Hybrid RF-Si Xerogels: A Cost-Effective Proposal for Insulator Materials
Abstract
1. Introduction
2. Experimental
2.1. Reagents
2.2. Synthesis of Hybrid Xerogels
- (a)
- Convection heating. The synthesis process was carried out in an electric oven for 72 h in a closed vessel to promote the gelation and aging step.
- (b)
- Microwave heating. The samples were under MW heating for 5 h. Initially, the reaction container was maintained closed for 3 h to promote the gelation and aging step. Then, the container was opened and kept under MW heating for 2 h to initiate the drying stage.
2.3. Textural and Chemical Characterization
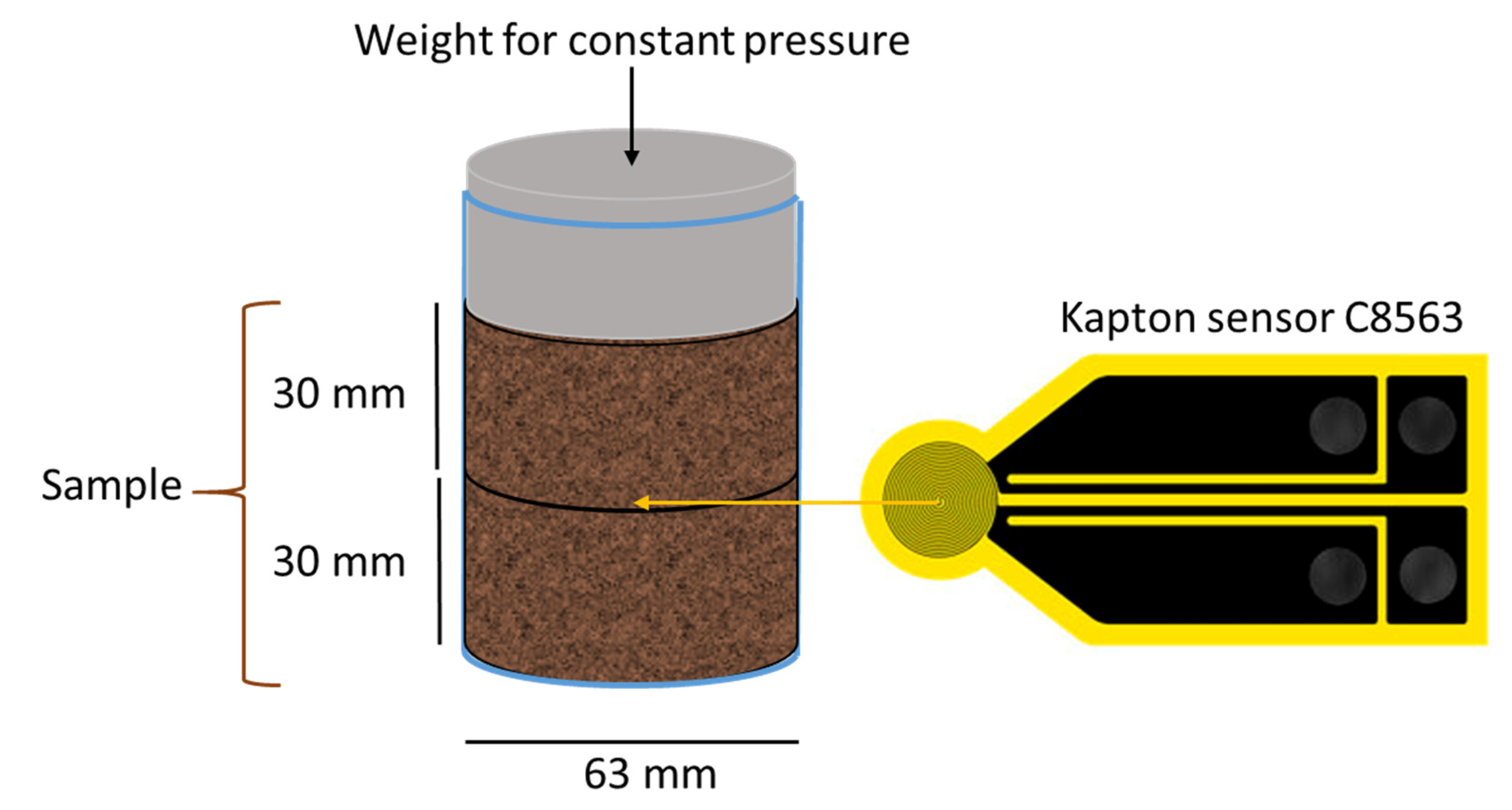
2.4. Thermal Conductivity Analysis
3. Results and Discussion
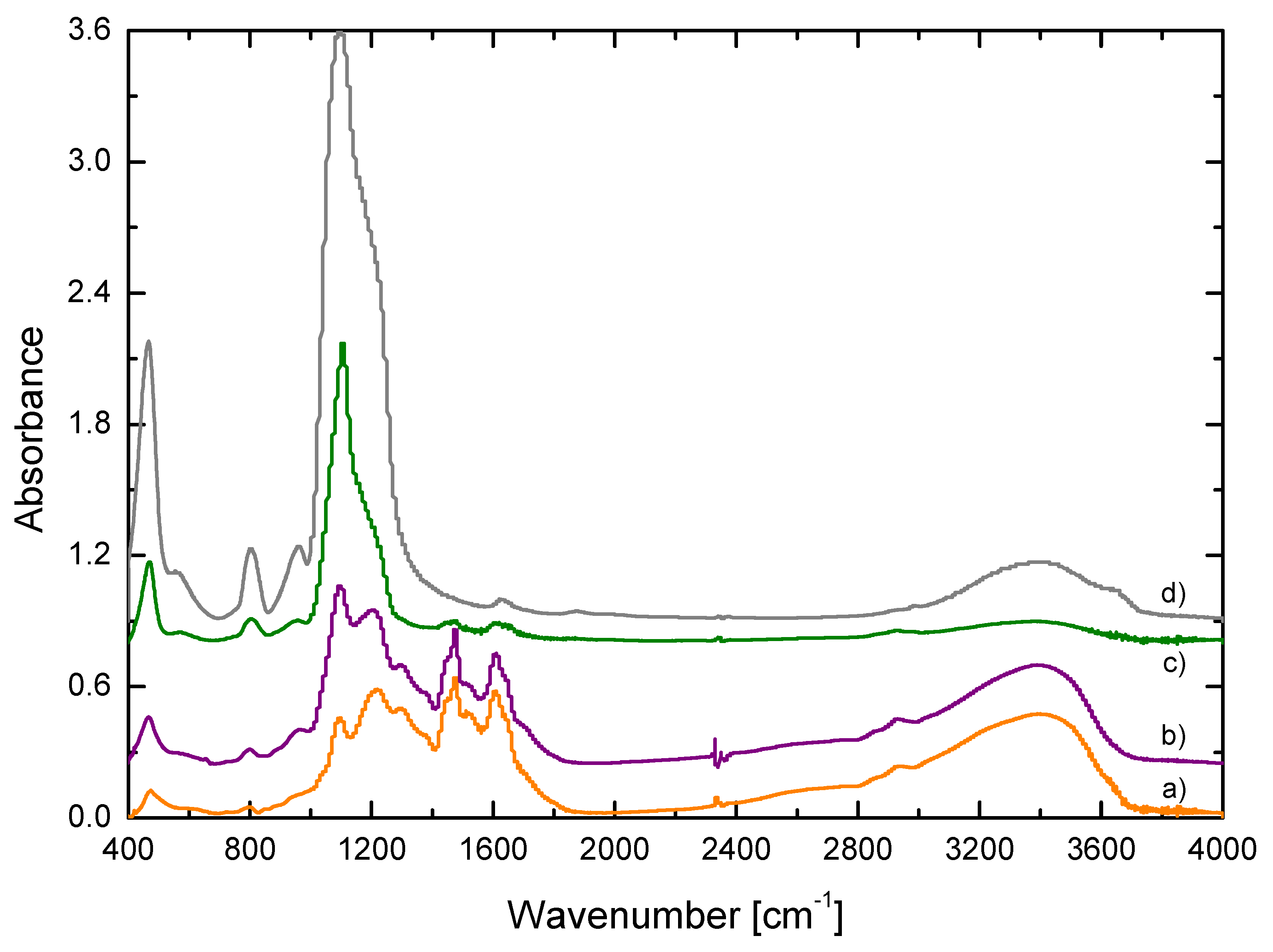
3.1. Chemical Composition Contribution
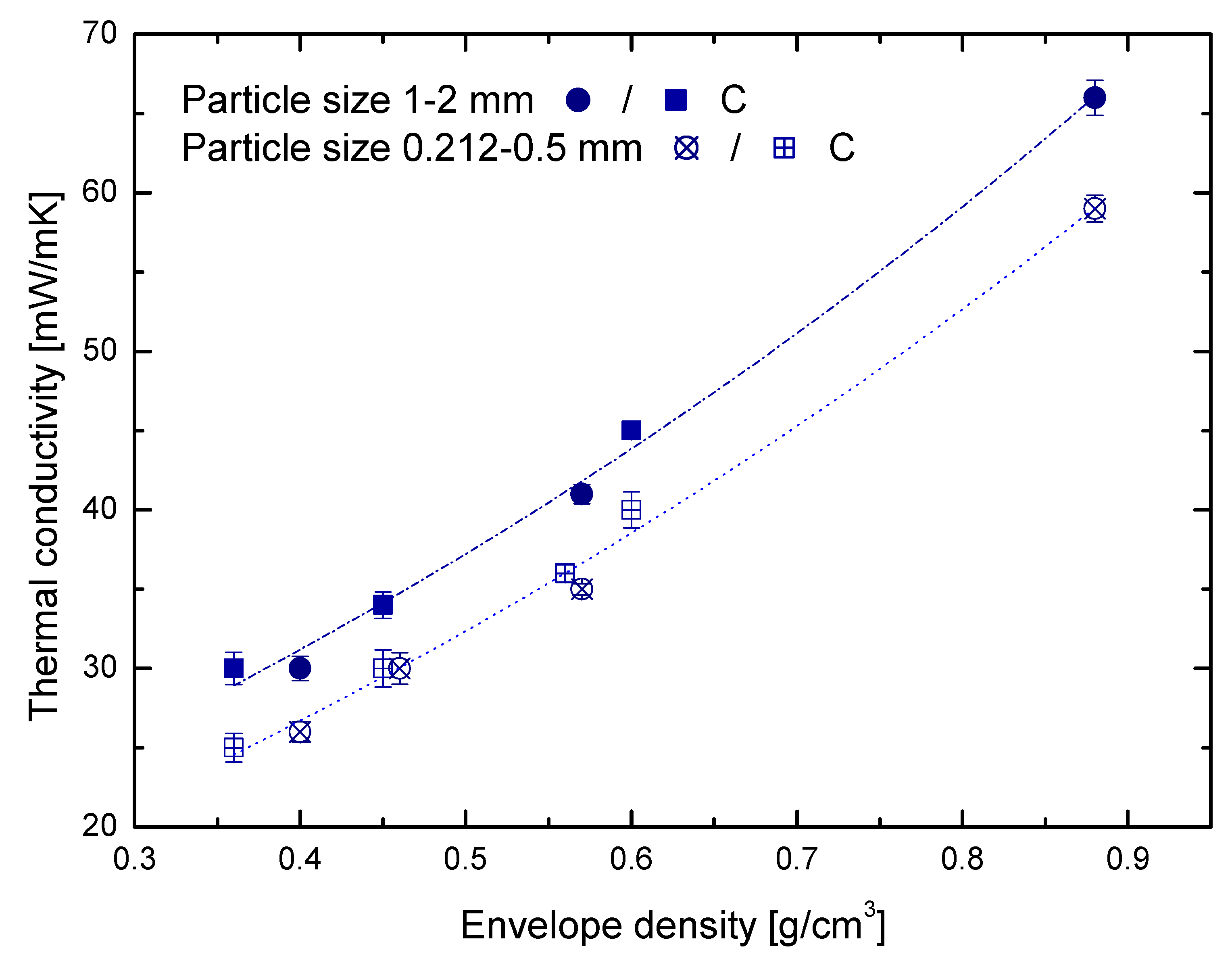
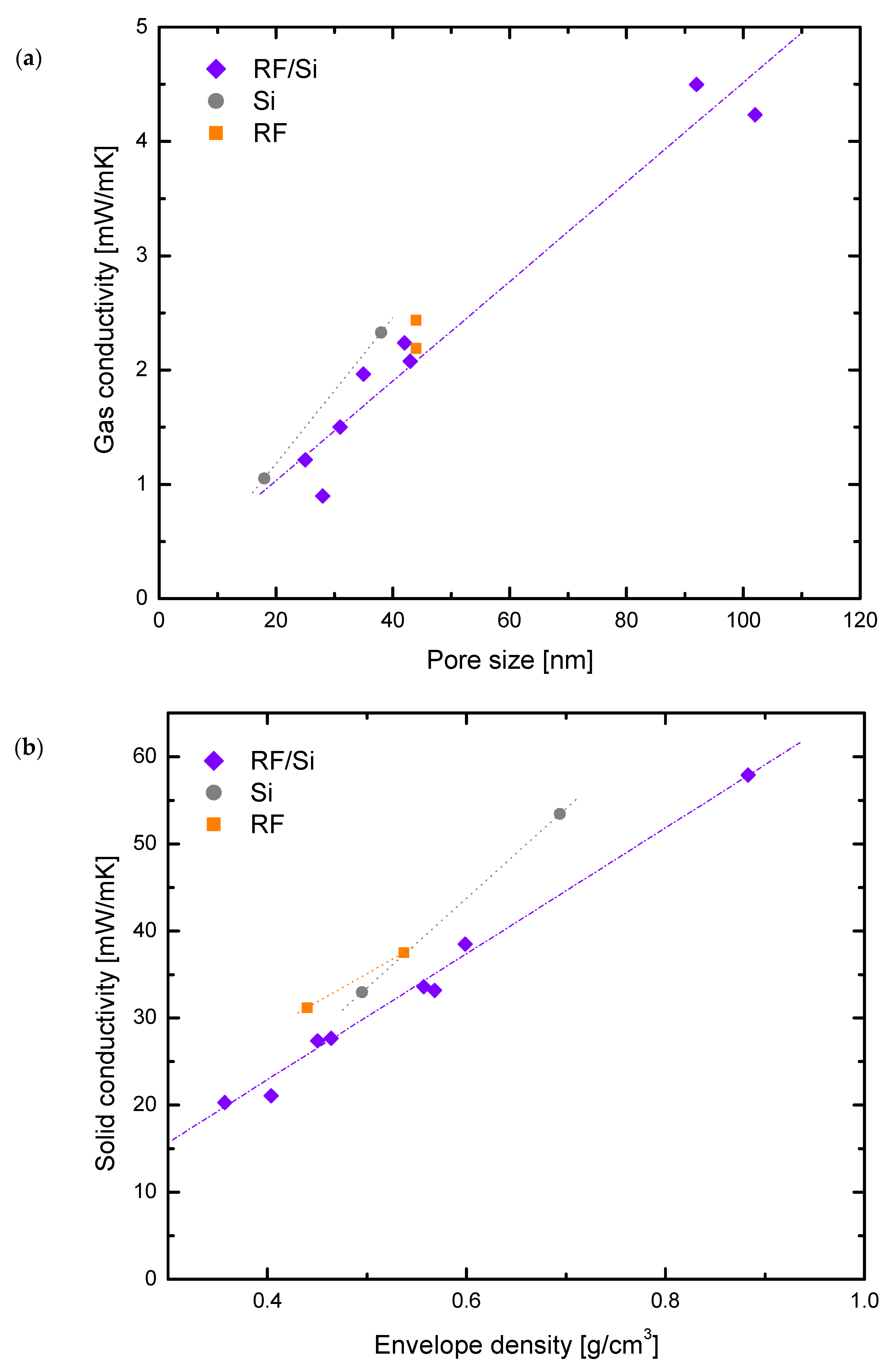
3.2. Porous Matrix Contribution
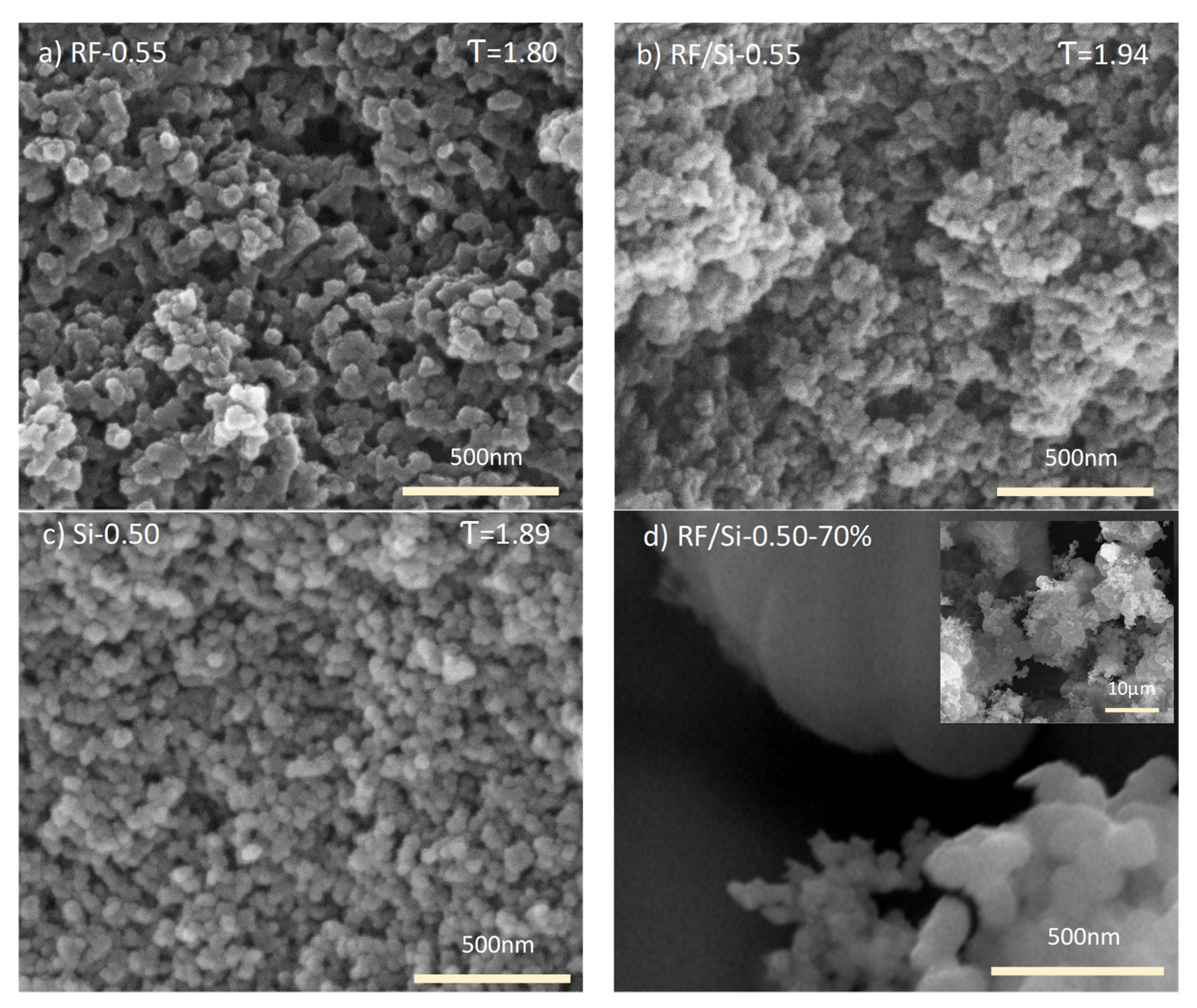
3.3. Solid Matrix Contribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thapliyal, P.C.; Singh, K. Aerogels as Promising Thermal Insulating Materials: An Overview. J. Mater. 2014, 2014, 127049. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review, Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef]
