Recent Advances in the Excipients Used in Modified Release Vaginal Formulations
Abstract
:1. Introduction
2. Anatomy and Physiology of the Vagina
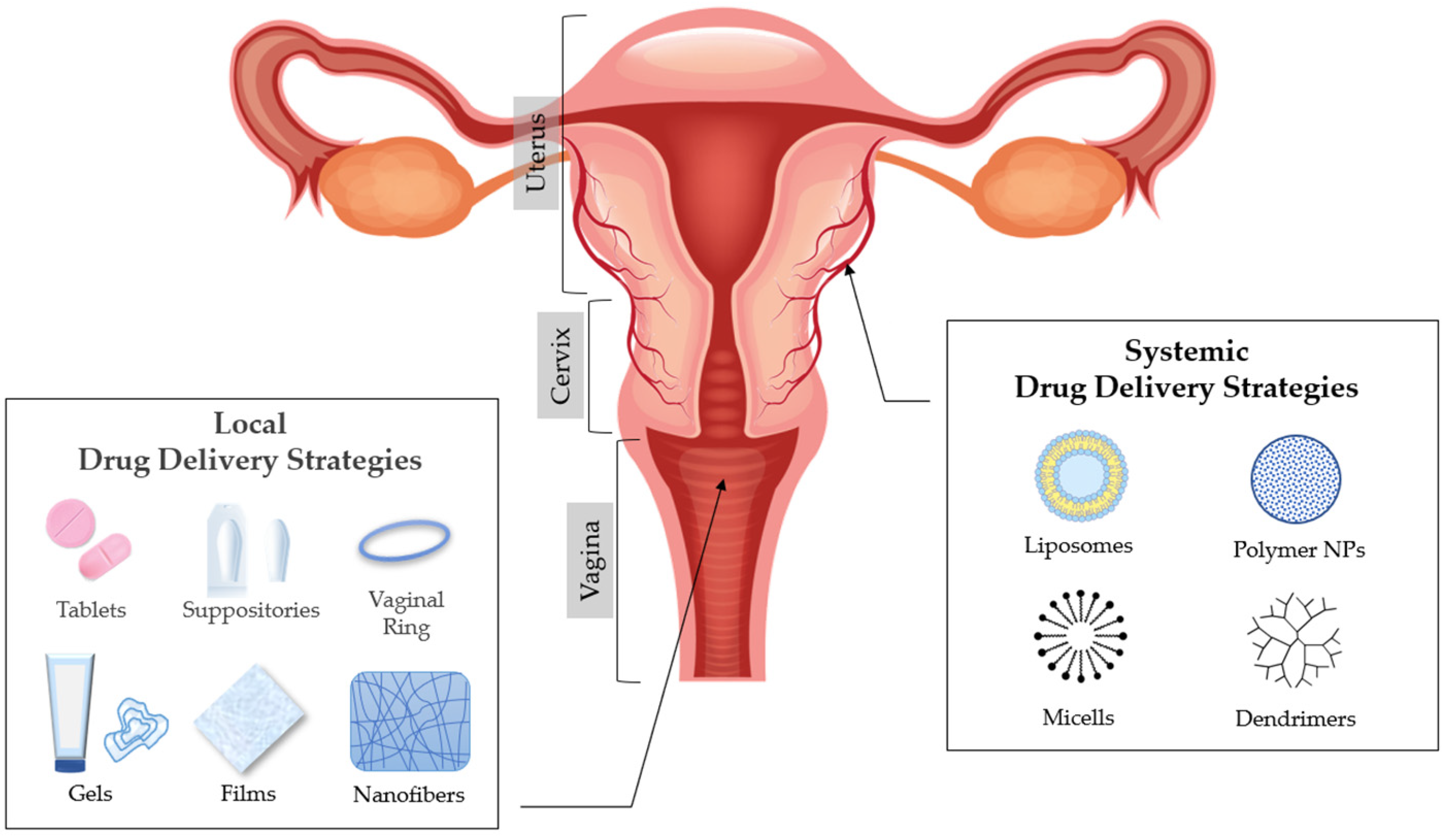
3. Vaginal Drug Delivery
4. Factors That Affect Drug Absorption in Vaginal Epithelium
5. Excipients Used in Modified Drug Release Pharmaceutical Dosage Forms for Vaginal Administration
5.1. Excipients Used in Matrix Tablets for Vaginal Administration
5.2. Excipients Used in Solid Dosage Forms for Vaginal Administration: Ovules and Vaginal Suppositories
5.3. Excipients Used in Semi-Solid Dosage Forms for Vaginal Administration: Gels
5.4. Excipients Used in Films for Vaginal Administration
5.5. Excipients Used in Devices for Vaginal Administration: Vaginal Rings
5.6. Excipients Used in Nanomedicine for Vaginal Administration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kar, M.; Chourasiya, Y.; Maheshwari, R.; Tekade, R.K. Current Developments in Excipient Science: Implication of Quantitative Selection of Each Excipient in Product Development. In Advances in Pharmaceutical Product Development and Research, Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 29–83. [Google Scholar] [CrossRef]
- Alexander, N.J.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12. [Google Scholar] [CrossRef]
- Forsberg, J.G. A morphologist’s approach to the vagina. Acta Obstet. Gynecol. Scand. Suppl. 1996, 163, 3–10. [Google Scholar]
- Sjoberg, I. The vagina: Morphological, functional and ecological aspects. Acta Obstet. Gynecol. Scand. Suppl. 1992, 71, 84–85. [Google Scholar] [CrossRef] [Green Version]
- Pendergrass, P.B.; Reeves, C.A.; Belovicz, M.W.; Molter, D.J.; White, J.H. The shape and dimensions of the human vagina as seen in three-dimensional vinyl polysiloxane casts. Gynecol. Obstet. Investig. 1996, 42, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, P.B.; Belovicz, M.W.; Reeves, C.A. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol. Obstet. Investig. 2003, 55, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.; Ruiz-Caro, R.; Mart, A. Polymer Gels in Vaginal Drug Delivery Systems. In Polymer Gels, Gels Horizons: From Science to Smart Materials; Thakur, V.K., Ed.; Springer: Singapore, 2018; pp. 197–246. [Google Scholar]
- Rohan, L.C.; Sassi, A.B. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009, 11, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Walker, D.C.; Brown, B.H.; Blackett, A.D.; Tidy, J.; Smallwood, R.H. A study of the morphological parameters of cervical squamous epithelium. Physiol. Meas. 2003, 24, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.M.; Rohan, L.C. The importance of the vaginal delivery route for antiretrovirals in HIV prevention. Ther. Deliv. 2011, 2, 1535–1550. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Vermani, K.; Kohli, G.; Kandarapu, R.; Tambwekar, K.R.; Garg, A.; Waller, D.P.; Zaneveld, L.J. Survey of vaginal formulations available on the Indian market: Physicochemical characterization of selected products. Int. J. Pharmaceut. Med. 2002, 16, 141–152. [Google Scholar] [CrossRef]
- Vermani, K.; Garg, S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364. [Google Scholar] [CrossRef]
