Characterizing the Chemical Structure of Ti3C2Tx MXene by Angle-Resolved XPS Combined with Argon Ion Etching
Abstract
:1. Introduction
2. Experimental
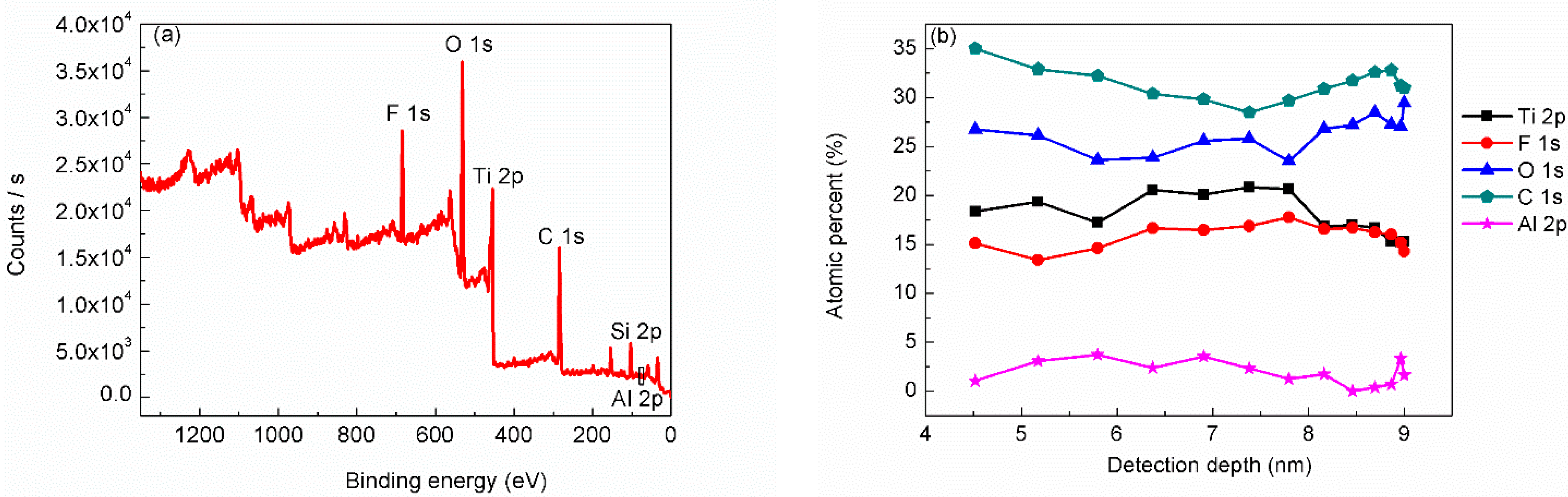
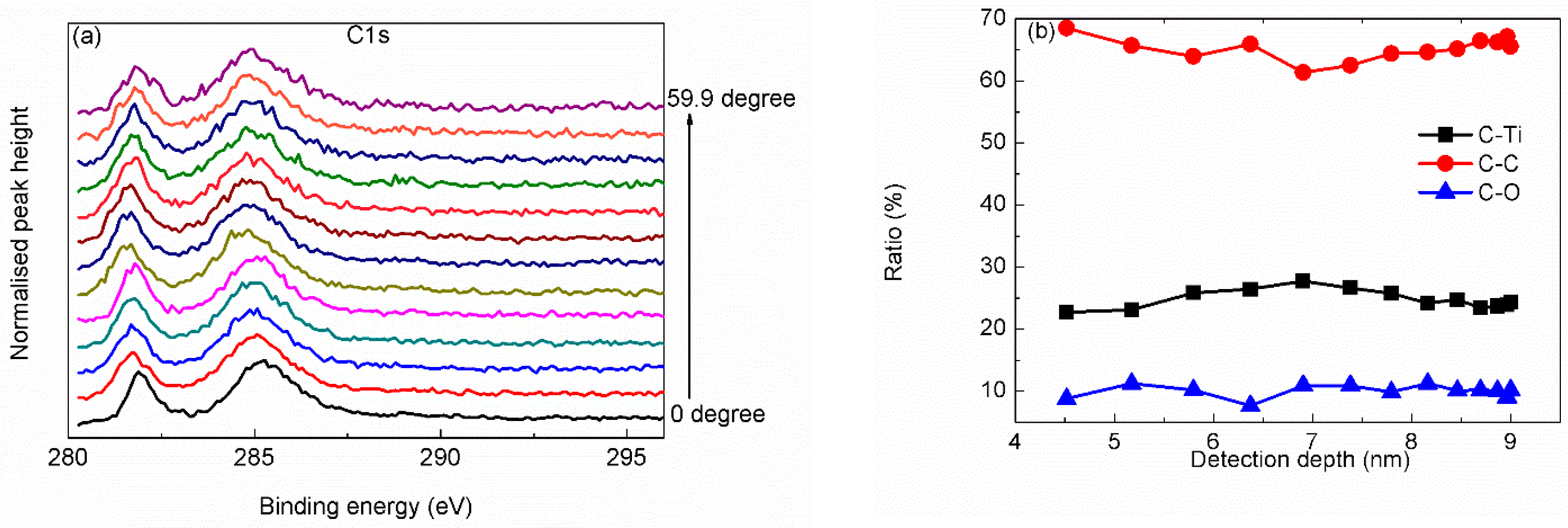
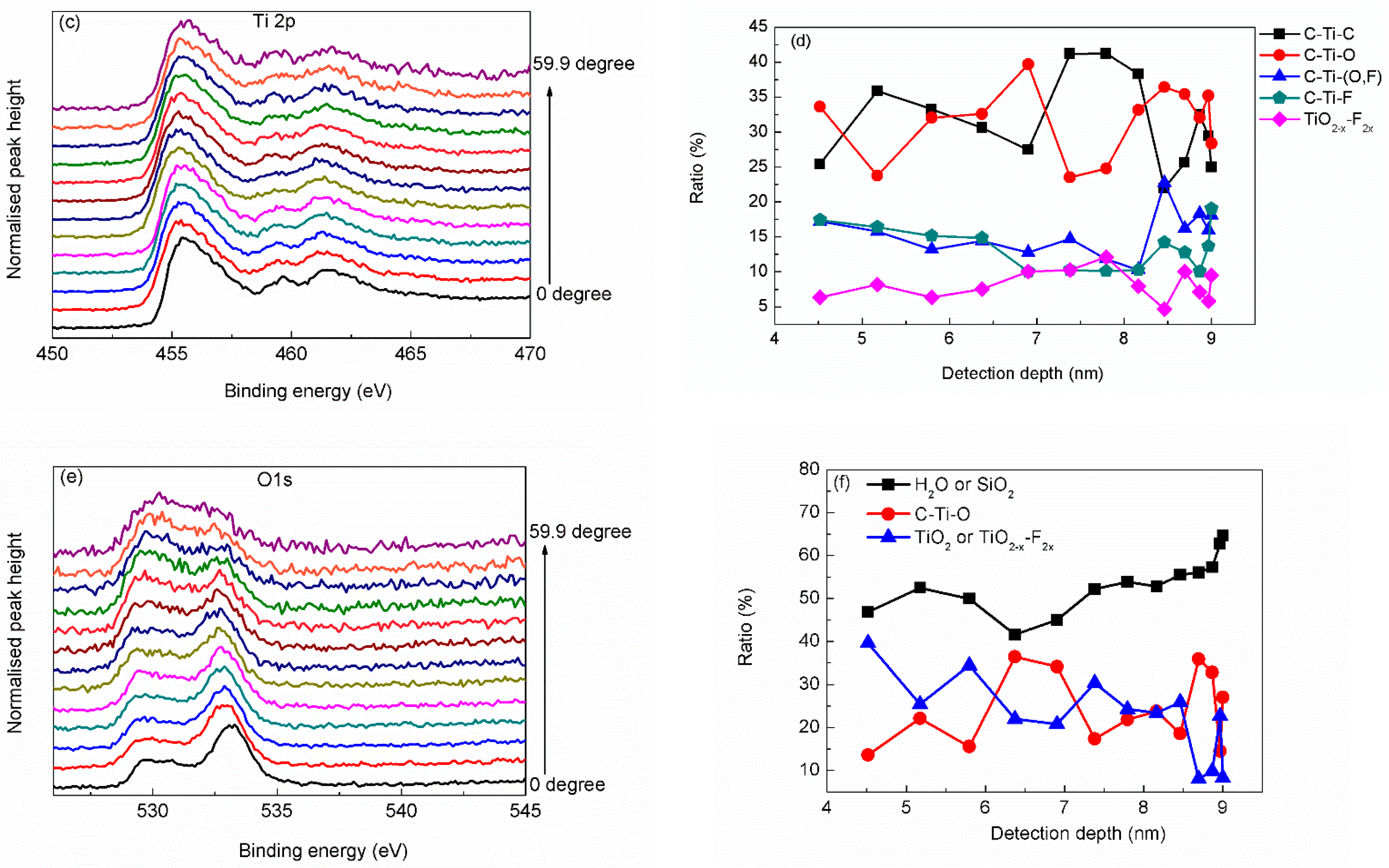
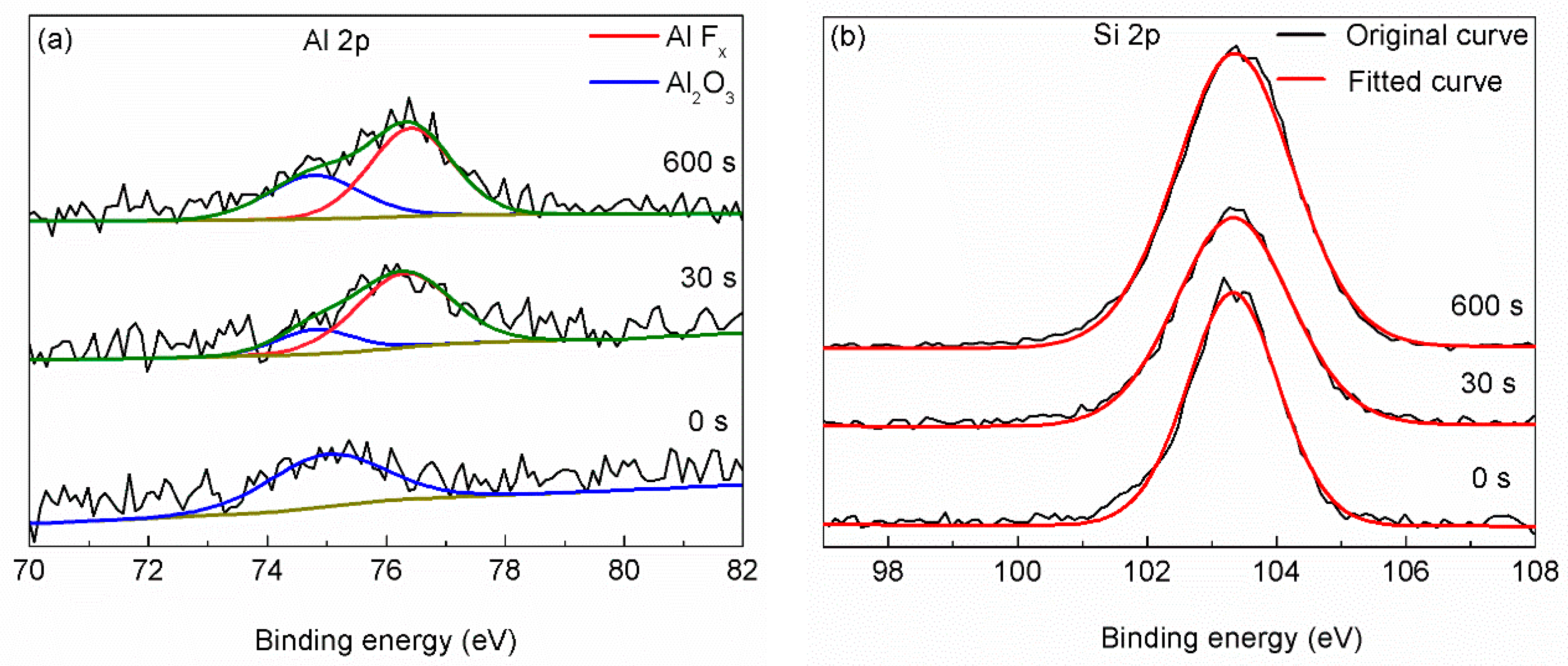
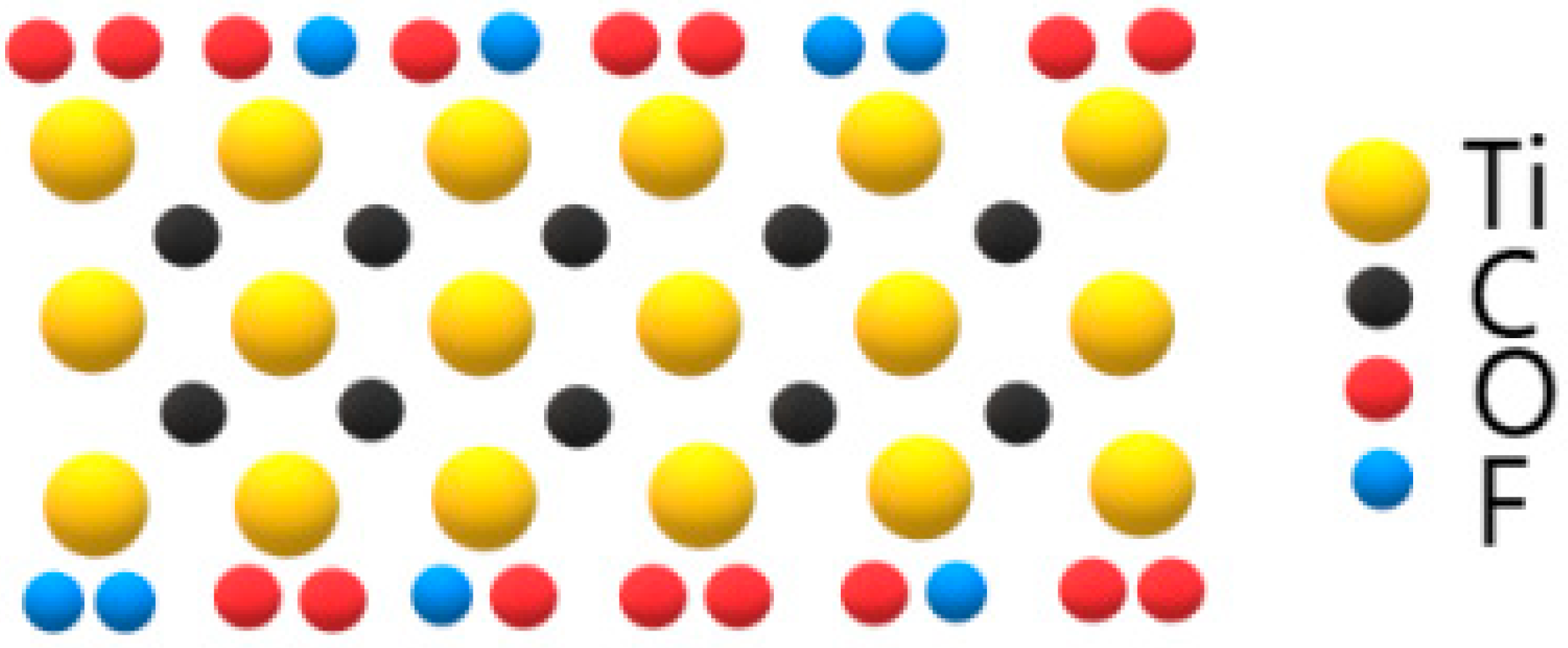
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically ThinMoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, X.; Zhao, R.; Liao, K.; Chan, V. Dual-functional Ti3C2Tx MXene for wastewater treatment and electrochemical energy storage. Sustain. Energy Fuels 2020, 4, 3566–3573. [Google Scholar] [CrossRef]
- Zhang, F.; Jia, Z.; Wang, C.; Feng, A.; Wang, K.; Hou, T.; Liu, J.; Zhang, Y.; Wu, G. Sandwich-like silicon/Ti3C2Tx MXene composite by electrostatic self-assembly for high performance lithium ion battery. Energy 2020, 195, 117047. [Google Scholar] [CrossRef]