- Zou, F.; Budtova, T. Polysaccharide-based aerogels for thermal insulation and superinsulation: An overview. Carbohydr. Polym. 2021, 266, 118130. [Google Scholar] [CrossRef]
- Boeri, A.; Antonini, E.; Gaspari, J.; Longo, D. Energy Design Strategies for Retrofitting: Methodology, Technologies and Applications; WIT Press: Southampton Boston, MA, USA, 2015. [Google Scholar]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.-E.; Fan, W.; Liu, T. Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications. Materials 2015, 8, 6806–6848. [Google Scholar] [CrossRef]
- Lu, X.; Arduini-Schuster, M.C.; Kuhn, J.; Nilsson, O.; Fricke, J.; Pekala, R.W. Thermal Conductivity of Monolithic Organic Aerogels. Science 1992, 255, 971–972. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Pekala, R.W. Thermal properties of organic and inorganic aerogels. J. Mater. Res. 1994, 9, 731–738. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Yang, H.; Zhang, H. Tailoring thermal properties of ambient pressure dried MTMS/TEOS co-precursor aerogels. Mater. Lett. 2016, 171, 91–94. [Google Scholar] [CrossRef]
- Niu, D.; Gao, H. Thermal Conductivity of Ordered Porous Structures Coupling Gas and Solid Phases: A Molecular Dynamics Study. Materials 2021, 14, 2221. [Google Scholar] [CrossRef]
- Gutzov, S.; Danchova, N.; Karakashev, S.I.; Khristov, M.; Ivanova, J.; Ulbikas, J. Preparation and thermal properties of chemically prepared nanoporous silica aerogels. J. Sol-Gel Sci. Technol. 2014, 70, 511–516. [Google Scholar] [CrossRef]
