The Effect of Reactive Electric Field-Assisted Sintering of MoS2/Bi2Te3 Heterostructure on the Phase Integrity of Bi2Te3 Matrix and the Thermoelectric Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Hexagonal Nanoplatelets of Bi2Te3 and Bi2Te3/MoS2 Heterostructures
2.2. Synthesis of Bi2Te3/X mol%MoS2 Bulk Samples
2.3. Chemical and Structural Characterization
2.4. Physical Property Measurements
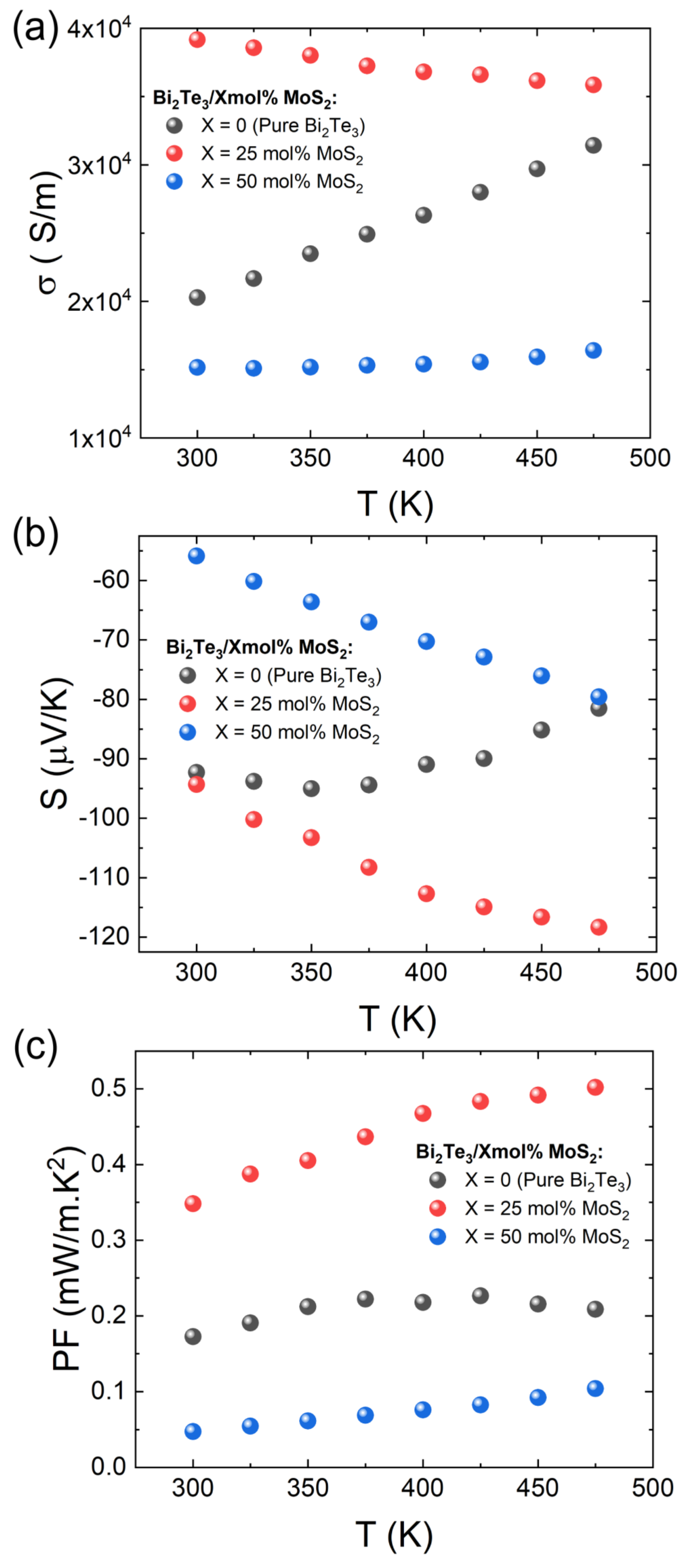
3. Results and Discussion
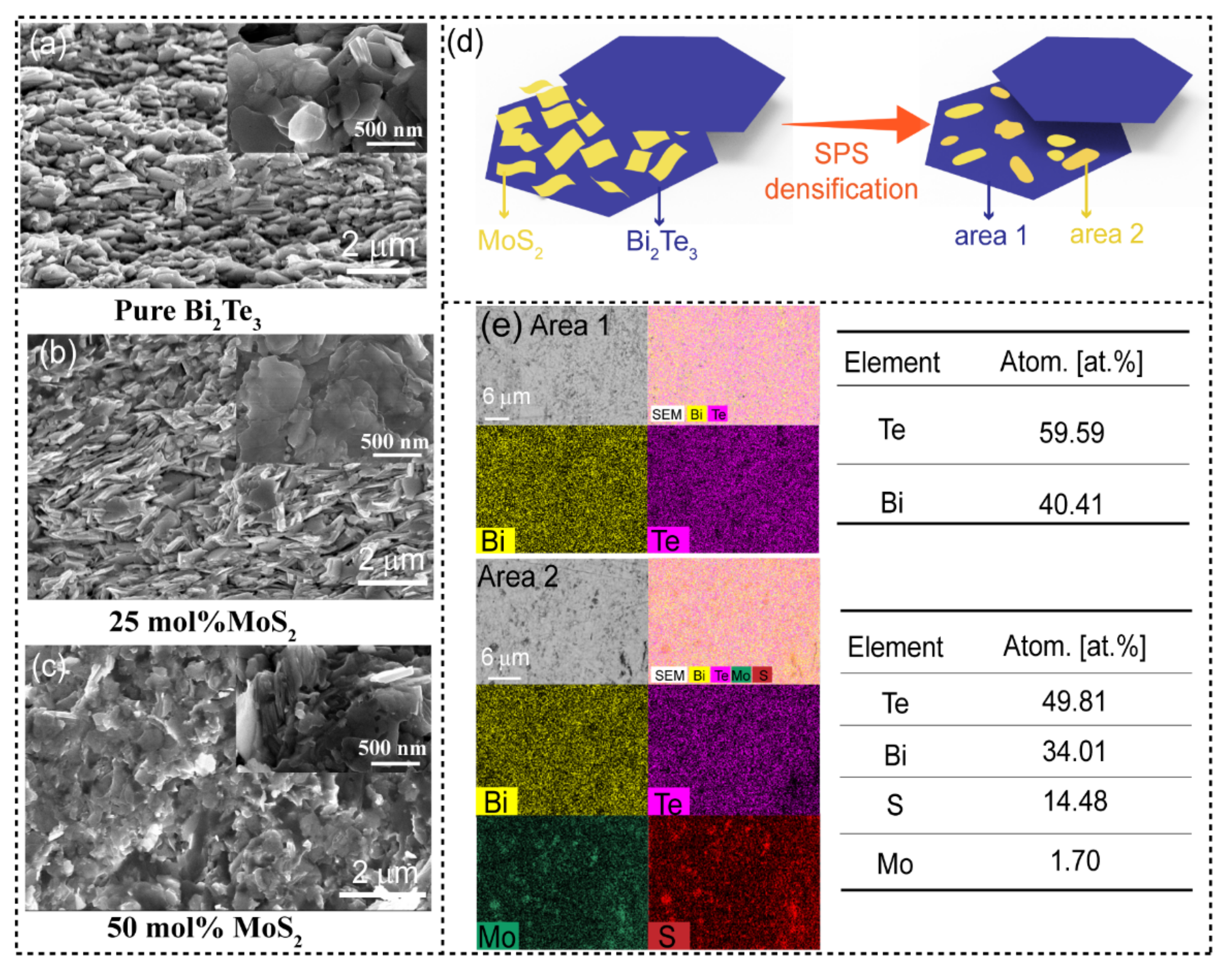
Microstructure and Chemical Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mori, T.; Priya, S. Materials for energy harvesting: At the forefront of a new wave. MRS Bull. 2018, 43, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.E. Cooling, Heating, Generating Power, and Recovering Waste Heat with Thermoelectric Systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Sato, N.; Gao, W.; Yubuta, K.; Kawamoto, N.; Mitome, M.; Kurashima, K.; Owada, Y.; Nagase, K.; Lee, C.-H.; et al. Demonstration of Ultrahigh Thermoelectric Efficiency of ∼7.3% in Mg3Sb2/MgAgSb Module for Low-Temperature Energy Harvesting. Joule 2021, 5, 1196–1208. [Google Scholar] [CrossRef]
- Mori, T. Novel Principles and Nanostructuring Methods for Enhanced Thermoelectrics. Small 2017, 13, 1702013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-Performance Bulk Thermoelectrics with All-Scale Hierarchical Architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Tang, X.; Yan, Y.; Zhang, Q.; Tritt, T.M. Unique Nanostructures and Enhanced Thermoelectric Performance of Melt-spun BiSbTe alloys. Appl. Phys. Lett. 2009, 94, 102111. [Google Scholar] [CrossRef]
- Jaworski, C.M.; Kulbachinskii, V.; Heremans, J.P. Resonant Level formed by Tin in Bi2Te3 and the Enhancement of Room-Temperature Thermoelectric Power. Phys. Rev. B 2009, 80, 233201. [Google Scholar] [CrossRef]