- Garg, S.; Tambwekar, K.R.; Vermani, K.; Kandarapu, R.; Garg, A.; Waller, D.P.; Zaneveld, L.J. Development pharmaceutics of microbicide formulations. Part II: Formulation, evaluation, and challenges. AIDS Patient Care STDs 2003, 17, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, A.D.; Malcolm, R.K.; Gallagher, R. Drug delivery by the intravaginal route. Crit. Rev. Ther. Drug Carr. Syst. 2000, 17, 509–555. [Google Scholar] [CrossRef]
- Baloglu, E.; Senyigit, Z.A.; Karavana, S.Y.; Bernkop-Schnurch, A. Strategies to prolong the intravaginal residence time of drug delivery systems. J. Pharm. Pharm. Sci. 2009, 12, 312–336. [Google Scholar] [CrossRef]
- Ozyazici, M.; Gökçe, E.; Hizarcioglu, S.Y.; Taner, M.S.; Köseoglu, K.; Ertan, G. Dissolution and vaginal absorption characteristics of metronidazole and ornidazole. Pharmazie 2006, 61, 855–861. [Google Scholar]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Wong, T.W.; Dhanawat, M.; Rathbone, M.J. Vaginal drug delivery: Strategies and concerns in polymeric nanoparticle development. Expert Opin. Drug Deliv. 2014, 11, 1419–1434. [Google Scholar] [CrossRef]
- Farage, M.; Maibach, H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obs. 2006, 273, 195–202. [Google Scholar] [CrossRef]
- Burruano, B.T.; Schnaare, R.L.; Malamud, D. Synthetic cervical mucus formulation. Contraception 2002, 66, 137–140. [Google Scholar] [CrossRef]
- Katz, D.F. Human cervical mucus: Research update. Am. J. Obstet. Gynecol. 1986, 165, 1984–1986. [Google Scholar] [CrossRef]
- Zavos, P.M.; Cohen, M.R. The pH of cervical mucus and the postcoital test. Fertil. Steril. 1980, 34, 234–238. [Google Scholar] [CrossRef]
- Larsen, B.; Monif, G.R. Understanding the bacterial flora of the female genital tract. Clin. Infect. Dis. 2001, 32, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, 120.e1–120.e5. [Google Scholar] [CrossRef] [PubMed]
- Nami, Y.; Haghshenas, B.; Khosroushahi, A.Y. Molecular Identification and Probiotic Potential Characterization of Lactic Acid Bacteria Isolated from Human Vaginal Microbiota. Adv. Pharm. Bull. 2018, 8, 683–695. [Google Scholar] [CrossRef]
- Lamont, R.F.; Sobel, J.D.; Akins, R.A.; Hassan, S.S.; Chaiworapongsa, T.; Kusanovic, J.P.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG 2011, 118, 533–549. [Google Scholar] [CrossRef] [Green Version]
- Medaglini, D.; Rush, C.M.; Sestini, P.; Pozzi, G. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: Vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 1997, 15, 1330–1337. [Google Scholar] [CrossRef]
- Liu, X.; Lagenaur, L.A.; Simpson, D.A.; Essenmacher, K.P.; Frazier-Parker, C.L.; Liu, Y.; Tsai, D.; Rao, S.S.; Hamer, D.H.; Parks, T.P.; et al. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob. Agents Chemother. 2006, 50, 3250–3259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.L.; Illum, L. Routes of delivery: Case studies. The vaginal route of peptide and protein drug delivery. Adv. Drug Deliv. Rev. 1992, 8, 341–366. [Google Scholar] [CrossRef]
- Olmsted, S.S.; Meyn, L.A.; Rohan, L.C.; Hillier, S.L. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex. Trans. Dis. 2003, 30, 257–261. [Google Scholar] [CrossRef]