- Meliță, L.; Croitoru, C.V. Aerogel, a high performance material for thermal insulation-A brief overview of the building applications. E3S Web Conf. 2019, 111, 06069. [Google Scholar] [CrossRef]
- Berkefeld, A.; Heyer, M.; Milow, B. Silica aerogel paper honeycomb composites for thermal insulations. J. Sol-Gel Sci. Technol. 2017, 84, 486–495. [Google Scholar] [CrossRef]
- Shang, L.; Lyu, Y.; Han, W. Microstructure and Thermal Insulation Property of Silica Composite Aerogel. Materials 2019, 12, 993. [Google Scholar] [CrossRef]
- Rezaei, S.; Zolali, A.M.; Jalali, A.; Park, C.B. Novel and simple design of nanostructured, super-insulative and flexible hybrid silica aerogel with a new macromolecular polyether-based precursor. J. Colloid Interface Sci. 2020, 561, 890–901. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, L.; Shen, Q.; Li, X.; Wu, T.; Zhang, J.; Nie, C. Rapid synthesis of dual-mesoporous silica aerogel with excellent adsorption capacity and ultra-low thermal conductivity. J. Non-Cryst. Solids. 2021, 555, 120547. [Google Scholar] [CrossRef]
- Du, D.; Liu, F.; Jiang, Y.; Feng, J.; Li, L.; Feng, J. Facile Preparation of High Strength Silica Aerogel Composites via a Water Solvent System and Ambient Pressure Drying without Surface Modification or Solvent Replacement. Materials 2021, 14, 3983. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Calvo, E.G.; Menéndez, J.A.; Arenillas, A. Exploring the potential of resorcinol-formaldehyde xerogels as thermal insulators. Microporous Mesoporous Mater. 2017, 244, 50–54. [Google Scholar] [CrossRef]
- Brilliantova, I.S.; Lebedev, I.V.; Gordienko, M.G.; Menshutina, N.V. Synthesis and Analysis of Silica–Resorcinol–Formaldehyde Aerogels. Russ. J. Phys. Chem. B. 2019, 13, 1174–1181. [Google Scholar] [CrossRef]
- Berthon-Fabry, S.; Hildenbrand, C.; Ilbizian, P. Lightweight superinsulating Resorcinol-Formaldehyde-APTES benzoxazine aerogel blankets for space applications. Eur. Polym. J. 2016, 78, 25–37. [Google Scholar] [CrossRef]
- Jaxel, J.; Markevicius, G.; Rigacci, A.; Budtova, T. Thermal superinsulating silica aerogels reinforced with short man-made cellulose fibers. Compos. Part A Appl. Sci. Manuf. 2017, 103, 113–121. [Google Scholar] [CrossRef]
- Yun, S.; Luo, H.; Gao, Y. Ambient-pressure drying synthesis of large resorcinol–formaldehyde-reinforced silica aerogels with enhanced mechanical strength and superhydrophobicity. J. Mater. Chem. A 2014, 2, 14542–14549. [Google Scholar] [CrossRef]
