Mechanical and Durability Properties of Cementless Concretes Made Using Three Types of CaO-Activated GGBFS Binders
Abstract
:1. Introduction
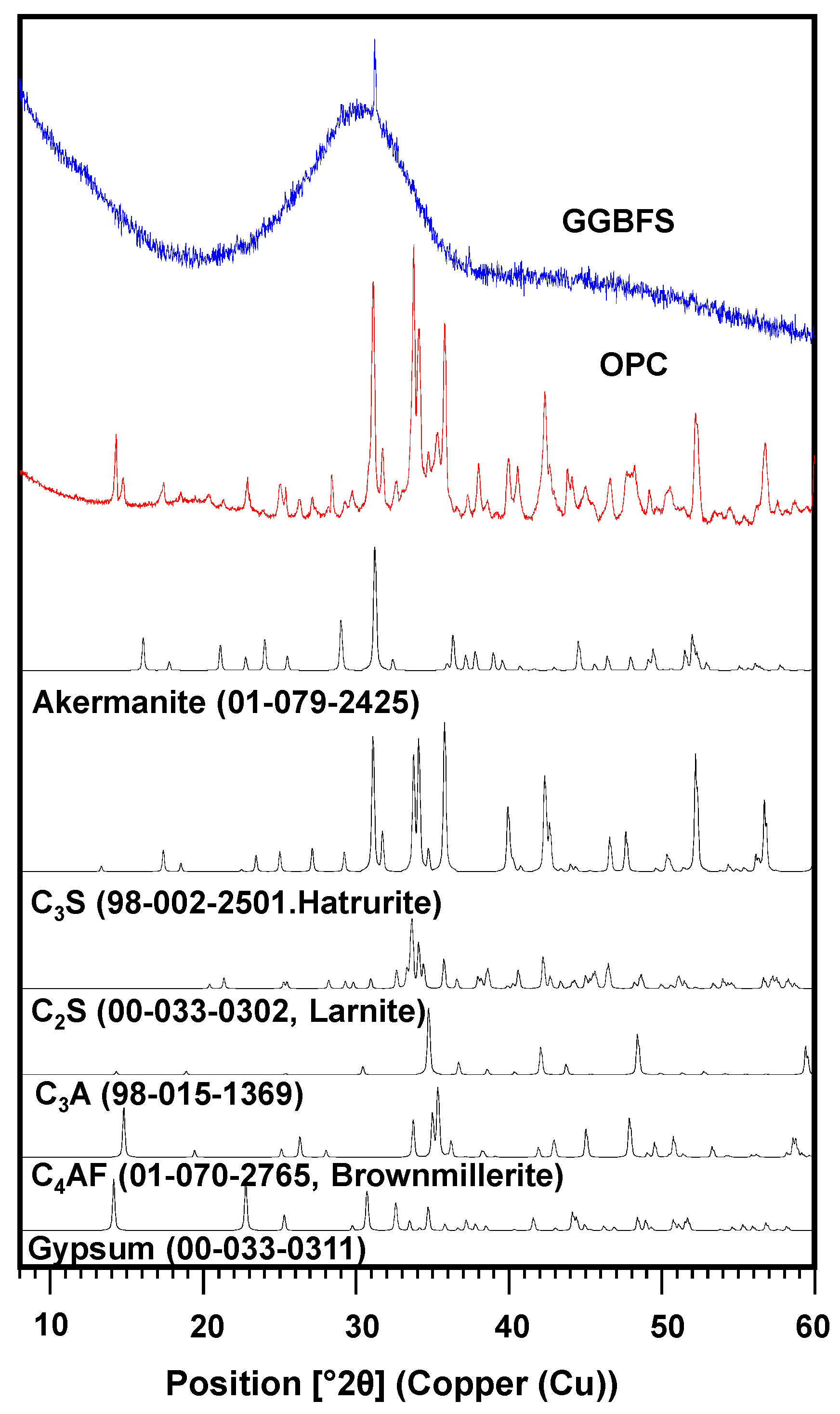
