A Study on High Strength, High Plasticity, Non-Heat Treated Die-Cast Aluminum Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Forming Characteristics of Non-Heat-Treated, Die-Cast Aluminum Alloy
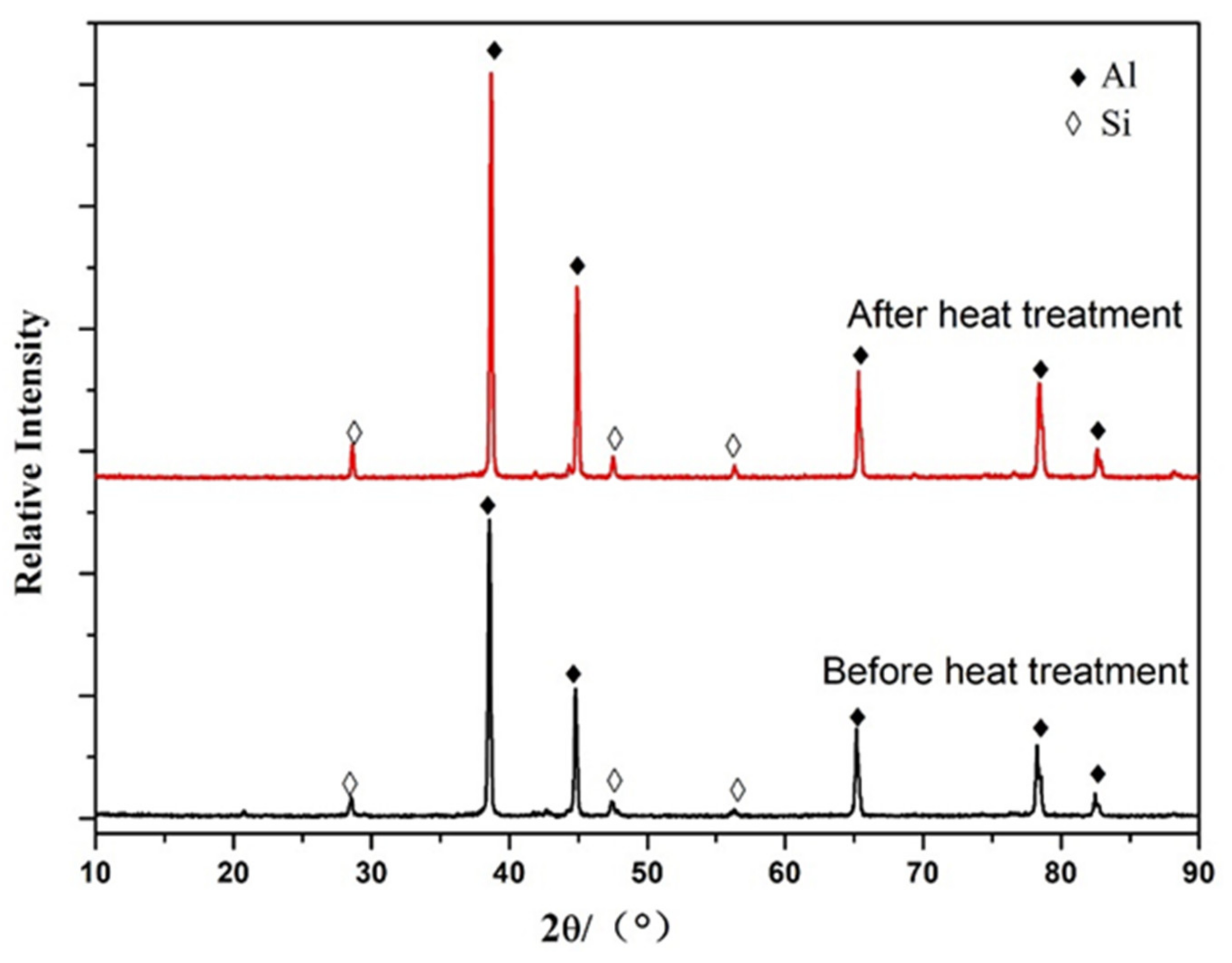
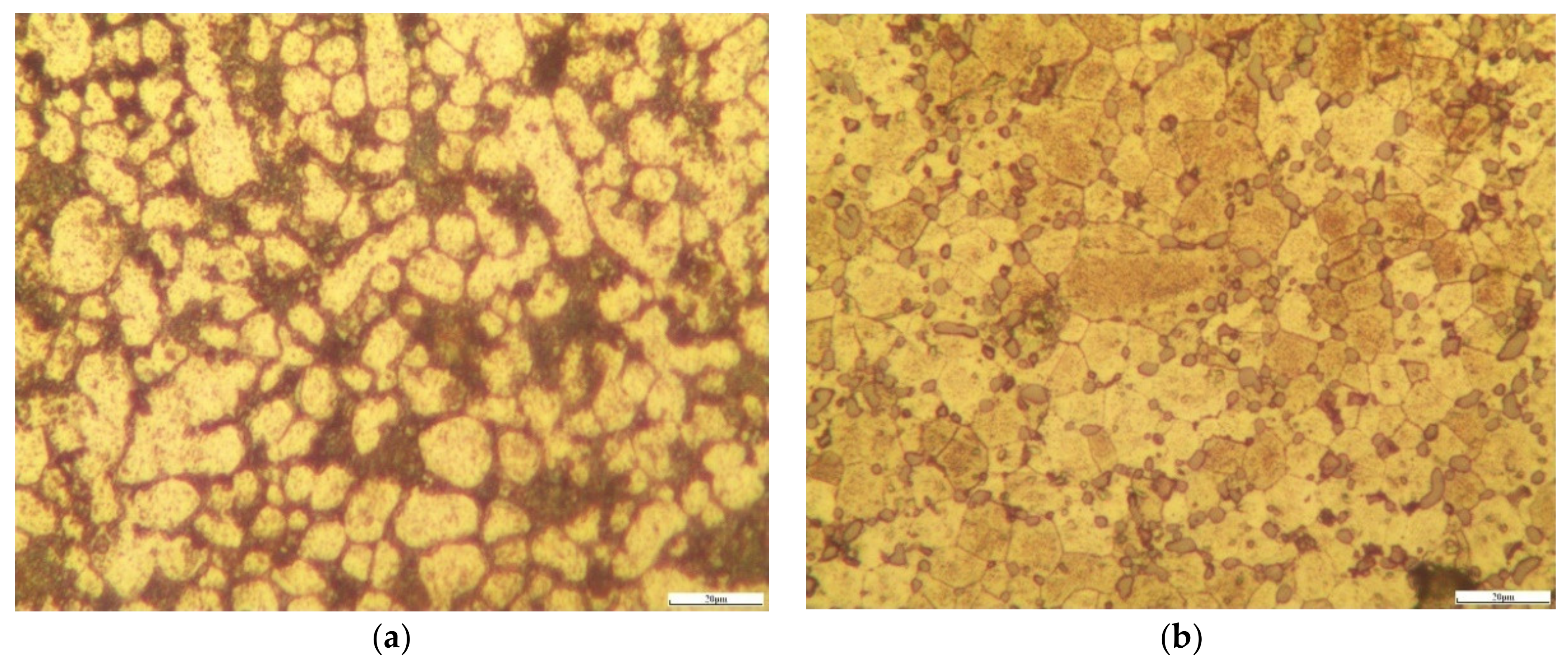
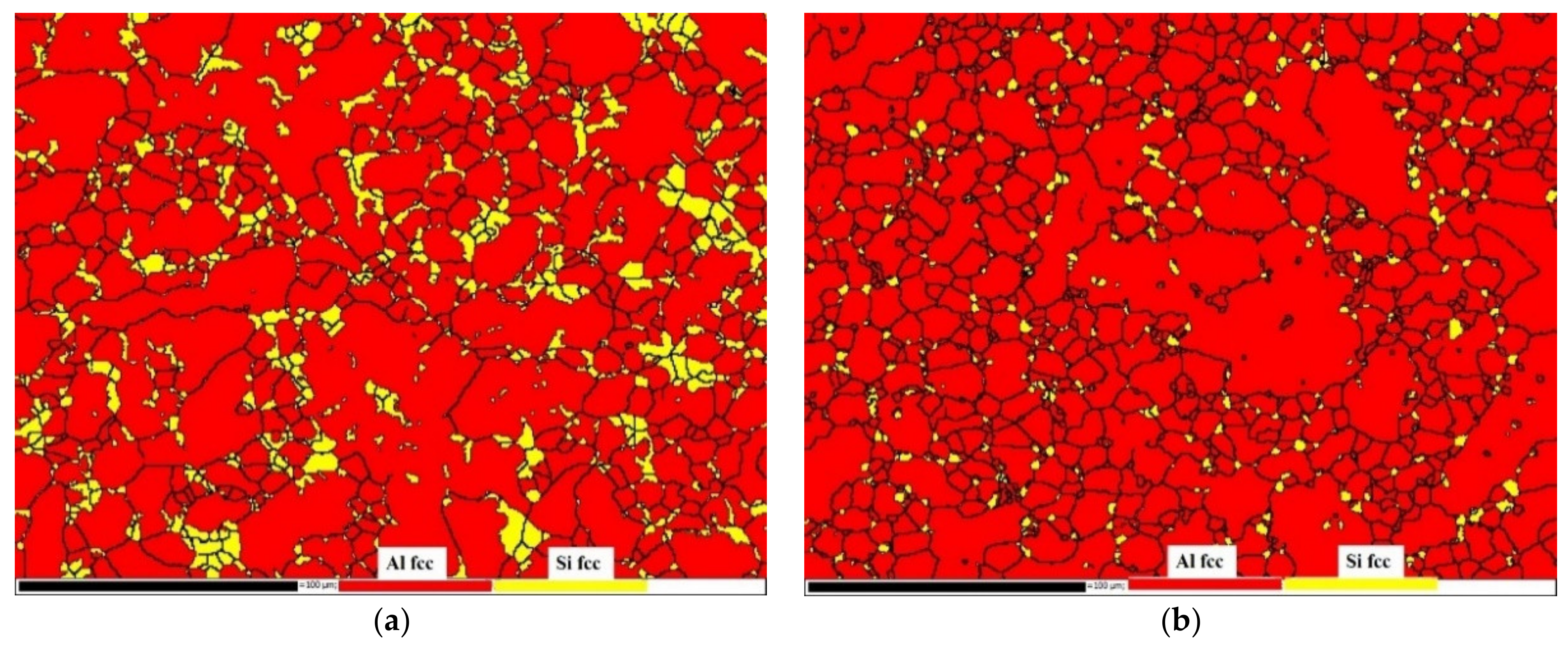
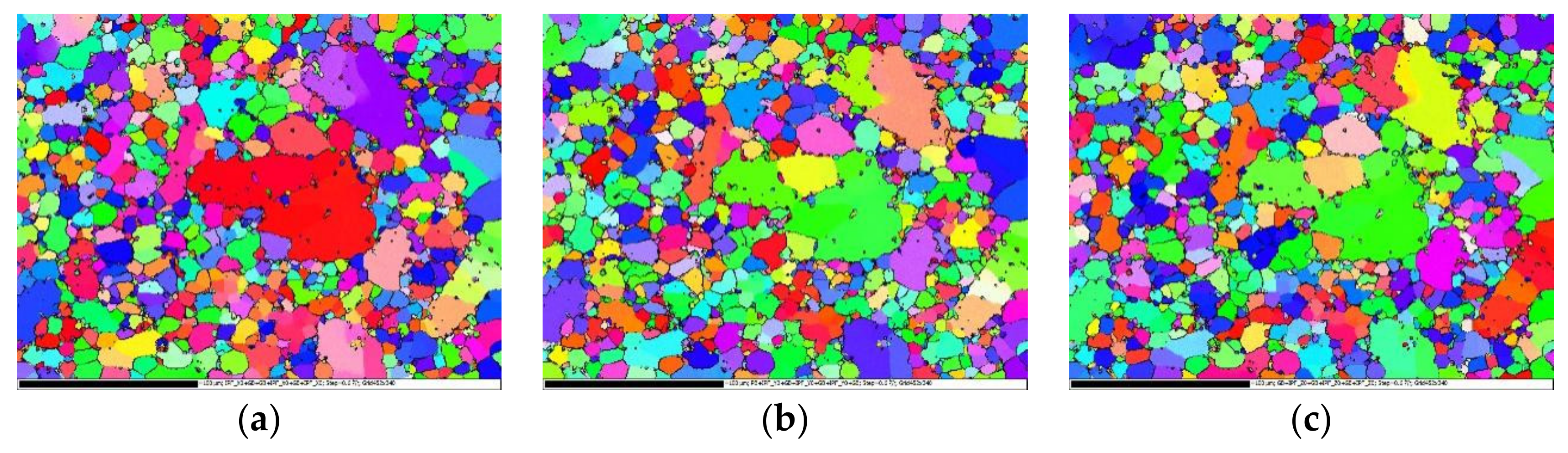
3.2. Non-Heat-Treated, Die-Cast Aluminum Alloy Composition and Structural Characteristics
3.3. Mechanical Properties of Non-Heat-Treated, Die-Cast Aluminum Alloy Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, F.; Yi, Y.; Huang, C.; Huang, S. Influence of cryogenic deformation on second-phase particles, grain structure, and mechanical properties of Al–Cu–Mn alloy. J. Alloy. Compd. 2020, 827, 154300. [Google Scholar] [CrossRef]
- Bahl, S.; Hu, X.; Hoar, E.; Cheng, J.; Haynes, J.A.; Shyam, A. Effect of copper content on the tensile elongation of Al–Cu–Mn–Zr alloys: Experiments and finite element simulations. Mater. Sci. Eng. A 2020, 772, 138801. [Google Scholar] [CrossRef]
- El-Batahgy, A.-M.; Klimova-Korsmik, O.; Akhmetov, A.; Turichin, G. High-Power Fiber Laser Welding of High-Strength AA7075-T6 Aluminum Alloy Welds for Mechanical Properties Research. Materials 2021, 14, 7498. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Ishihara, S.; Shibata, H.; Oguma, N. Effect of Rod-like Structure on Fatigue Life, Short Surface Crack Initiation and Growth Characteristics of Extruded Aluminum Alloy A2024 (Analysis via Modified Linear Elastic Fracture Mechanics). Materials 2021, 14, 7538. [Google Scholar] [CrossRef]