- Buckheit, R.W.; Watson, K.M.; Morrow, K.M.; Ham, A.S. Development of topical microbicides to prevent the sexual transmission of HIV. Antivir. Res. 2010, 85, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Khanna, N.; Dalby, R.; Tan, M.; Arnold, S.; Stern, J.; Frazer, N. Phase I/II clinical safety studies of terameprocol vaginal ointment. Gynecol. Oncol. 2007, 107, 554–562. [Google Scholar] [CrossRef]
- Khanna, N.; Dalby, R.; Connor, A.; Church, A.; Stern, J.; Frazer, N. Phase I clinical trial of repeat dose terameprocol vaginal ointment in healthy female volunteers. Sex. Transm. Dis. 2008, 35, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.N.; Hendrix, C.W.; Chaturvedula, A. Population pharmacokinetics of tenofovir and tenofovir-diphosphate in healthy women. J. Clin. Pharmacol. 2015, 55, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Laeyendecker, O.; Redd, A.D.; Nason, M.; Longosz, A.F.; Karim, Q.A.; Naranbhai, V.; Garrett, N.; Eshleman, S.H.; Abdool Karim, S.S.; Quinn, T.C. Antibody maturation in women who acquire HIV infection while using antiretroviral preexposure prophylaxis. J. Infect. Dis. 2015, 212, 754–759. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2015, 372, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.R.; Peet, M.M.; Davis, S.; Doncel, G.F.; Friend, D.R. Evaluation of rapidly disintegrating vaginal tablets of Tenofovir, Emtricitabine and their combination for HIV-1 prevention. Pharmaceutics 2014, 6, 616–631. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Sánchez, M.P.; Martín-Illana, A.; Ruiz-Caro, R.; Bermejo, P.; Abad, M.J.; Carro, R.; Bedoya, L.M.; Tamayo, A.; Rubio, J.; Fernández-Ferreiro, A.; et al. Chitosan and Kappa-Carrageenan vaginal acyclovir formulations for prevention of genital herpes. In vitro and ex vivo evaluation. Mar. Drugs 2015, 13, 5976–5992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnyk, G.; Yarnykh, T.; Herasymova, I. Analytical review of the modern range of suppository bases. Syst. Rev. Pharm. 2020, 11, 503–508. [Google Scholar]
- Akil, A.; Agashe, H.; Dezzutti, C.S.; Moncla, B.J.; Hillier, S.L.; Devlin, B.; Shi, Y.; Uranker, K.; Rohan, L.C. Formulation and characterization of polymeric films containing combinations of antiretrovirals (ARVs) for HIV prevention. Pharm. Res. 2015, 32, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.J.; Gupta, K.M.; Fabian, J.; Albright, T.H.; Kiser, P.F. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 2010, 39, 203–212. [Google Scholar] [CrossRef]
- Smith, J.M.; Srinivasan, P.; Teller, R.S.; Lo, Y.; Dinh, C.T.; Kiser, P.F.; Herold, B.C. Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate- treated macaques from multiple SHIV exposures. J. Acquir. Immune Defic. Syndr. 2015, 68, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, P.; Dinh, C.; Zhang, J.; Pau, C.P.; McNicholl, J.M.; Lo, Y.; Herold, B.C.; Teller, R.; Kiser, P.; Smith, J.M. Pharmacokinetic evaluation of tenofovir disoproxil fumarate released from an intravaginal ring in pigtailed macaques after 6 months of continuous use. J. Med. Primatol. 2014, 43, 364–369. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Cazorla-Luna, R.; Notario-Pérez, F.