2. Materials and Methods
3. Results
3.1. Slump and Air Content
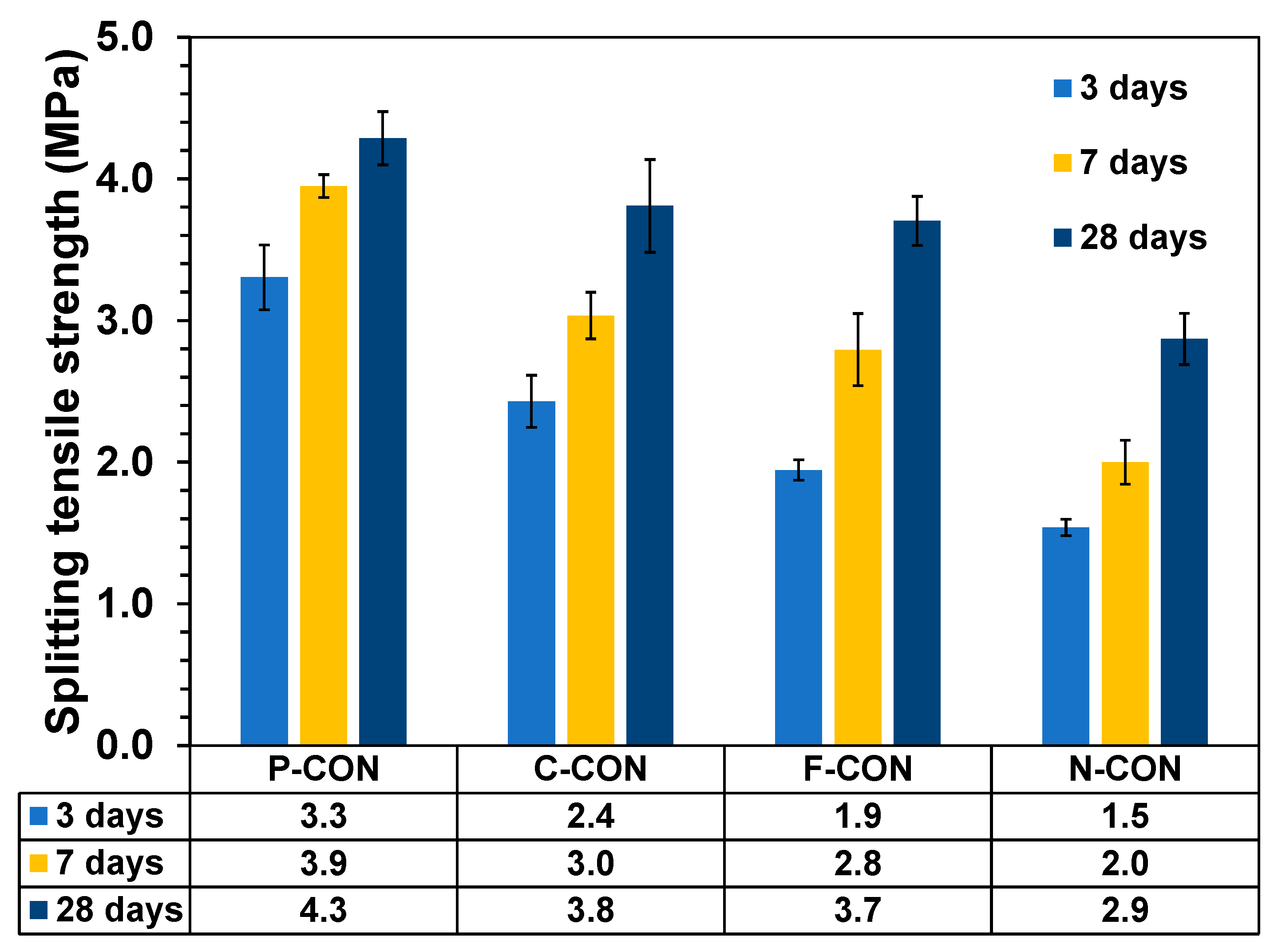
3.2. Compressive and Split Strength
3.3. Chord Elastic Modulus
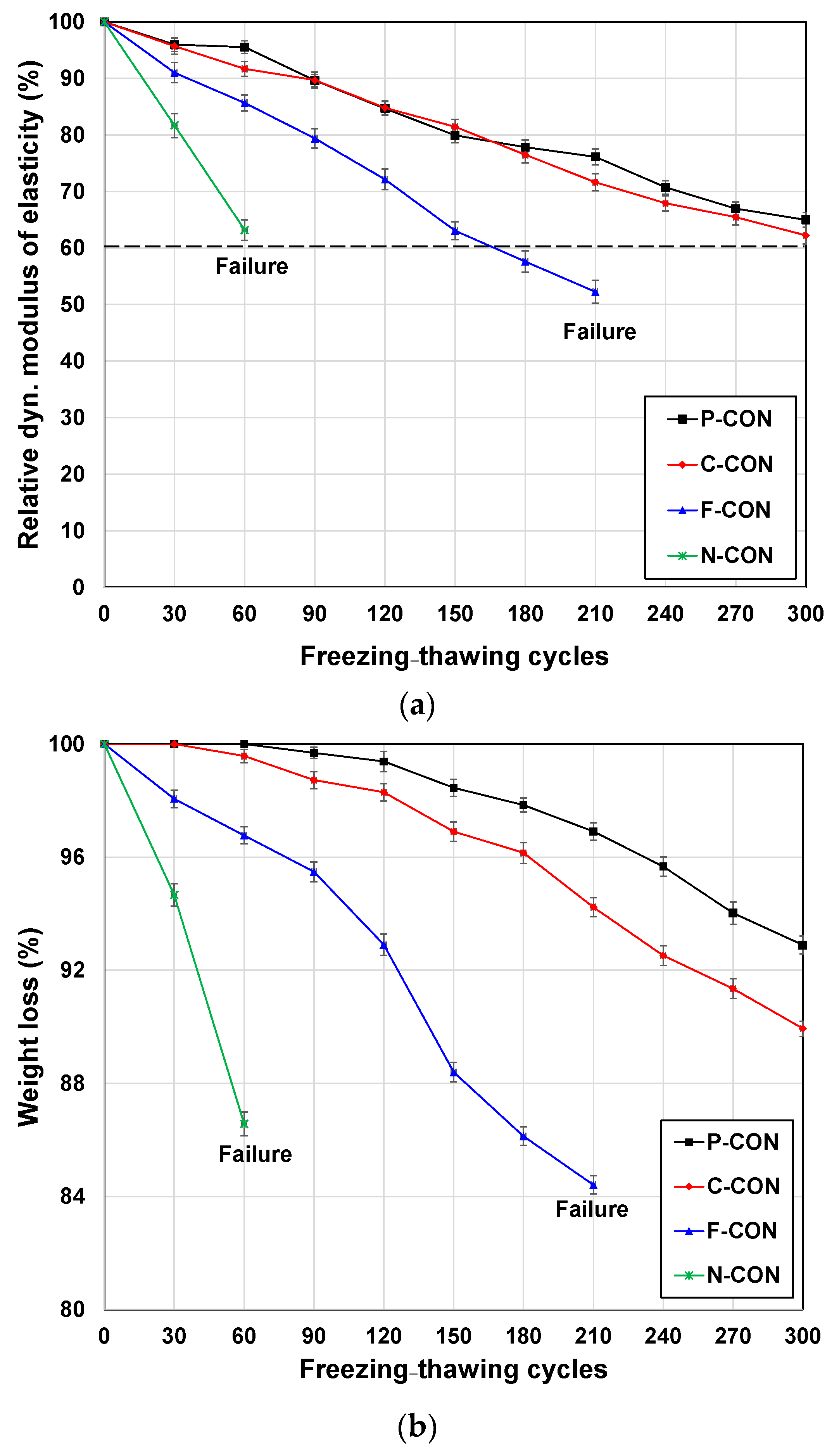
