In Cheung, C.; Chin, Y.-C.; Chen, H.-C.; Chiu, Y.-C.; et al. Off-Resonance SERS Nanoprobe-Targeted Screen of Biomarkers for Antigens Recognition of Bladder Normal and Aggressive Cancer Cells. Anal. Chem. 2019, 91, 8213–8220. [Google Scholar] [CrossRef]
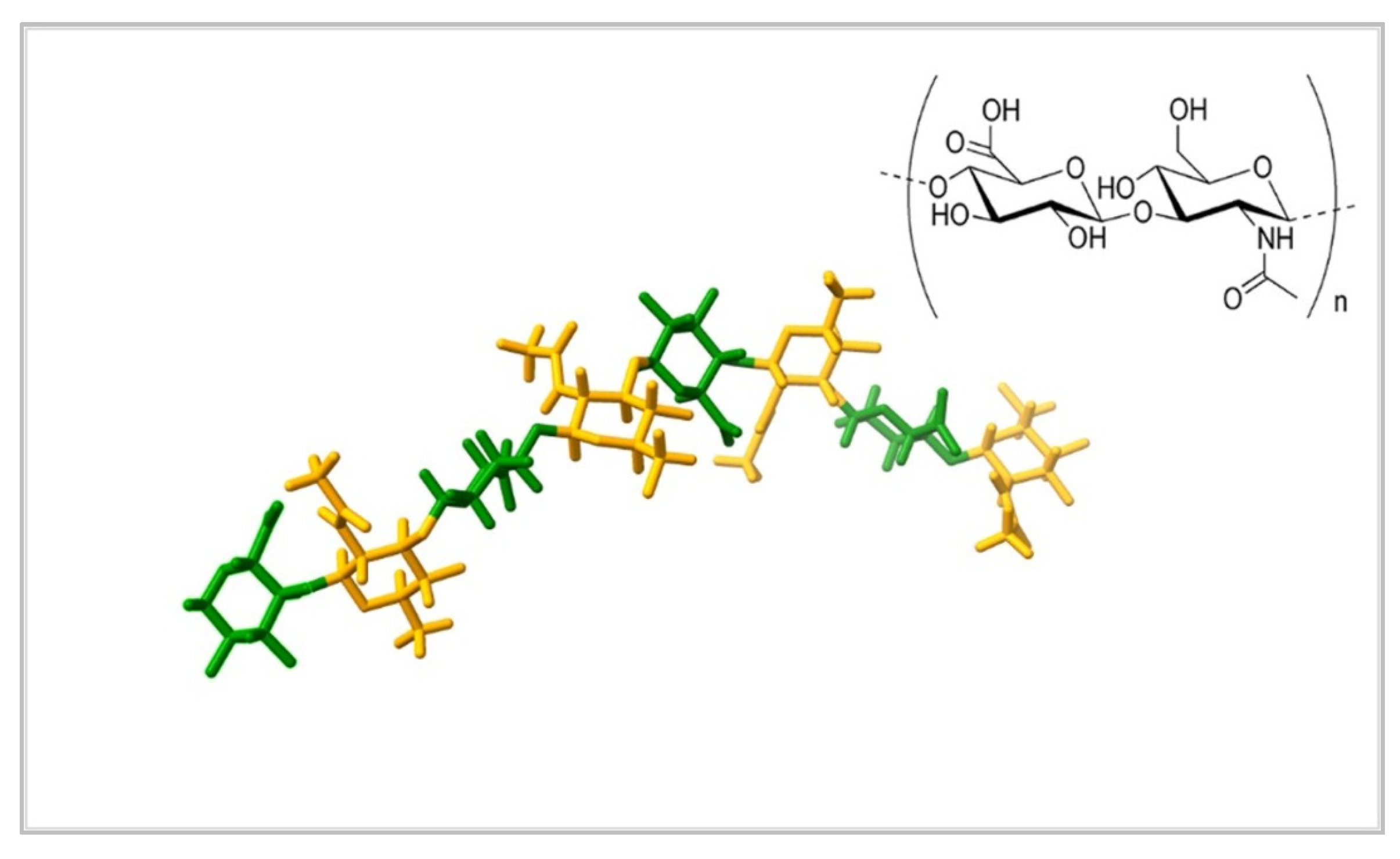



| Nanocomposite | Properties | Potential Applications | References |
|---|---|---|---|
| GO-HA-AgNPs | antibacterial against S. aureus, low toxicity to mammalian cells | diagnosis and treatment of bacterial infections | [97] |
| HA-Ag (nanofibers, nanosheets) | good mechanical properties, bactericidal against E. coli K12, nontoxic to human keratinocyte cells HaCaT; positive effect on chronic ulcerations | wound and chronic ulcer treatment | [101] |
| Chitosan/L-GA/HA/AgNPs (sponges) | good mechanical properties, swelling, water retention capacity, inhibition of S. aureus and E. coli; nontoxic to L929 fibroblast cells at low concentrations, promotion of wound healing | antibacterial wound dressings | [98] |
| CARR, HA, LID, AgNPs freeze-dried wafers | good porosity, bactericidal against E. coli, P. aeruginosa and S. aureus, nontoxic to human keratinocyte cells, fast release of lidocaine | treatment of pain in chronic leg ulcers | [99] |
| Ti-PTL-HA-CS/Ag | protection against planktonic and fixed forms of S. aureus, concentration-dependent silver cytotoxicity on MC3T3 mice osteoblasts | medical devices—catheters, wound dressings, bone cement | [100] |
| Hyal/Ag, Hyal-L/Ag (foils) | elasticity, water solubility, bactericidal against S. aureus, S. epidermidis and E. coli | prophylaxis and therapy | [26] |
| (Ag-PGA/HA) -PVA nanofibers | effective against gram-positive bacterial strains; Bacillus subtilis and S. aureus, as well as Gram-negative bacterial strain; E. coli | quick healing of wound infection | [29] |
| CSNMs with a PEG/PCL/Ag shell (PPA) and HA core | provides lubrication effect and reduced fibroblast attachment, bactericidal and anti-inflammatory properties, | management of post-surgical tendon adhesion | [30] |