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Chitosan-based mucoadhesive vaginal tablets for controlled release of the anti-HIV drug tenofovir. Pharmaceutics 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, M.T.; Ruiz, M.A.; Castán, H.; Morales, M.E. A novel double-layer mucoadhesive tablet containing probiotic strain for vaginal administration: Design, development and technological evaluation. Eur. J. Pharm. Sci. 2018, 112, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lupo, N.; Fodor, B.; Muhammad, I.; Yaqoob, M.; Matuszczak, B.; Bernkop-Schnürch, A. Entirely S-protected chitosan: A promising mucoadhesive excipient for metronidazole vaginal tablets. Acta Biomater. 2017, 64, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.R.; Shivakumar, H.G. Bioadhesive controlled release clotrimazole vaginal tablets. Trop. J. Pharm. Res. 2010, 9, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Acarturk, F. Mucoadhesive Vaginal Drug Delivery Systems. Recent Pat. Drug Deliv. 2009, 3, 193–205. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food A Critical Review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Reddy, R.S.; Kumar, L.; Pydi, C.R.; Reddy, M.S.; Verma, R. Development of fluconazole suppositories for the treatment of candida infection of genitourinary tract. Indian J. Pharm. Educ. Res. 2018, 52, S16–S22. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Li, X.; Mao, L.; Xiong, J.; Gao, C.; Shen, H.; Wang, L.; Zhu, D.; Ding, W.; Wang, H. An effective and biocompatible polyethylenimine based vaginal suppository for gene delivery. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101994. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Mohd Amin, M.C.; Zulfakar, M.H. Development and physical characterization of polymer-fish oil bigel (hydrogel/oleogel) system as a transdermal drug delivery vehicle. J. Oleo Sci. 2014, 63, 961–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagiri, S.S.; Singh, V.K.; Kulanthaivel, S.; Banerjee, I.; Basak, P.; Battachrya, M.K.; Pal, K. Stearate organogel-gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J. Mech. Behav. Biomed. Mater. 2015, 43, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Coggins, C.; Elias, C.J.; Atisook, R.; Bassett, M.T.; Ettiègne-Traoré, V.; Ghys, P.D.; Jenkins-Woelk, L.; Thongkrajai, E.; VanDevanter, N.L. Women’s preferences regarding the formulation of over-the-counter vaginal spermicides. AIDS 1998, 12, 1389–1391. [Google Scholar] [CrossRef] [Green Version]
- Hardy, E.; Jiménez, A.L.; de Padua, K.S.; Zaneveld, L.J.D. Women’s preferences for vaginal antimicrobial contraceptives III: Choice of a formulation, applicator, and packaging. Contraception 1998, 58, 245–249. [Google Scholar] [CrossRef]
- Rosen, R.K.; Morrow, K.M.; Carballo-Diéguez, A.; Mantell, J.E.; Hoffman, S.; Gai, F.; Maslankowski, L.; El-Sadr, W.M.; Mayer, K.H. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: A mixed-methods study. J. Women’s Health 2008, 17, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Tien, D.; Schnaare, R.L.; Kang, F.; Cohl, G.; McCormick, T.J.; Moench, T.R.; Doncel, G.; Watson, K.; Buckheit, R.W.; Lewis, M.G.; et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res. Hum. Retrovir. 2005, 21, 845–853. [Google Scholar] [CrossRef]