- Huo, X.; Liu, Y.; Li, R.; Li, J. Two-dimensional Ti3C2Tx@S as cathode for room temperature sodium-sulfur batteries. Ionics 2019, 25, 5373–5382. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Liao, Y.; Xiang, Q.; Li, Y.; Wen, T.; Zhong, Z. Accordion-like composite of carbon-coated Fe3O4 nanoparticle decorated Ti3C2 MXene with enhanced electrochemical performance. J. Mater. Sci. 2021, 56, 2486–2496. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Ma, W.; Fan, W.; Sun, Y.; Yin, H.; Shi, X.; Liu, X.; Ding, Y. Enhanced low-temperature Li-ion storage in MXene titanium carbide by surface oxygen termination. 2D Mater. 2019, 6, 045025. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Li, G.; Zhang, X.; Wang, Z. Surface modified MXene Ti3C2 multilayers by aryl diazonium salts leading to large-scale delamination. Appl. Surf. Sci. 2016, 384, 287–293. [Google Scholar] [CrossRef]
- Qian, A.; Hyeon, S.E.; Seo, J.Y.; Chung, C.-H. Capacitance changes associated with cation-transport in free-standing flexible Ti3C2Tx (T O, F, OH) MXene film electrodes. Electrochim. Acta 2018, 266, 86–93. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, 191, 33–40. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Levitt, A.; Gogotsi, Y. Selective Etching of Silicon from Ti3SiC2 (MAX) To Obtain 2D Titanium Carbide (MXene). Angew. Chem. Int. Ed. 2018, 57, 5444–5448. [Google Scholar] [CrossRef] [PubMed]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, E.A.; Anasori, B.; et al. Synthesis of Mo4VAlC4 