- Giovanni, M.D.; Kaduk, J.A.; Srirangam, P. Modification of Al-Si Alloys by Ce or Ce with Sr. JOM-US 2019, 71, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Zawada-Michałowska, M.; Pieśko, P. Post-Machining Deformations of Thin-Walled Elements Made of EN AW-2024 T351 Aluminum Alloy as Regards the Mechanical Properties of the Applied, Rolled Semi-Finished Products. Materials 2021, 14, 7591. [Google Scholar] [CrossRef]
- Penghuai, F.; Liming, P.; Wenjiang, D. Automobile Lightweight Technology: Development Trends of Aluminum/Magnesium Alloys and Their Forming Technologies. Eng. Sci. 2018, 20, 84–90. [Google Scholar]
- Kawajiri, K.; Kobayashi, M.; Sakamoto, K. Lightweight materials equal lightweight greenhouse gas emissions? A historical analysis of greenhouse gases of vehicle material substitution. J. Clean. Prod. 2020, 253, 119805. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Gao, B. Extraction of Al from Coarse Al–Si Alloy by The Selective Liquation Method. Materials 2021, 14, 3680. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, S.; Cao, F.; Shen, H.; Zhang, L.; Gu, X.; Song, H.; Sun, J. Thermal Expansion Behavior of Co-Spray Formed Al-20Si/7075 Bimetallic Gradient Alloy. Materials 2021, 14, 4100. [Google Scholar] [CrossRef]
- Agarwal, J.; Sahoo, S.; Mohanty, S.; Nayak, S.K. Progress of novel techniques for lightweight automobile applications through innovative eco-friendly composite materials: A review. J. Thermoplast. Compos. Mater. 2020, 33, 089270571881553. [Google Scholar] [CrossRef]