- Forbes, C.J.; Lowry, D.; Geer, L.; Veazey, R.S.; Shattock, R.J.; Klasse, P.J.; Mitchnick, M.; Goldman, L.; Doyle, L.A.; Muldoon, B.C.; et al. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J. Control. Release 2011, 156, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, C.J.; McCoy, C.F.; Murphy, D.J.; Woolfson, A.D.; Moore, J.P.; Evans, A.; Shattock, R.J.; Malcolm, R.K. Modified silicone elastomer vaginal gels for sustained release of antiretroviral HIV microbicides. J. Pharm. Sci. 2014, 103, 1422–1432. [Google Scholar] [CrossRef] [Green Version]
- Jespers, V.A.; Van Roey, J.M.; Beets, G.I.; Buve, A.M. Dose-ranging Phase I study of TMC120, a promising vaginal microbicide, in HIV-negative and HIV-positive female volunteers. J. Acquir. Immune Defic. Syndr. 2007, 44, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Lacey, C.J.; Woodhall, S.; Qi, Z.; Sawant, S.; Cowen, M.; McCormack, S.; Jiang, S. Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase I trial. Int. J. STD AIDS 2010, 21, 714–717. [Google Scholar] [CrossRef]
- Nel, A.M.; Coplan, P.; van de Wijgert, J.H.; Kapiga, S.H.; von Mollendorf, C.; Geubbels, E.; Vyankandondera, J.; Rees, H.V.; Masenga, G.; Kiwelu, I.; et al. Safety, tolerability, and systemic absorption of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS 2009, 23, 1531–1538. [Google Scholar] [CrossRef]
- Nel, A.M.; Smythe, S.C.; Habibi, S.; Kaptur, P.E.; Romano, J.W. Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs. Hydroxyethyl cellulose-based universal placebo gel. J. Acquir. Immune Defic. Syndr. 2010, 55, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Kovalevsky, G.; Lai, J.J.; Ballagh, S.A.; McCormick, T.; Douville, K.; Mauck, C.K.; Callahan, M.M. A randomized six-day safety study of an antiretroviral microbicide candidate UC781, a non-nucleoside reverse transcriptase inhibitor. Sex. Transm. Dis. 2008, 35, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: Development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control. Release 2004, 94, 177–186. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Gibin, S.; Caramella, C. Chitosan citrate as multifunctional polymer for vaginal delivery. Evaluation of penetration enhancement and peptidase inhibition properties. Eur. J. Pharm. Sci. 2008, 33, 166–176. [Google Scholar] [CrossRef]
- Martín-Illana, A.; Cazorla-Luna, R.; Notario-Pérez, F.; Bedoya, L.M.; Ruiz-Caro, R.; Veiga, M.D. Freeze-dried bioadhesive vaginal bigels for controlled release of Tenofovir. Eur. J. Pharm. Sci. 2019, 127, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Saikia, K.; Kalita, B. Formulation and evaluation of metronidazole microspheres-loaded bioadhesive vaginal gel. Asian J. Pharm. Clin. Res. 2017, 10, 418–424. [Google Scholar] [CrossRef]
- Regev, G.; Patel, S.K.; Moncla, B.J.; Twist, J.; Devlin, B.; Rohan, L.C. Novel Application of Hot Melt Extrusion for the Manufacturing of Vaginal Films Containing Microbicide Candidate Dapivirine. AAPS PharmSciTech 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Ham, A.S.; Rohan, L.C.; Boczar, A.; Yang, L.; Buckheit, K.W.; Buckheit, R.W. Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm. Res. 2012, 29, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Vermani, K.; Garg, A.; Anderson, R.A.; Rencher, W.B.; Zaneveld, L.J.D. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm. Res. 2005, 22, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.L.; Svetaz, L.A.; Alvarez, V.; Quiroga, A.D.; Lamas, M.C.; Leonardi, D. Chitosan-hydroxypropyl methylcellulose tioconazole films: A promising alternative dosage form for the treatment of vaginal candidiasis. Int. J. Pharm. 2019, 556, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Martín-Illana, A.; Chinarro, E.; Cazorla-Luna, R.; Notario-Perez, F.; Veiga-Ochoa, M.D.; Rubio, J.; Tamayo, A. Optimized hydration dynamics in mucoadhesive xanthan-based trilayer vaginal films for the controlled release of tenofovir. Carbohydr. Polym. 2022, 278, 118958. [Google Scholar] [CrossRef]
- Calvo, N.L.; Tejada, G.; Svetaz, L.A.; Quiroga, A.D.; Alvarez, V.A.; Lamas, M.C.; Leonardi, D. Development and optimization of a new tioconazole vaginal mucoadhesive film using an experimental design strategy. Physicochemical and biological characterization. J. Pharm. Biomed. Anal. 2021, 205, 114303. [Google Scholar] [CrossRef]
- Jalil, A.; Asim, M.H.; Le, N.M.N.; Laffleur, F.; Matuszczak, B.; Tribus, M.; Bernkop-Schnürch, A. S-protected gellan gum: Decisive approach towards mucoadhesive antimicrobial vaginal films. Int. J. Biol. Macromol. 2019, 130, 148–157. [Google Scholar] [CrossRef]
- McBride, J.W.; Boyd, P.; Dias, N.; Cameron, D.; Offord, R.E.; Hartley, O.; Kett, V.L.; Malcolm, R.K. Vaginal rings with exposed cores for sustained delivery of the HIV CCR5 inhibitor 5P12-RANTES. J. Control. Release 2019, 298, 1–11. [Google Scholar] [CrossRef]
- Tietz, K.; Klein, S. In Vitro Methods for Evaluating Drug Release of Vaginal Ring Formulations—A Critical Review. Pharmaceutics 2019, 11, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstraelen, H.; Vervaet, C.; Remon, J.P. Rationale and safety assessment of a novel intravaginal drug-delivery system with sustained DL-lactic acid release, intended for long-term protection of the vaginal microbiome. PLoS ONE 2016, 11, 1–15. [Google Scholar]
- Gupta, V.K.; Assmus, M.W.; Beckert, T.E.; Price, J.C. A novel pH- and time-based multi-unit potential colonic drug delivery system. II. Optimization of multiple response variables. Int. J. Pharm. 2001, 213, 93–102. [Google Scholar] [CrossRef]
- Santos, S.S.; Lorenzoni, A.; Ferreira, L.M.; Mattiazzi, J.; Adams, A.I.H.; Denardi, L.B.; Alves, S.H.; Schaffazick, S.R.; Cruz, L. Clotrimazole-loaded Eudragit® RS100 nanocapsules: Preparation, characterization and in vitro evaluation of antifungal activity against Candida species. Mater. Sci. Eng. C 2013, 33, 1389–1394. [Google Scholar] [CrossRef]
- de Lima, J.A.; Paines, T.C.; Motta, M.H.; Weber, W.B.; dos Santos, S.S.; Cruz, L.; da Silva, C.D.B. Novel Pemulen/Pullulan blended hydrogel containing clotrimazole-loaded cationic nanocapsules: Evaluation of mucoadhesion and vaginal permeation. Mater. Sci. Eng. C 2017, 79, 886–893. [Google Scholar] [CrossRef]
- Yoo, J.; Giri, N.; Lee, C.H. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int. J. Pharm. 2011, 403, 262–267. [Google Scholar] [CrossRef]