3.4. Freezing and Thawing Cycle Tests
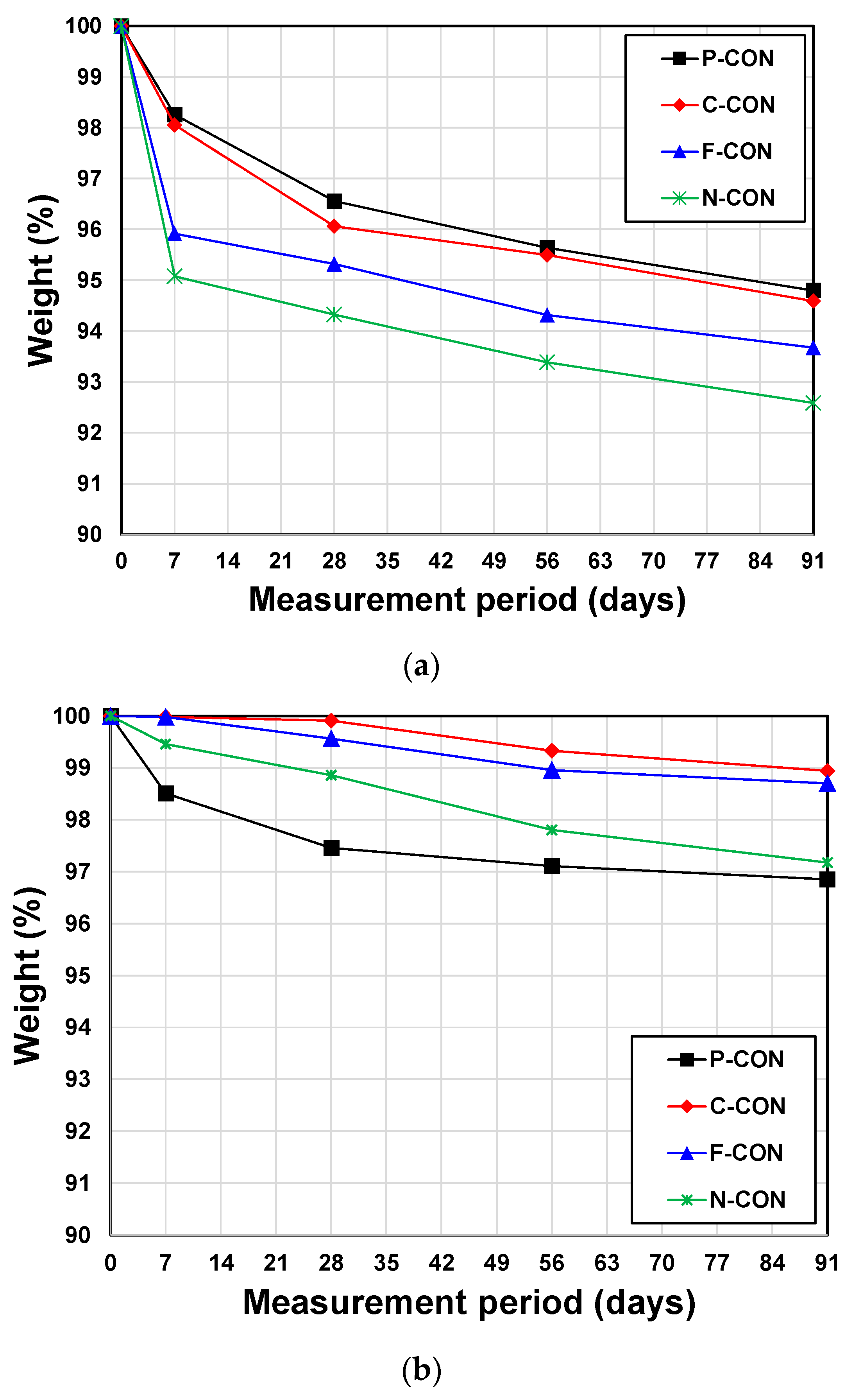
3.5. Chemical Resistance Tests (5% HCl and 5% H2SO4)
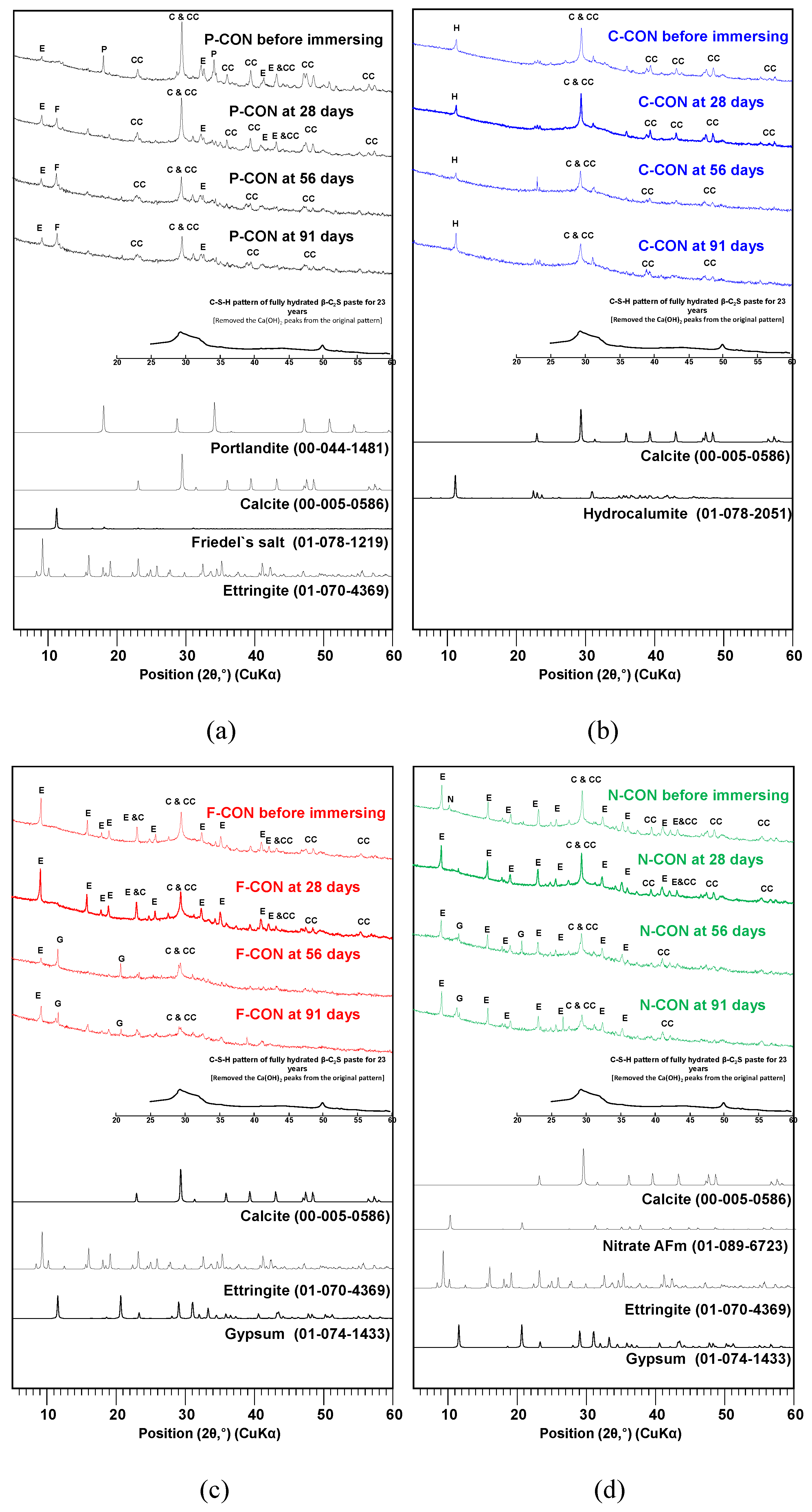