- Choudry, S.A.; Kaspar, J.; Greinacher, F.; Vielhaber, M. A Prediction-Model for an Improved Decision-making of Joining Technologies in the Early Stage of Automobile Body Development. Procedia CIRP 2019, 80, 10–14. [Google Scholar] [CrossRef]
- Anonymous. Research and Markets; Materials for Next Generation Automobiles: Smart Technologies and the Demand for Lightweight, Fuel-Efficient Vehicles Spur Growth of Future Automobiles Materials. Nanotechnol. Bus. J. 2009. [Google Scholar]
- Hanlin, L.; Xin, S.; Lijun, Y.; Zhongchao, M.; Leyu, S.; Bo, M.; Rong, J. Production and testing of non-heat-treated 1120 medium strengt aluminum alloy stranded conductors. Opt. Fiber Electr. Cable Appl. 2020, 1, 24–26+38. [Google Scholar]
- Nellippallil, A.B.; De, P.S.; Gupta, A.; Goyal, S.; Singh, A.K. Hot Rolling of a Non-heat Treatable Aluminum Alloy: Thermo-Mechanical and Microstructure Evolution Model. Trans. Indian Inst. Met. 2016, 70, 1387–1398. [Google Scholar] [CrossRef]
- Li, F.; Chen, S.; Chen, K.; Huang, L. The role of Si on microstructure, mechanical and local corrosion behaviors of an Al–Cu–Mg–Si alloy with high Cu/Mg ratio. J. Alloy. Compd. 2020, 819, 152977. [Google Scholar] [CrossRef]
- Xu, R.; Lin, B.; Li, H.-Y.; Xiao, H.-Q.; Zhao, Y.-L.; Zhang, W.-W. Microstructure evolution and mechanical properties of Al–6.5Cu–0.6Mn–0.5Fe alloys with different Si additions. Trans. Nonferr. Metal Soc. 2019, 29, 1583–1591. [Google Scholar] [CrossRef]