- Zhang, T.; Sturgis, T.F.; Youan, B.C. European Journal of Pharmaceutics and Biopharmaceutics pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur. J. Pharm. Biopharm. 2011, 79, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Brako, F.; Raimi-Abraham, B.T.; Mahalingam, S.; Craig, D.Q.M.; Edirisinghe, M. The development of progesterone-loaded nanofibers using pressurized gyration: A novel approach to vaginal delivery for the prevention of pre-term birth. Int. J. Pharm. 2018, 540, 31–39. [Google Scholar] [CrossRef]
- Ham, A.S.; Cost, M.R.; Sassi, A.B.; Dezzutti, C.S.; Rohan, L.C. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm. Res. 2009, 26, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Gu, K.; Dash, A.; Sayre, C.L.; Davies, N.M.; Ho, E.A. Novel intravaginal nanomedicine for the targeted delivery of saquinavir to CD4 + immune cells. Int. J. Nanomed. 2013, 8, 2847–2858. [Google Scholar]
- Cunha-Reis, C.; Machado, A.; Barreiros, L.; Araújo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; Segundo, M.A.; Sarmento, B.; das Neves, J. Nanoparticles-in-film for the combined vaginal delivery of anti-HIV microbicide drugs. J. Control. Release 2016, 243, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Shibata, A.; Goede, M.; Sanford, B.; La, K.; Belshan, M.; Destache, C.J. Development and evaluation of a thermosensitive vaginal gel containing raltegravir + efavirenz loaded nanoparticles for HIV prophylaxis. Antivir. Res. 2012, 96, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogstad, E.A.; Ramanathan, R.; Nhan, C.; Kraft, J.C.; Blakney, A.K.; Cao, S.; Ho, R.J.Y.; Woodrow, K.A. Nanoparticle-releasing nanofiber composites for enhanced in vivo vaginal retention. Biomaterials 2017, 144, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Ahmad, S.; Mallick, M.N.; Manzoor, N.; Talegaonkar, S.; Iqbal, Z. Development of a novel synergistic thermosensitive gel for vaginal candidiasis: An in vitro, in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A vaginal drug delivery model. Drug Deliv. 2016, 23, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and characterization of chitosan nanoparticles loaded nanofiber hybrid system for vaginal controlled release of benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801. [Google Scholar] [CrossRef] [PubMed]

| Formualtion/ Release Rate * | API(s) | Excipients Used | Reference |
|---|---|---|---|
| Solid dosage forms: tablets | |||
| controlled | tenofovir | CS, pectin, locust bean gum, MS | [45] |
| fast and extended (double layer) | lactobacillus cells | lactose monohydrate, maize starch, ascorbic acid, stearic acid, NaCMC (1500–4500), sodium citrate dehydrate, glucose anhydrous, talc, MS, carbopol® 934, CS | [46] |
| controlled | metronidazol | CS-NAC-MNA (S-protected CS) | [47] |
| controlled | clotrimazole | HPMC, NaCMC, GG, MS, dicalcium phosphate | [48] |
| Solid dosage forms: suppositories | |||
| sustained | floconazole | HPMC K100, agar, PG, gelatin, glycerin, cocoa butter, bees wax | [51] |
| sustained | genes HeLa and HEK293 | PEI (MW: 25 kDa), Suppocire® BM pellets, polysorbate 80 | [52] |
| Semi-solid dosage forms: gels | |||
| ≈6 h | acyclovir, ciprofloxacin HCl | CS citrate (medium MW and acetylation degree of 10%) | [69] |
| controlled | tenofovir | GG, sesame oil, sorbitan monostearate 60, polysorbate 60 | [70] |
| sustained | metronidazole | HPMC K4M, Carbopol 934P, PCL, triethanolamine, PVA | [71] |