- Sawicki, B.; Karolewicz, M.; Tomaszewicz, E.; Oboz, M.; Gron, T.; Kukula, Z.; Pawlus, S.; Nowok, A.; Duda, H. Effect of Gd3+ Substitution on Thermoelectric Power Factor of Paramagnetic Co2+-Doped Calcium Molybdato-Tungstates. Materials 2021, 14, 3692. [Google Scholar] [CrossRef] [PubMed]
- Poudeu, P.F.; D’Angelo, J.; Downey, A.D.; Short, J.L.; Hogan, T.P.; Kanatzidis, M.G. High Thermoelectric Figure of Merit and Nanostructuring in Bulk p-type Na1-xPbmSbyTem+2. Angew. Chem. Int. Ed. Engl. 2006, 45, 3835–3839. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, A.; Guo, Q.; Nicolosi, V.; Mori, T. Enhanced thermoelectric performance of Bi–Sb–Te/Sb2O3 nanocomposites by energy filtering effect. J. Mater. Chem. A 2018, 6, 21341–21349. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of Electronic Bands for High Performance Bulk Thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef]
- Vaney, J.B.; Aminorroaya Yamini, S.; Takaki, H.; Kobayashi, K.; Kobayashi, N.; Mori, T. Magnetism-Mediated Thermoelectric Performance of the Cr-doped Bismuth Telluride Tetradymite. Mater. Today Phys. 2019, 9, 100090. [Google Scholar] [CrossRef]
- Naohito, T.; Akinori, N.; Jun, H.; Takao, M. Observation of Enhanced Thermopower due to Spin Fluctuation in Weak Itinerant Ferromagnet. Sci. Adv. 2019, 5, eaat5935. [Google Scholar]
- Liu, W.; Yan, X.; Chen, G.; Ren, Z. Recent Advances in Thermoelectric Nanocomposites. Nano Energy 2012, 1, 42–56. [Google Scholar] [CrossRef]
- Mori, T.; Hara, T. Hybrid Effect to Possibly Overcome the Trade-off Between Seebeck Coefficient and Electrical Conductivity. Scr. Mater. 2016, 111, 44–48. [Google Scholar] [CrossRef]
- Zianni, X. The annealed-nanograin phase: A Route to Simultaneous Increase of the Conductivity and the Seebeck Coefficient and High Thermoelectric Performance. J. Appl. Phys. 2019, 126, 194301. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liu, C.-J.; Mori, T. Polymer Based Thermoelectric Nanocomposite Materials and Devices: Fabrication and Characteristics. Nano Energy 2020, 78, 105186. [Google Scholar] [CrossRef]