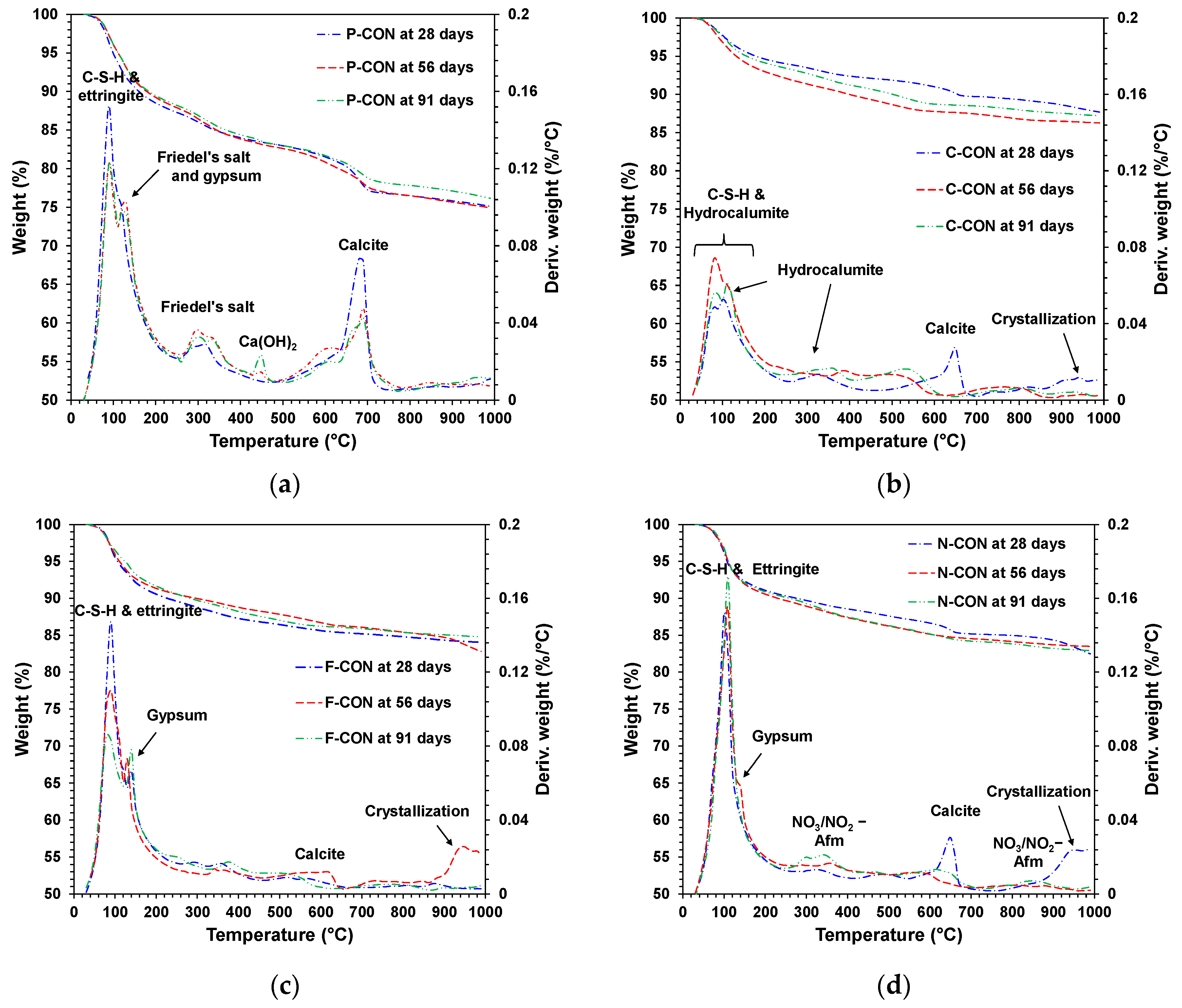
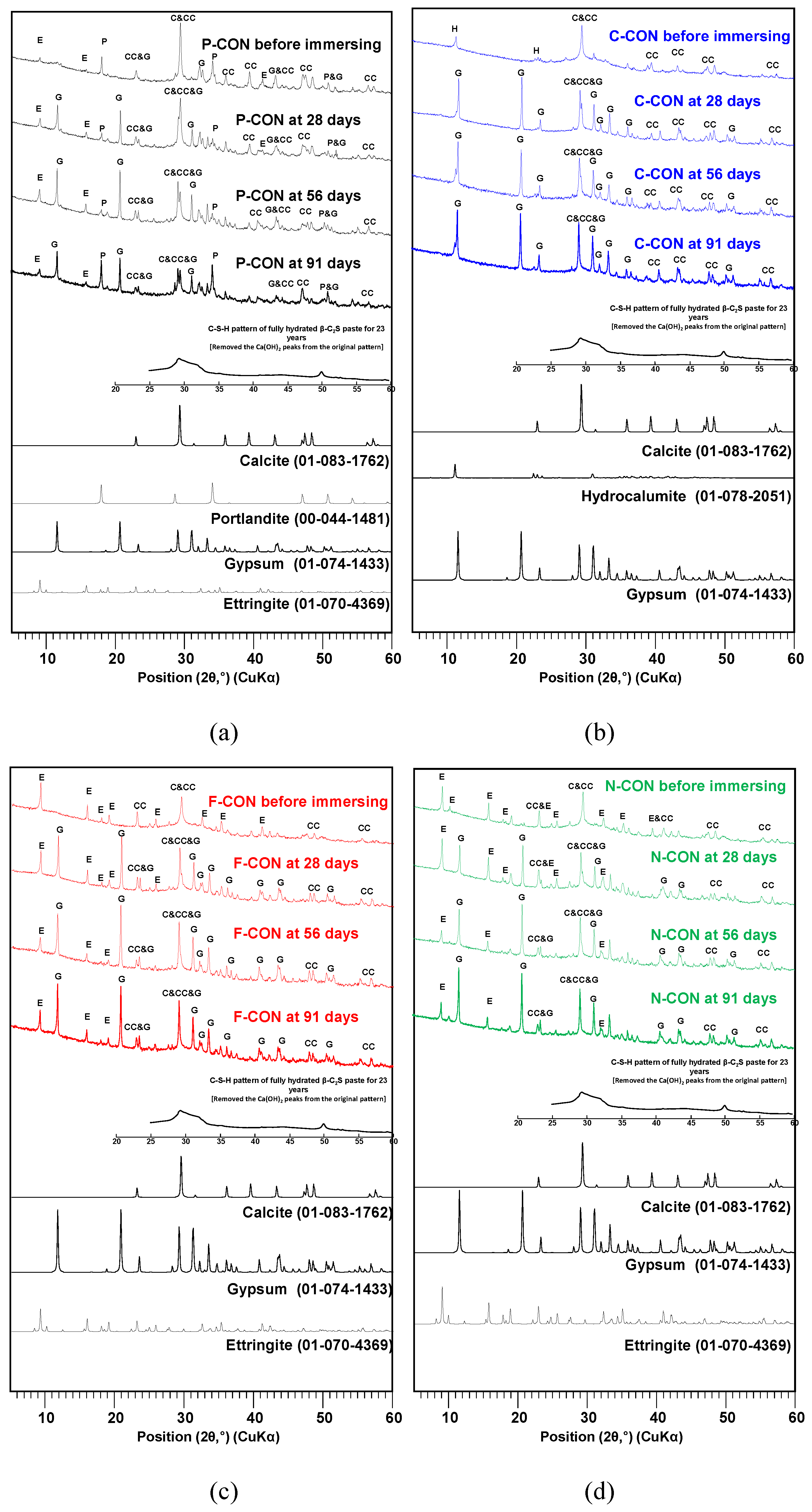
XRD and TG/DTG Analysis in Chemical Resistance Tests
4. Conclusions
- All CaO-activated GGBFS concretes satisfied the targeted air content and slump ranges. However, they exhibited lower mechanical properties (compressive strength, split strength, and elastic modulus) than OPC concrete.