- Kong, Y.; Zhong, Y.; Shen, X.; Cui, S.; Fan, M. Effect of silica sources on nanostructures of resorcinol–formaldehyde/silica and carbon/silicon carbide composite aerogels. Microporous Mesoporous Mater. 2014, 197, 77–82. [Google Scholar] [CrossRef]
- Flores-López, S.L.; Montes-Morán, M.A.; Arenillas, A. Carbon/silica hybrid aerogels with controlled porosity by a quick one-pot synthesis. J. Non-Cryst. Solids. 2021, 569, 120992. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Arenillas, A.; Menéndez, J.A. A visual validation of the combined effect of pH and dilution on the porosity of carbon xerogels. Microporous Mesoporous Mater. 2016, 223, 89–93. [Google Scholar] [CrossRef][Green Version]
- Rey-Raap, N.; Menéndez, J.A.; Arenillas, A. RF xerogels with tailored porosity over the entire nanoscale. Microporous Mesoporous Mater. 2014, 195, 266–275. [Google Scholar] [CrossRef]
- Flores-López, S.L.; Villanueva, S.F.; Montes-Morán, M.A.; Cruz, G.; Garrido, J.J.; Arenillas, A. Advantages of microwave-assisted synthesis of silica gels. Colloids Surf. Physicochem. Eng. Asp. 2020, 604, 125248. [Google Scholar] [CrossRef]
- Musgo, J.; Echeverría, J.C.; Estella, J.; Laguna, M.; Garrido, J.J. Ammonia-catalyzed silica xerogels: Simultaneous effects of pH, synthesis temperature, and ethanol:TEOS and water:TEOS molar ratios on textural and structural properties. Microporous Mesoporous Mater. 2009, 118, 280–287. [Google Scholar] [CrossRef]