- Madavali, B.; Sharief, P.; Park, K.-T.; Song, G.; Back, S.-Y.; Rhyee, J.-S.; Hong, S.-J. Development of High-Performance Thermoelectric Materials by Microstructure Control of P-Type BiSbTe Based Alloys Fabricated by Water Atomization. Materials 2021, 14, 4870. [Google Scholar] [CrossRef]
- Hines, M.; Lenhardt, J.; Lu, M.; Jiang, L.; Xiao, Z. Cooling Effect of Nanoscale Bi2Te3/Sb2Te3 Multilayered Thermoelectric Thin Films. J. Vac. Sci. Technol. A 2012, 30, 041509. [Google Scholar] [CrossRef]
- Saleemi, M.; Toprak, M.S.; Li, S.; Johnsson, M.; Muhammed, M. Synthesis, Processing, and Thermoelectric Properties of Bulk Nanostructured Bismuth Telluride (Bi2Te3). J. Mater. Chem. 2012, 22, 725–730. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Bismuth Telluride and Its Alloys as Materials for Thermoelectric Generation. Materials 2014, 7, 2577–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-Thermoelectric Performance of Nanostructured Bismuth Antimony Telluride Bulk Alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Cai, K.; Cui, J.; Yin, J.; Shen, S. Preparation and Thermoelectric Properties of MoS2/Bi2Te3 Nanocomposites. Ceram. Int. 2016, 42, 17972–17977. [Google Scholar] [CrossRef]
- Keshavarz, M.K.; Vasilevskiy, D.; Masut, R.A.; Turenne, S. Synthesis and Characterization of Bismuth Telluride-based Thermoelectric Nanocomposites Containing MoS2 Nano-inclusions. Mater. Charact. 2014, 95, 44–49. [Google Scholar] [CrossRef]
- Grasso, S.; Tsujii, N.; Jiang, Q.; Khaliq, J.; Maruyama, S.; Miranda, M.; Simpson, K.; Mori, T.; Reece, M.J. Ultra Low Thermal Conductivity of Disordered Layered P-Type Bismuth Telluride. J. Mater. Chem. C 2013, 1, 2362. [Google Scholar]
- Kim, C.; Lopez, D.H. Effects of the Interface between Inorganic and Organic Components in a Bi2Te3-Polypyrrole Bulk Composite on Its Thermoelectric Performance. Materials 2021, 14, 3080. [Google Scholar] [CrossRef]