- In the CaO-activated GGBFS system, the additive CaCl2 was the most effective at increasing strength and elastic modulus, while the additive Ca(NO3)2 was the least effective.
- In the freezing and thawing cycle test, the CaO-activated GGBFS concretes with added Ca(HCOO)2 and Ca(NO3)2 were destroyed before 300 cycles; however, the one made with CaCl2 had a relatively comparable resistance in the freezing and thawing cycles test to OPC concrete.
- The strength was a less accurate indicator than the dynamic modulus for estimating the ability to resist freezing and thawing cycles in this study.
- The chemical resistance tests using 5% HCl and 5% H2SO4 solutions showed significantly different results depending on the type of acid solutions. In a 5% HCl solution, although weight loss was observed in all immersed samples, the OPC concrete had the most excellent resistance, while the CaO-activated GGBFS concrete with Ca(NO3)2 was the most vulnerable. However, in 5% H2SO4 aqueous, the most considerable weight loss occurred in the OPC concrete.
- In XRD, all concrete with added sulfate ion (SO42−) (i.e., Portland cement concrete and CaO-activated GGBFS concretes with Ca(HCOO)2 and Ca(NO3)2) had ettringite before immersion. When they were immersed in HCl solution, their DTG results showed that ettringite tended to decrease, and gypsum was generated; however, the CaO-activated GGBFS concrete with CaCl2 did not change the type of reaction product in the HCl solution, possibly due to the absence of ettringite and Ca(OH)2 before immersion.
- When immersed in H2SO4 solution, similar to the case of HCl, the DTG peaks of ettringite decreased, and the gypsum DTG peaks tended to increase. However, in the H2SO4 solution, gypsum was synthesized from the reaction between Ca(OH)2 and H2SO4 in all concrete samples. In particular, the concrete without ettringite (i.e., CaO-activated GGBFS concrete with CaCl2) had a considerable amount of gypsum formation; in this concrete, dissolved C-S-H and calcite, due to the low pH, likely produced Ca2+ ions, and gypsum formed from the reaction between Ca2+ and H2SO4.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerr, R.A. Global warming is changing the world. Science 2007, 316, 188–190. [Google Scholar] [CrossRef] [Green Version]
- Drake, F. Global Warming; Routledge: Abingdon, UK, 2014. [Google Scholar]
- Aïtcin, P.-C. Cements of yesterday and today: Concrete of tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Aydin, E. Novel coal bottom ash waste composites for sustainable construction. Constr. Build. Mater. 2016, 124, 582–588. [Google Scholar] [CrossRef]
- Poudyal, L.; Adhikari, K. Environmental sustainability in cement industry: An integrated approach for green and economical cement production. Resour. Environ. Sustain. 2021, 4, 100024. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Huynh, T.-P. Engineering, Evaluation of the performance and microstructure of ecofriendly construction bricks made with fly ash and residual rice husk ash. Adv. Mater. Sci. Eng. 2015, 2015, 891412. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.; Zain, M.F.M.; Jamil, M.; Lai, F. Fabrication of a non-cement binder using slag, palm oil fuel ash and rice husk ash with sodium hydroxide. Constr. Build. Mater. 2013, 49, 894–902. [Google Scholar] [CrossRef]
- Billong, N.; Melo, U.; Kamseu, E.; Kinuthia, J.; Njopwouo, D.J.C. Improving hydraulic properties of lime–rice husk ash (RHA) binders with metakaolin (MK). Constr. Build. Mater. 2011, 25, 2157–2161. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.; Blanco, M. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, X.; Vollpracht, A. Pervious concrete made of alkali activated slag and geopolymers. Constr. Build. Mater. 2018, 189, 797–803. [Google Scholar] [CrossRef]
- Ariffin, M.; Bhutta, M.; Hussin, M.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Yurt, Ü. High performance cementless composites from alkali activated GGBFS. Constr. Build. Mater. 2020, 264, 120222. [Google Scholar] [CrossRef]
- Rostami, M.; Behfarnia, K. The effect of silica fume on durability of alkali activated slag concrete. Constr. Build. Mater. 2017, 134, 262–268. [Google Scholar] [CrossRef]
- Messina; Ferone; Colangelo; Roviello; Cioffi. Alkali activated waste fly ash as sustainable composite: Influence of curing and pozzolanic admixtures on the early-age physico-mechanical properties and residual strength after exposure at elevated temperature. Compos. Part B Eng. 2018, 132, 161–169. [Google Scholar] [CrossRef]