- Calvo, E.G.; Juárez-Pérez, E.J.; Menéndez, J.A.; Arenillas, A. Fast microwave-assisted synthesis of tailored mesoporous carbon xerogels. J. Colloid Interface Sci. 2011, 357, 541–547. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Menéndez, J.A.; Arenillas, A. Optimization of the process variables in the microwave-induced synthesis of carbon xerogels. J. Sol-Gel Sci. Technol. 2013, 69, 488–497. [Google Scholar] [CrossRef]
- Flores-López, S.L.; Ramírez-Montoya, L.A.; Casal, M.D.; Montes-Morán, M.A.; Menéndez, J.A.; Arenillas, A. Tortuosity of the porous structure of carbon gels. Carbon 2021, 171, 921–930. [Google Scholar] [CrossRef]
- Alshrah, M.; Mark, L.H.; Zhao, C.; Naguib, H.E.; Park, C.B. Nanostructure to thermal property relationship of resorcinol formaldehyde aerogels using the fractal technique. Nanoscale 2018, 10, 10564–10575. [Google Scholar] [CrossRef]
- Alonso-Buenaposada, I.D.; Rey-Raap, N.; Calvo, E.G.; Menéndez, J.A.; Arenillas, A. Acid-based resorcinol-formaldehyde xerogels synthesized by microwave heating. J. Sol-Gel Sci. Technol. 2017, 84, 60–69. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, H.; Li, Y.; Xie, S.; Li, B. Sintered metal fibers@carbon molecular sieve membrane (SMFs@CMSM) composites for the adsorptive removal of low concentration isopropanol. RSC Adv. 2017, 7, 37604–37611. [Google Scholar] [CrossRef]