- Li, Y.; Hu, B.; Liu, B.; Nie, A.; Gu, Q.; Wang, J.; Li, Q. Insight into Si poisoning on grain refinement of Al-Si/Al-5Ti-B system. Acta Mater. 2020, 187, 51–65. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Ren, F.; Stock, H.-R. Effect of different Cu contents on the microstructure and hydrogen production of Al–Cu-Ga-In-Sn alloys for dissolvable materials. J. Alloy. Compd. 2020, 821, 153489. [Google Scholar] [CrossRef]
- Arif, M.A.M.; Omar, M.Z.; Sajuri, Z.; Salleh, M.S. Effects of Cu and Mg on thixoformability and mechanical properties of aluminium alloy 2014. Trans. Nonferr. Metal Soc. 2020, 30, 275–287. [Google Scholar] [CrossRef]
- Salleh, M.S.; Omar, M.Z. Influence of Cu content on microstructure and mechanical properties of thixoformed Al–Si–Cu–Mg alloys. Trans. Nonferr. Metal Soc. 2015, 25, 3523–3538. [Google Scholar] [CrossRef]
- Javidani, M.; Larouche, D.; Grant Chen, X. Assessment of Post-eutectic Reactions in Multicomponent Al-Si Foundry Alloys Containing Cu, Mg, and Fe. Metall. Mater. Trans. A 2015, 46, 2933–2946. [Google Scholar] [CrossRef]
- Zeren, M.; Karakulak, E.; GÜMÜŞ, S. Influence of Cu addition on microstructure and hardness of near-eutectic Al-Si-xCu-alloys. Trans. Nonferr. Metal Soc. 2011, 21, 1698–1702. [Google Scholar] [CrossRef]
- Ke, C.; Fubao, Z.; Hongjun, N.; Yunyang, J.; Yu, Z.; Mingyu, H. Effection of Mn on microstructure and properties of AlSiMgMn aluminum alloy. Nonferrous Met. Eng. 2020, 10, 37–43. [Google Scholar]