MAX Phase and Two-Dimensional Mo4VC4 MXene with Five Atomic Layers of Transition Metals. ACS Nano 2020, 14, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Yu, J.; Zhao, H.; Zhang, H.; Li, W. One-step synthesis for cations intercalation of two-dimensional carbide crystal Ti3C2 MXene. Appl. Surf. Sci. 2020, 505, 144538. [Google Scholar] [CrossRef]
- Persson, I.; Lars-Åke, N.; Halim, J.; Barsoum, M.W.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O. Å On the organization and thermal behavior of functional groups on Ti 3 C 2 MXene surfaces in vacuum. 2D Mater. 2017, 5, 015002. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Lars-Åke, N.; Persson, P.O.Å.; Rosen, J. X-ray Photoelectron Spectroscopy of Ti3AlC2, Ti3C2Tz, and TiC Provides Evidence for the Electrostatic Interaction between Laminated Layers in MAX-Phase Materials. J. Phys. Chem. C 2020, 124, 27732–27742. [Google Scholar] [CrossRef]
- Verpoort, F.; Bossuyt, A.; Verdonck, L. Olefin metathesis catalyst.: III. Angle-resolved XPS and depth profiling study of a tungsten oxide layer on silica. J. Electron Spectrosc. Relat. Phenom. 1996, 82, 151–163. [Google Scholar] [CrossRef]
- Xia, Q.X.; Fu, J.; Yun, J.M.; Mane, R.S.; Kim, K.H. High volumetric energy density annealed-MXene-nickel oxide/MXene asymmetric supercapacitor. RSC Adv. 2017, 7, 11000–11011. [Google Scholar] [CrossRef] [Green Version]
- Safyari, M.; Moshtaghi, M.; Hojo, T.; Akiyama, E. Mechanisms of hydrogen embrittlement in high-strength aluminum alloys containing coherent or incoherent dispersoids. Corros. Sci. 2021, 194, 109895. [Google Scholar] [CrossRef]