- Maazoun, A.; Mezghani, A.; Moussa, A.B. Numerical Study of Thermal Properties of a Silica Aerogel Material. J. Heat Transf. 2021, 143, 034502. [Google Scholar] [CrossRef]
- Hu, B.; Wang, J.; Ma, Z.; Sang, S. Permeability and thermal conductivity models of shale matrix with a bundle of tortuous fractal tree-like branching micropore networks. Int. J. Therm. Sci. 2021, 164, 106876. [Google Scholar] [CrossRef]
- Kou, J.; Wu, F.; Lu, H.; Xu, Y.; Song, F. The effective thermal conductivity of porous media based on statistical self-similarity. Phys. Lett. A 2009, 374, 62–65. [Google Scholar] [CrossRef]


| Reference | Inputs | Results * | |||
|---|---|---|---|---|---|
| Power Heat | Analysis Time | Thermal Conductivity | Thermal Diffusivity | Specific Heat | |
| W | S | mW/mK | mm2/s | MJ/m3 K | |
| Foamglass® | 0.02 | 160 | 42.7 (42) | 0.43 (0.42) | 0.10 (0.10) |
| CASSPIR® | 0.02 | 160 | 22.2 (22) | 0.28 | 0.08 |
| Lumira® | 0.01 | 640 | 19.9 (21) | 0.12 (0.12) | 0.17 (0.18 a) |
| Sample | ρHe | ρenv | P | VT | dp | SBET |
|---|---|---|---|---|---|---|
| (g/cm3) | (g/cm3) | % | (cm3/g) | (nm) | (m2/g) | |
| Si-0.50 | 2.05 | 0.50 | 76 | 1.54 | 38 | 568 |
| Si-0.70 | 2.08 | 0.69 | 67 | 0.98 | 18 | 543 |
| RF-0.45 | 1.42 | 0.44 | 69 | 1.55 | 44 | 107 |
| RF-0.55 | 1.43 | 0.54 | 62 | 1.16 | 44 | 110 |
| RF/Si-0.35-C | 1.46 | 0.36 | 76 | 2.11 | 102 | 76 |