- Mengen, L.; Yilei, R.; Li, B. Effect of Alloying Elements Cu and Mn on Microstructure and Mechanical Properties of As-cast Al-20Si Alloy. Hot Work. Technol. 2019, 48, 76–78. [Google Scholar]
- Rakhmonov, J.; Liu, K.; Pan, L.; Breton, F.; Chen, X.G. Enhanced mechanical properties of high-temperature-resistant Al–Cu cast alloy by microalloying with Mg. J. Alloy. Compd. 2020, 827, 154305. [Google Scholar] [CrossRef]
- Alhawari, K.S.; Omar, M.Z.; Ghazali, M.J.; Salleh, M.S.; Mohammed, M.N. Microstructural evolution during semisolid processing of Al–Si–Cu alloy with different Mg contents. Trans. Nonferr. Metal Soc. 2017, 27, 1483–1497. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Wang, K.; Zhang, Z.-M.; Li, B.; Xue, H.-S.; Yang, D.-Z. Effects of Mg content on primary Mg2Si phase in hypereutectic Al–Si alloys. Trans. Nonferr. Metal Soc. 2015, 25, 3197–3203. [Google Scholar] [CrossRef]
- Kim, B.H.; Jung, S.K.; Yoon, Y.O.; Lim, H.K.; Kim, S.K. Effect of Mg Content on Age Hardening of an Extruded Al-Si-Mg-Mn Alloy. Adv. Mat. Res. 2012, 630, 54–58. [Google Scholar] [CrossRef]
- Ravi, K.R.; Manivannan, S.; Phanikumar, G.; Murty, B.S.; Sundarraj, S. Influence of Mg on Grain Refinement of Near Eutectic Al-Si Alloys. Metall. Mater. Trans. A 2011, 42, 2028–2039. [Google Scholar] [CrossRef]
- Jin, L.; Liu, K.; Chen, X.G. Evolution of Fe-Rich Intermetallics in Al-Si-Cu 319 Cast Alloy with Various Fe, Mo, and Mn Contents. Metall. Mater. Trans. B 2019, 50, 1896–1907. [Google Scholar] [CrossRef]