| AgNPs-HA coated HA-UF monolith | satisfactory extraction efficiency towards unsaturated compounds | microextraction of monounsaturated fatty acid methyl esters | [25] |
| CH-PUL-HA/CS with AgNPs | good propensity to promote fibroblast proliferation, good biocompatibility, antimicrobial properties against S. aureus | treatment of chronic wounds (venous leg ulcers, diabetic foot, bed sores, burns and surgical lesions) | [24] |
| PGA-HA with AgNPs | antioxidative and anti-inflammatory, antibacterial against Gram + and Gram—bacteria, hydrophilicity, | quick healing of wound infections | [29] |
| Chi@Ag NPs/HA composite coating | bactericidal: sensitivity to bacteria secreting hyaluronidase for the controlled delivery of Ag ions, a good effect on the inhibition of bacterial growth | antibacterial surface with a controlled release of Ag ions | [20] |
| HA-Ag@S- nitrosothiols core-shell nanoparticles | “light-to-heat” transformation ability, first successful synergistic tumour targeting therapy performance | chemo- and photothermal synergistic tumor target therapy | [73] |
| Ce-MoF-HA-AgNPs-HRP | a broad linear response range, good reproducibility, selectivity, stability, without matrix effect | sensitive detection of carcinoembryonic antigen (CEA) | [74] |
| HA-MAgNPs | anticancer, fluorescence and photoactive properties | local treatment of cancer | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dulińska-Litewka, J.; Dykas, K.; Felkle, D.; Karnas, K.; Khachatryan, G.; Karewicz, A. Hyaluronic Acid-Silver Nanocomposites and Their Biomedical Applications: A Review. Materials 2022, 15, 234. https://doi.org/10.3390/ma15010234
Dulińska-Litewka J, Dykas K, Felkle D, Karnas K, Khachatryan G, Karewicz A. Hyaluronic Acid-Silver Nanocomposites and Their Biomedical Applications: A Review. Materials. 2022; 15(1):234. https://doi.org/10.3390/ma15010234
Chicago/Turabian StyleDulińska-Litewka, Joanna, Kacper Dykas, Dominik Felkle, Karolina Karnas, Gohar Khachatryan, and Anna Karewicz. 2022. "Hyaluronic Acid-Silver Nanocomposites and Their Biomedical Applications: A Review" Materials 15, no. 1: 234. https://doi.org/10.3390/ma15010234