- Van Deventer, J.S.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Yang, K.-H.; Song, J.-K.; Song, K.-I. Assessment of CO2 reduction of alkali-activated concrete. J. Clean. Prod. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.M.; Palomo, A.; Lopez-Hombrados, C. Engineering properties of alkali-activated fly ash concrete. ACI Mater. J. 2006, 103, 106. [Google Scholar]
- Atiş, C.D.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of carbonation on alkali-activated slag paste. J. Am. Ceram. Soc. 2006, 89, 3211–3221. [Google Scholar] [CrossRef]
- Yum, W.S.; Suh, J.-I.; Sim, S.; Yoon, S.; Jun, Y.; Oh, J.E. Influence of calcium and sodium nitrate on the strength and reaction products of the CaO-activated GGBFS system. Constr. Build. Mater. 2019, 215, 839–848. [Google Scholar] [CrossRef]
- Suh, J.-I.; Yum, W.S.; Jeong, Y.; Park, H.-G.; Oh, J.E. The cation-dependent effects of formate salt additives on the strength and microstructure of CaO-activated fly ash binders. Constr. Build. Mater. 2019, 194, 92–101. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Yum, W.S.; Jeong, Y.; Yoon, S.; Jeon, D.; Jun, Y.; Oh, J.E. Effects of CaCl2 on hydration and properties of lime (CaO)-activated slag/fly ash binder. Cem. Concr. Compos. 2017, 84, 111–123. [Google Scholar] [CrossRef]
- Yum, W.S.; Suh, J.-I.; Jeon, D.; Oh, J.E. Strength enhancement of CaO-activated slag system through addition of calcium formate as a new auxiliary activator. Cem. Concr. Compos. 2020, 109, 103572. [Google Scholar] [CrossRef]
- Jeon, D.; Yum, W.S.; Jeong, Y.; Oh, J.E. Properties of quicklime (CaO)-activated Class F fly ash with the use of CaCl2. Cem. Concr. Res. 2018, 111, 147–156. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete Microstructure, Properties and Materials; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Ministry of Land, I.T. Korea, Korea Concrete Specification; Ministry of Land: Daejeon, Korea, 2017. [Google Scholar]
- Korean Standards Association. Standard Test Method for Making Concrete Specimens; KS F 2403; Korean Standards Association: Seoul, Korea, 2019. [Google Scholar]
- Korean Standards Association. Method of Test for Slump of Concrete; KS F 2402; Korean Standards Association: Seoul, Korea, 2012. [Google Scholar]
- Korean Standards Association. Standard Test Method for Air Content of Fresh Concrete by the Pressure Method (Air Receiver Method); KS F 2421; Korean Standards Association: Seoul, Korea, 2011. [Google Scholar]
- ACI Committee. Building Code Requirements for Structural Concrete (ACI 318-08) and Commentary; American Concrete Institute: Farmington Hills, MI, USA, 2008. [Google Scholar]
- Comite Euro-International Du Beton. CEB-FIP Model Code 1990, Design Code; Institution of Civil Engineers Publishing: London, UK, 1993. [Google Scholar]
- Korean Standards Association. Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing; KS F 2456; Korean Standards Association: Seoul, Korea, 2013. [Google Scholar]
- ASTM. Standard Test Methods for Chemical Resistance of Mortars, Grouts, and Monolithic Surfacings and Polymer Concretes; ASTM C 267; ASTM: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.-H.; Oh, J.E. Microstructural verification of the strength performance of ternary blended cement systems with high volumes of fly ash and GGBFS. Constr. Build. Mater. 2015, 95, 96–107. [Google Scholar] [CrossRef]
- Ministry of Land, Infrastructure and Transport. Korea, Concrete Standard Specification; Korean Standards Association: Seoul, Korea, 2016. [Google Scholar]
- Jo, B.-W.; Shon, Y.-H.; Kim, Y.-J. The evalution of elastic modulus for steel fiber reinforced concrete. Russ. J. Nondestruct. Test. 2001, 37, 152–161. [Google Scholar] [CrossRef]
- Kim, J.-K.; Han, S.H.; Song, Y.C. Effect of temperature and aging on the mechanical properties of concrete: Part I. Experimental results. Cem. Concr. Res. 2002, 32, 1087–1094. [Google Scholar] [CrossRef]
- Alsalman, A.; Dang, C.N.; Prinz, G.S.; Hale, W.M. Evaluation of modulus of elasticity of ultra-high performance concrete. Constr. Build. Mater. 2017, 153, 918–928. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Acidic corrosion of hydrated cement based materials. Ceram. Silikáty 2000, 44, 152–160. [Google Scholar]