- Becker, H.; Thum, A.; Distl, B.; Kriegel, M.J.; Leineweber, A. Effect of Melt Conditioning on Removal of Fe from Secondary Al-Si Alloys Containing Mg, Mn, and Cr. Metall. Mater. Trans. A 2018, 49, 6375–6389. [Google Scholar] [CrossRef]
- Yang, W.C.; Gao, F.; Ji, S.X. Formation and sedimentation of Fe-rich intermetallics in Al–Si–Cu–Fe alloy. Trans. Nonferr. Metal Soc. 2015, 25, 1704–1714. [Google Scholar] [CrossRef] [Green Version]
- Budsarakham, P.; Riyaphan, C.; Canyook, R.; Taweesup, K. Effects of Cr on anodising and microstructure of cast aluminium alloys. Mater. Today Proc. 2018, 5, 9417–9423. [Google Scholar] [CrossRef]
- Tocci, M.; Donnini, R.; Angella, G.; Pola, A. Effect of Cr and Mn addition and heat treatment on AlSi3Mg casting alloy. Mater. Charact. 2017, 123, 75–82. [Google Scholar] [CrossRef]
- Tocci, M.; Pola, A.; Angella, G.; Donnini, R.; Vecchia, G.M.L. Dispersion hardening of an AlSi3Mg alloy with Cr and Mn addition. Mater. Today Proc. 2019, 10, 319–326. [Google Scholar] [CrossRef]
- Buraś, J.; Szucki, M.; Piwowarski, G.; Krajewski, W.K.; Krajewski, P.K. Strength properties examination of high zinc aluminium alloys inoculated with Ti addition. China Foundry 2017, 14, 211–215. [Google Scholar] [CrossRef]
- Nassirirad, A.; Emamy, M.; Pourbahari, B. The effects of Zr and Ti on the microstructure and tensile properties of Al–Cu–Mg aluminium alloy. Can. Metall. Q. 2018, 57, 470–480. [Google Scholar] [CrossRef]
- GB/T 15114-2009; Aluminum Alloy Die Castings; Standardization Administration of the People’s Republic of China: Beijing, China, 2009; pp. 1–12.