| RF/Si-0.40 | 1.43 | 0.40 | 72 | 1.91 | 92 | 83 |
| RF/Si-0.45-C | 1.47 | 0.45 | 69 | 1.59 | 42 | 216 |
| RF/Si-0.45 | 1.46 | 0.46 | 68 | 1.35 | 35 | 128 |
| RF/Si-0.55-C | 1.41 | 0.56 | 60 | 1.12 | 43 | 110 |
| RF/Si-0.55 | 1.45 | 0.57 | 61 | 1.14 | 31 | 111 |
| RF/Si-0.60-C | 1.47 | 0.60 | 59 | 1.01 | 25 | 229 |
| RF/Si-0.85 | 1.56 | 0.88 | 43 | 0.48 | 28 | 75 |
| RF/Si-0.50–70% | 1.68 | 0.48 | 71 | 1.48 | 5395 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-López, S.L.; Villanueva, S.F.; Rey-Raap, N.; Arenillas, A. Hybrid RF-Si Xerogels: A Cost-Effective Proposal for Insulator Materials. Materials 2022, 15, 265. https://doi.org/10.3390/ma15010265
Flores-López SL, Villanueva SF, Rey-Raap N, Arenillas A. Hybrid RF-Si Xerogels: A Cost-Effective Proposal for Insulator Materials. Materials. 2022; 15(1):265. https://doi.org/10.3390/ma15010265
Chicago/Turabian StyleFlores-López, Samantha L., Sara F. Villanueva, Natalia Rey-Raap, and Ana Arenillas. 2022. "Hybrid RF-Si Xerogels: A Cost-Effective Proposal for Insulator Materials" Materials 15, no. 1: 265. https://doi.org/10.3390/ma15010265
APA StyleFlores-López, S. L., Villanueva, S. F., Rey-Raap, N., & Arenillas, A. (2022). Hybrid RF-Si Xerogels: A Cost-Effective Proposal for Insulator Materials. Materials, 15(1), 265. https://doi.org/10.3390/ma15010265