| Films | |||
| >60 min | dapivirine | PEO N10 and N80, HPC, PEG 400, PEG 4000, vitamin E acetate | [72] |
| >60 min | pyrimidinedione IQP-0528 | PVA-403, glycerin, PEG 400, HPMC | [73] |
| extended | PSS | PVA (MW: 30–70 kDa, 89–98 kDa) HEC, HPMC K4M, sorbitol, PEG 600, PG, triacetin | [74] |
| controlled | tioconazole | CS (MW: 230 kDa), HPMC K15M, liquid and solid vaseline, PEG 400 | [75] |
| controlled | tenofovir | karaya gum, pectin, xanthan gum, CS (viscosity: 37 mPa⋅S, degree of N- deacetylation: 54.7 ± 4.2%), ethylcellulose | [76] |
| extended | tioconazole | HPMC (MW ∼ 250 kDa), CS (MW ∼ 230 KDa; 80.6% of N-deacetylation), PEG 400, PG | [77] |
| prolonged in situ gelling system | metronidazole | S-protected gellan gum: cysteamine and 2-MNA (2-mercaptonicotinic acid) | [78] |
| Devices: ring | |||
| 7 days | DL-Lactic acid | EVA 28, Eudragit® L100 | [81] |
| Nanomedicine | |||
| prolonged cationic nanocapsules | clotrimazole | Eudragit® RS100, sorbitan monooleate 80, polysorbate 80 | [83] |
| prolonged hydrogels containing clotrimazole-loaded nanocapsules | clotrimazole | Pemulen® TR1, pullulan, Eudragit® RS100, sorbitan monooleate 80, polyssorbate 80, methylparaben and propylparaben, triethanolamine, imidazolidinyl urea | [84] |
| controlled pH-sensitive NPs | model compounds: sodium fluorescein and nile red | Eudragit® S100, PVA (MW: 30–70 kDa) | [85] |
| controlled pH-responsive NPs | tenofovir | PLGA (MW: 76-116 kDa), Eudragit® S100, poloxamer 407 | [86] |
| prolonged mucoadhesive nanofibers | progesterone | NaCMC (MW: ~250 kDa), PEO (MW: ~200 kDa) | [87] |
| controlled NPs | PSC-RANTES | PLGA | [88] |
| sustained release NPs | saquinavir | PLGA, PVA (MW: 31–50 kDa), MES, coumarin-6, EDC, NHS | [89] |
| prolonged NPs in film | tenofovir and efavirenz | PLGA (MW: ~17 kDa), poloxamer 407, HPMC E4M, PVA (MW: 30–70 kDa) | [90] |
| sustained release NPs loaded gel | raltegravir and efavirenz | PLGA, poloxamer 407, poloxamer 188, PVA (MW: 88 kDa) | [91] |
|
| [92] | |
| prolonged muco-and bio-adhesion thermosensitive gel (nanoemulsion) | itraconazole, tea tree oil | poloxamer 407, PEG-8 caprylic/ capric glycerides, triisostearin PEG-6 esters, ethoxydiglycol | [93] |
| prolonged mucoadhesive and thermosensitive gel SLNs | itraconazole | poloxamer 188 (MW: 162.23 Da), stearic acid, compritol 888 (1059.8 Da), sodium taurocholate | [94] |
| controlled release nanoparticles, loaded nanofiber hybrid system | benzydamide | PVP, CS (MW: 50,000–190,000 Da), HPMC K100M | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedeloudi, A.; Siamidi, A.; Pavlou, P.; Vlachou, M. Recent Advances in the Excipients Used in Modified Release Vaginal Formulations. Materials 2022, 15, 327. https://doi.org/10.3390/ma15010327
Dedeloudi A, Siamidi A, Pavlou P, Vlachou M. Recent Advances in the Excipients Used in Modified Release Vaginal Formulations. Materials. 2022; 15(1):327. https://doi.org/10.3390/ma15010327
Chicago/Turabian StyleDedeloudi, Aikaterini, Angeliki Siamidi, Panagoula Pavlou, and Marilena Vlachou. 2022. "Recent Advances in the Excipients Used in Modified Release Vaginal Formulations" Materials 15, no. 1: 327. https://doi.org/10.3390/ma15010327