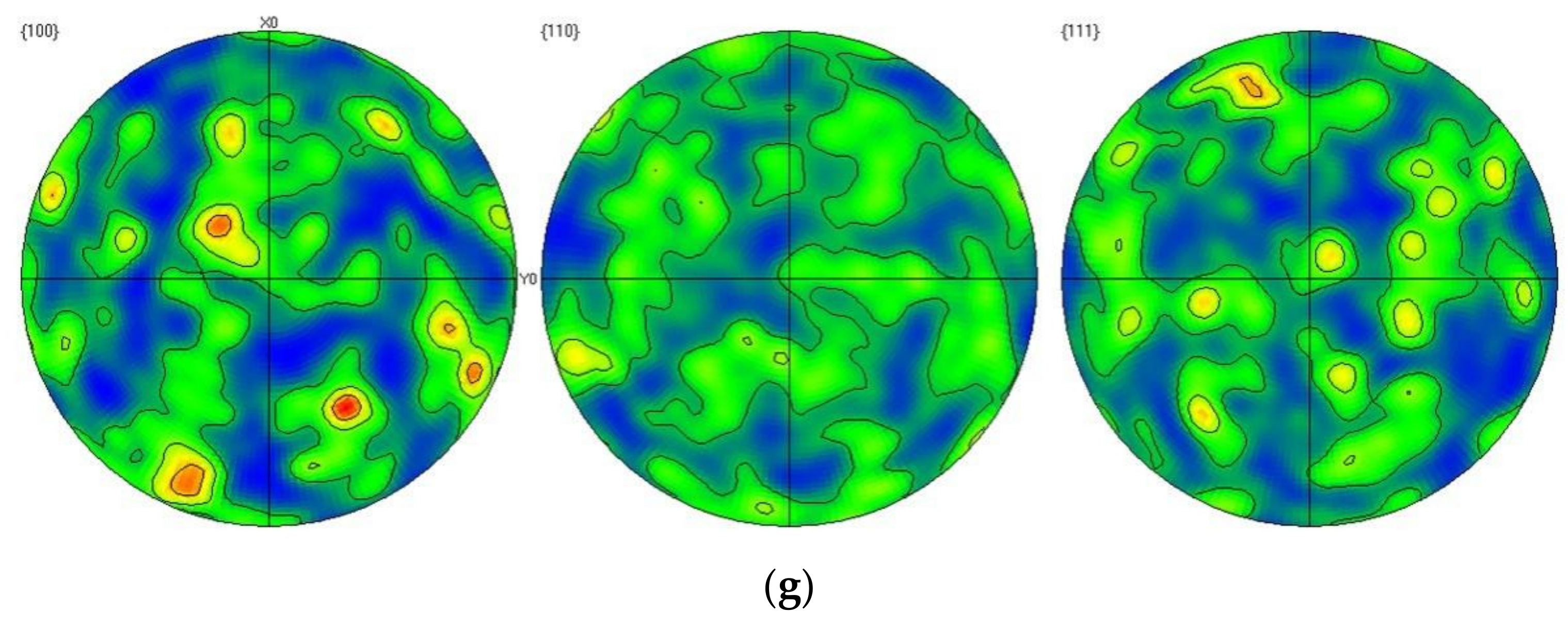

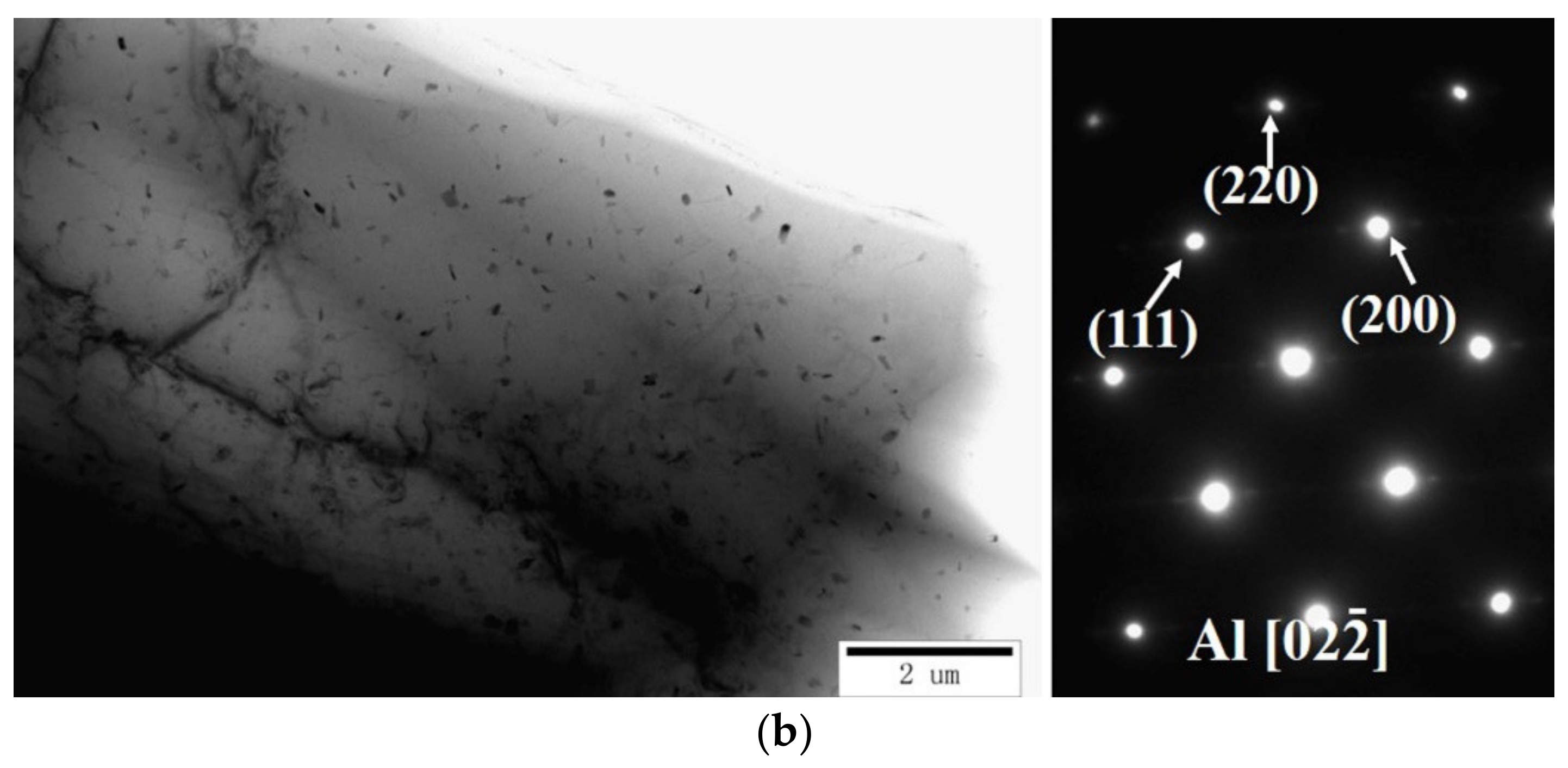


| Element | Fe | Cu | Mg | Mn | Ti | Si | Cr | Sr | Al |
|---|---|---|---|---|---|---|---|---|---|
| Sample | 0.208 | 2.50 | 0.326 | 0.660 | 0.091 | 8.55 | 0.088 | 0.02 | Bal. |
| Sample | 1 | 2 | 3 | 4 | 5 | AVG | Errors |
|---|---|---|---|---|---|---|---|
| Before heat treatment | 108 | 122 | 112 | 111 | 118 | 114 | 5.7 |
| After heat treatment | 124 | 111 | 116 | 124 | 128 | 121 | 6.9 |
| Sample | Yield Strength/MPa | Tensile Strength/MPa | Elongation (%) |
|---|---|---|---|
| Before heat treatment | 158 ± 6 | 310 ± 4 | 6.9 ± 0.4 |
| After heat treatment | 290 ± 10 | 375 ± 6 | 4.3 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Guo, C.; Ma, M. A Study on High Strength, High Plasticity, Non-Heat Treated Die-Cast Aluminum Alloy. Materials 2022, 15, 295. https://doi.org/10.3390/ma15010295
Hu R, Guo C, Ma M. A Study on High Strength, High Plasticity, Non-Heat Treated Die-Cast Aluminum Alloy. Materials. 2022; 15(1):295. https://doi.org/10.3390/ma15010295
Chicago/Turabian StyleHu, Ruizhang, Chun Guo, and Mingliang Ma. 2022. "A Study on High Strength, High Plasticity, Non-Heat Treated Die-Cast Aluminum Alloy" Materials 15, no. 1: 295. https://doi.org/10.3390/ma15010295
APA StyleHu, R., Guo, C., & Ma, M. (2022). A Study on High Strength, High Plasticity, Non-Heat Treated Die-Cast Aluminum Alloy. Materials, 15(1), 295. https://doi.org/10.3390/ma15010295