- Wang, T.; Sun, X.; Guo, X.; Zhang, J.; Yang, J.; Tao, S.; Guan, J.; Zhou, L.; Han, J.; Wang, C.; et al. Ultraefficiently Calming Cytokine Storm Using Ti3C2Tx MXene. Small Methods 2021, 5, 2001108. [Google Scholar] [CrossRef] [PubMed]
- Natu, V.; Benchakar, M.; Canaff, C.; Habrioux, A.; Célérier, S.; Barsoum, M.W. A critical analysis of the X-ray photoelectron spectra of Ti3C2Tz MXenes. Matter 2021, 4, 1224–1251. [Google Scholar] [CrossRef]
- Li, D.; Liu, G.; Zhang, Q.; Qu, M.; Fu, Y.Q.; Liu, Q.; Xie, J. Virtual sensor array based on MXene for selective detections of VOCs. Sens. Actuators B Chem. 2021, 331, 129414. [Google Scholar] [CrossRef]
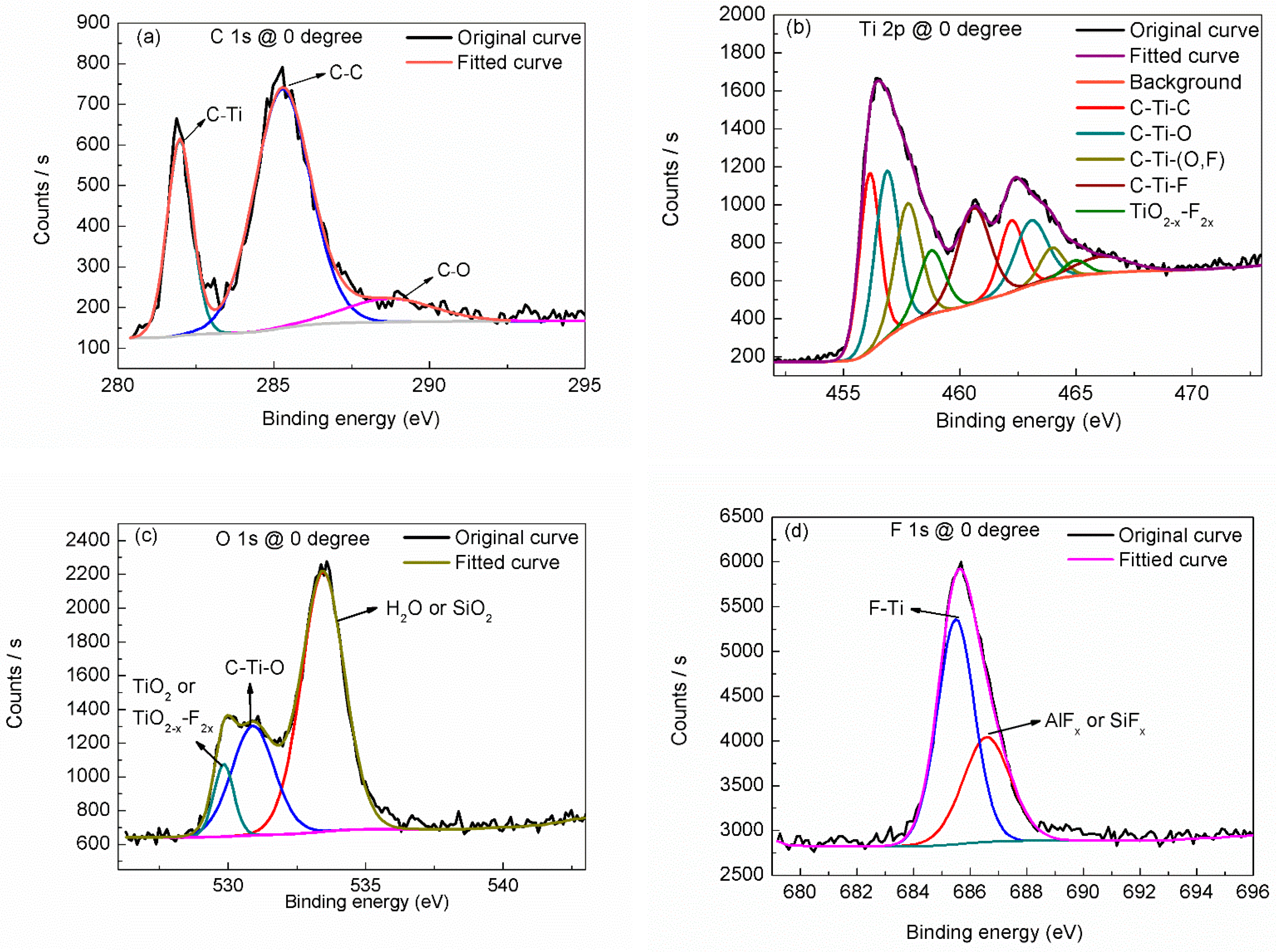
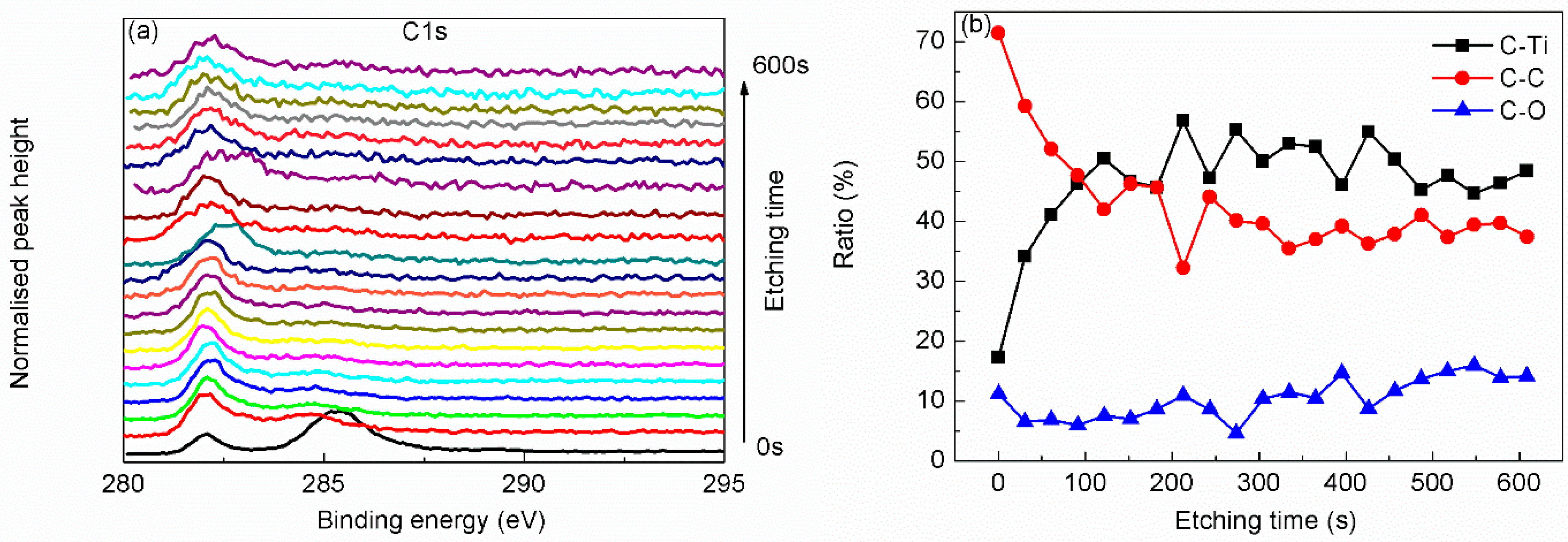
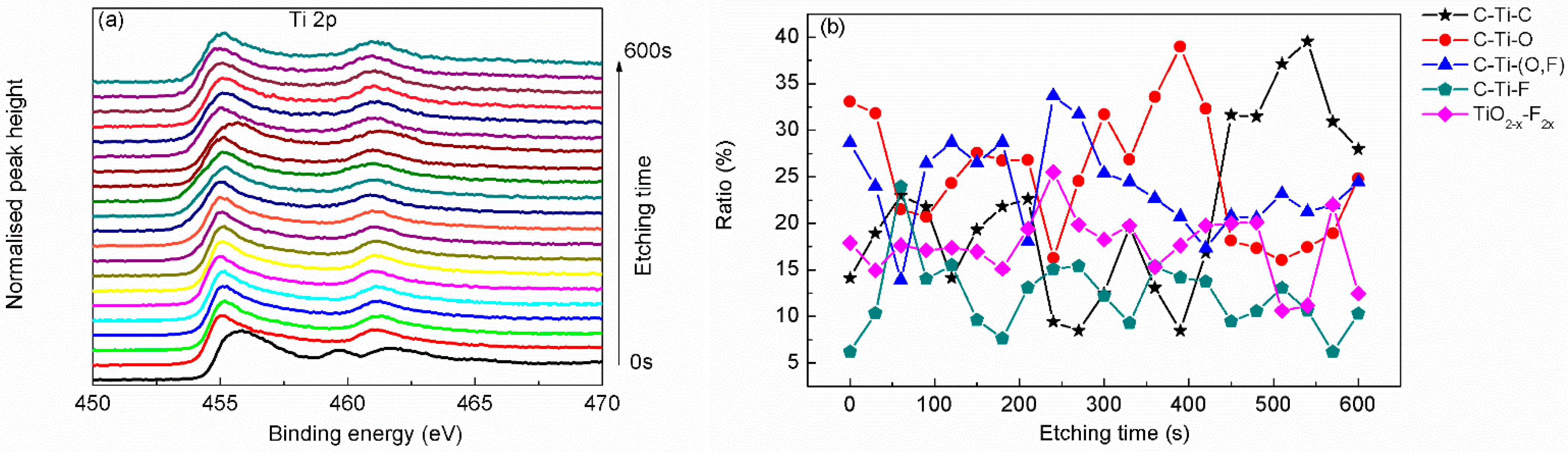
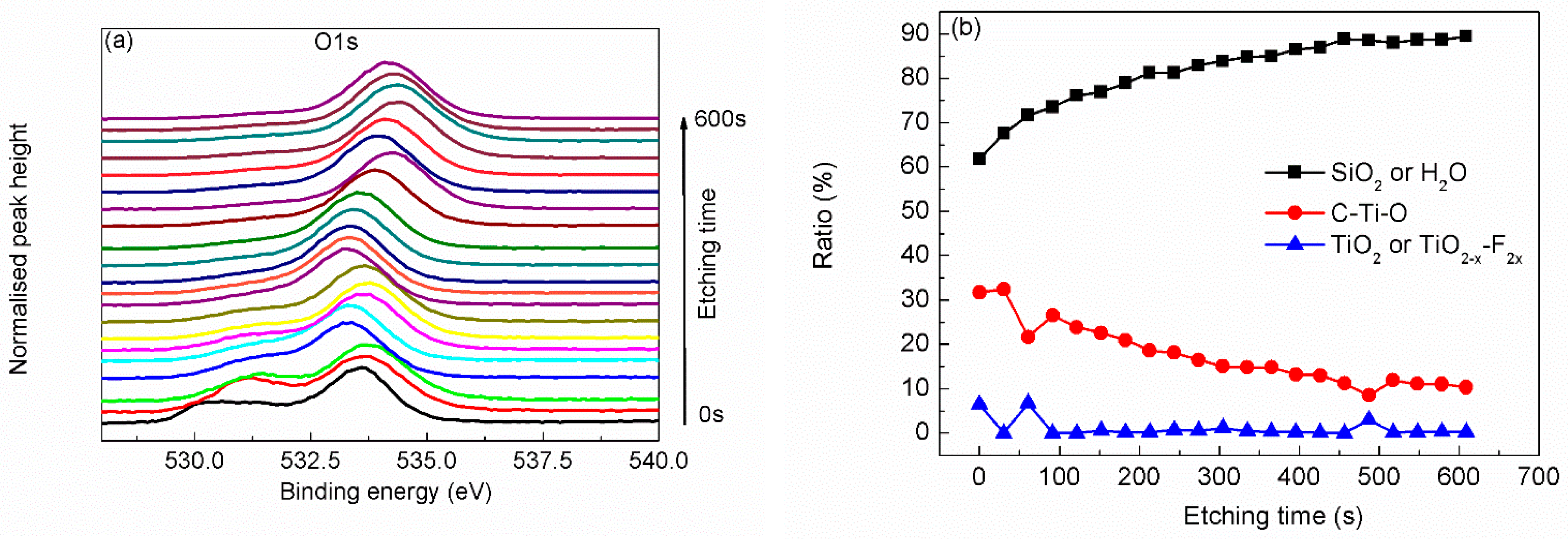