- Shi, Z.; Geiker, M.R.; Lothenbach, B.; De Weerdt, K.; Garzón, S.F.; Enemark-Rasmussen, K.; Skibsted, J. Friedel’s salt profiles from thermogravimetric analysis and thermodynamic modelling of Portland cement-based mortars exposed to sodium chloride solution. Cem. Concr. Compos. 2017, 78, 73–83. [Google Scholar] [CrossRef]
- Goyal, S.; Kumar, M.; Sidhu, D.S.; Bhattacharjee, B. Resistance of mineral admixture concrete to acid attack. J. Adv. Concr. Technol. 2009, 7, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Arjunan, P.; Silsbee, M. Effect of silica fume, metakaolin, and low-calcium fly ash on chemical resistance of concrete. Cem. Concr. Res. 2001, 31, 1809–1813. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry; Thomas Telford: London, UK, 1997; Volume 2. [Google Scholar]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Song, H.; Jeong, Y.; Bae, S.; Jun, Y.; Yoon, S.; Eun Oh, J. A study of thermal decomposition of phases in cementitious systems using HT-XRD and TG. Constr. Build. Mater. 2018, 169, 648–661. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Li, W.; Geng, G.; Huang, J.; Gao, Y.; Mu, S.; Hong, J. Effects of pH on the nano/micro structure of calcium silicate hydrate (CSH) under sulfate attack. Cem. Concr. Res. 2021, 140, 106306. [Google Scholar] [CrossRef]
- Sverdrup, H.U. Calcite dissolution and acidification mitigation strategies. Lake Reserv. Manag. 1984, 1, 345–355. [Google Scholar] [CrossRef]
- Pedrosa, E.T.; Fischer, C.; Morales, L.F.; Rohlfs, R.D.; Luttge, A. Influence of chemical zoning on sandstone calcite cement dissolution: The case of manganese and iron. Chem. Geol. 2021, 559, 119952. [Google Scholar] [CrossRef]
- Feng, P.; Miao, C.; Bullard, J.W. A model of phase stability, microstructure and properties during leaching of portland cement binders. Cem. Concr. Compos. 2014, 49, 9–19. [Google Scholar] [CrossRef]




| Oxide | OPC | GGBFS |
|---|---|---|
| CaO | 64.05 | 59.48 |
| SiO2 | 18.59 | 23.05 |
| Al2O3 | 4.45 | 9.72 |
| MgO | 3.14 | 2.54 |
| SO3 | 3.55 | 1.37 |
| TiO2 | 0.29 | 0.98 |
| MnO | 0.19 | 0.83 |
| FexOy | 3.61 | 0.68 |
| K2O | 1.23 | 0.68 |
| Na2O | 0.29 | 0.28 |
| Others | 0.61 | 0.4 |
| Binder Type | OPC | GGBFS | CaO | CaCl2 | Ca(HCOO)2 | Ca(NO3)2 | CaSO4 | Sum |
|---|---|---|---|---|---|---|---|---|
| P-binder | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| C-binder | 0 | 94 | 4 | 2 | 0 | 0 | 0 | 100 |
| F-binder | 0 | 88 | 4 | 0 | 3 | 0 | 5 | 100 |
| N-binder | 0 | 86 | 4 | 0 | 0 | 5 | 5 | 100 |
| Sample | Binder Type | Gmax (mm) | w/b (wt%) | s/a (vol%) | Unit Weight (kg/m3) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Water | Binder | FA | CA | Plasticizer | |||||
| P-CON | P-binder | 25 | 36.73 | 42.31 | 176.63 | 480.82 | 687.81 | 955.86 | 0.14 |
| C-CON | C-binder | 676.34 | 939.93 | 0.14 | |||||
| F-CON | F-binder | 673.87 | 936.48 | 0.14 | |||||
| N-CON | N-binder | 673.87 | 936.48 | 0.14 | |||||
| Sample Label | Compressive Strength at 28 Days (MPa) | Density (kg/m3) | Elastic Modulus (GPa) | ||
|---|---|---|---|---|---|
| Exp. | ACI 318 | CEB-FIP | |||
| P-CON | 44.8 | 2415.5 | 28.8 | 34.1 | 41.5 |
| C-CON | 30.5 | 2372.6 | 20.9 | 27.4 | 35.7 |
| F-CON | 29.5 | 2349.7 | 19.1 | 26.6 | 34.7 |
| N-CON | 20.7 | 2360.2 | 17.2 | 19.7 | 29.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yum, W.S.; Yu, J.; Jeon, D.; Song, H.; Sim, S.; Kim, D.H.; Oh, J.E. Mechanical and Durability Properties of Cementless Concretes Made Using Three Types of CaO-Activated GGBFS Binders. Materials 2022, 15, 271. https://doi.org/10.3390/ma15010271
Yum WS, Yu J, Jeon D, Song H, Sim S, Kim DH, Oh JE. Mechanical and Durability Properties of Cementless Concretes Made Using Three Types of CaO-Activated GGBFS Binders. Materials. 2022; 15(1):271. https://doi.org/10.3390/ma15010271
Chicago/Turabian StyleYum, Woo Sung, Juan Yu, Dongho Jeon, Haemin Song, Sungwon Sim, Do Hoon Kim, and Jae Eun Oh. 2022. "Mechanical and Durability Properties of Cementless Concretes Made Using Three Types of CaO-Activated GGBFS Binders" Materials 15, no. 1: 271. https://doi.org/10.3390/ma15010271
APA StyleYum, W. S., Yu, J., Jeon, D., Song, H., Sim, S., Kim, D. H., & Oh, J. E. (2022). Mechanical and Durability Properties of Cementless Concretes Made Using Three Types of CaO-Activated GGBFS Binders. Materials, 15(1), 271. https://doi.org/10.3390/ma15010271