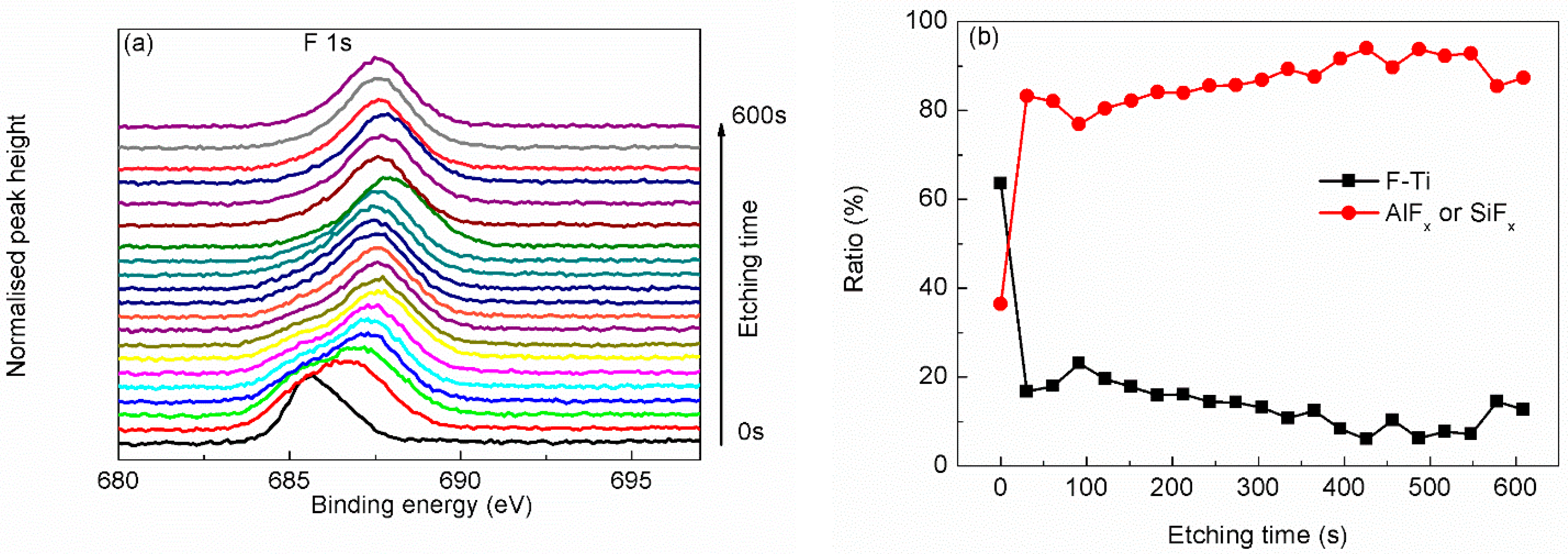
| Region | BE (eV) | FWHM (eV) | Assignment | References |
|---|---|---|---|---|
| C 1s | 282.0 | 0.83 | C–Ti | [22,23,28] |
| 285.3 | 1.77 | C–C | [21,22] | |
| 288.7 | 2.5 | C–O | [21,22,23,28] | |
| Ti 2p3 (2p1) | 455.1 (461.3) | 1.04 (1.23) | C–Ti–C | [23,25] |
| 456.0 (462.0) | 1.22 (1.63) | C–Ti–O | [21,23,28] | |
| 456.9 (463.0) | 1.31 (2.29) | C–Ti–(O, F) | [21,23,28] | |
| 457.9 (464.0) | 1.67 (2.73) | C–Ti–F | [22,28] | |
| 459.7 (465.2) | 1.34 (1.24) | TiO2–x–F2x | [22,28] | |
| O 1s | 529.8 | 0.89 | TiO2 or TiO2–x–F2x | [22,23,25,28] |
| 530.9 | 1.9 | C–Ti–O | [23,28] | |
| 533.4 | 1.92 | adsorbed H2O or SiO2 | [22,28] | |
| F 1s | 685.6 | 1.67 | F–Ti | [21,22,28] |
| 687.0 | 1.38 | AlFx or SiFx | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Li, D.; Liu, F. Characterizing the Chemical Structure of Ti3C2Tx MXene by Angle-Resolved XPS Combined with Argon Ion Etching. Materials 2022, 15, 307. https://doi.org/10.3390/ma15010307
Lu Y, Li D, Liu F. Characterizing the Chemical Structure of Ti3C2Tx MXene by Angle-Resolved XPS Combined with Argon Ion Etching. Materials. 2022; 15(1):307. https://doi.org/10.3390/ma15010307
Chicago/Turabian StyleLu, Yangfan, Dongsheng Li, and Fu Liu. 2022. "Characterizing the Chemical Structure of Ti3C2Tx MXene by Angle-Resolved XPS Combined with Argon Ion Etching" Materials 15, no. 1: 307. https://doi.org/10.3390/ma15010307
APA StyleLu, Y., Li, D., & Liu, F. (2022). Characterizing the Chemical Structure of Ti3C2Tx MXene by Angle-Resolved XPS Combined with Argon Ion Etching. Materials, 15(1), 307. https://doi.org/10.3390/ma15010307