- Kou, L.; Ma, Y.; Sun, Z.; Heine, T.; Chen, C. Two-Dimensional Topological Insulators: Progress and Prospects. J. Phys. Chem. Lett. 2017, 8, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E. The Birth of Topological Insulators. Nature 2010, 464, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Nethravathi, C.; Dattatreya Manganahalli, A.; Rajamathi, M. Bi2Te3–MoS2 Layered Nanoscale Heterostructures for Electron Transfer Catalysis. ACS Appl. Nano Mater. 2019, 2, 2005–2012. [Google Scholar] [CrossRef]
- Babaei, H.; Khodadadi, J.M.; Sinha, S. Large theoretical Thermoelectric Power Factor of Suspended Single-Layer MoS2. Appl. Phys. Lett. 2014, 105, 193901. [Google Scholar] [CrossRef]
- Wickramaratne, D.; Zahid, F.; Lake, R.K. Electronic and Thermoelectric Properties of Few-layer Transition Metal Dichalcogenides. J. Chem. Phys. 2014, 140, 124710. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Cui, Y.; Li, Q.; Dun, C.; Zhou, W.; Huang, W.; Chen, L.; Hewitt, C.A.; Carroll, D.L. Metallic 1T Phase MoS2 Nanosheets for High-Performance Thermoelectric Energy Harvesting. Nano Energy 2016, 26, 172–179. [Google Scholar] [CrossRef]
- Bourgès, C.; Rajamathi, R.; Nethravathi, C.; Rajamathi, M.; Mori, T. Induced 2H-Phase Formation and Low Thermal Conductivity by Reactive Spark Plasma Sintering of 1T-Phase Pristine and Co-Doped MoS2 Nanosheets. ACS Omega 2021, 48, 32783–32790. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z. Graphene-Analogous Low-Dimensional Materials. Prog. Mater. Sci. 2013, 58, 1244–1315. [Google Scholar] [CrossRef]
- Keshavarz, M.K.; Vasilevskiy, D.; Masut, R.A.; Turenne, S. Effect of Suppression of Grain Growth of Hot Extruded (Bi0.2Sb0.8)2Te3 Thermoelectric Alloys by MoS2 Nanoparticles. J. Electron. Mater. 2014, 43, 2239–2246. [Google Scholar] [CrossRef]
- Gangwar, P.; Kumar, S.; Khare, N. Ultrahigh Thermoelectric Performance of 2H–MoS2 Nanosheets with Incorporated Conducting Secondary Phase. Mater. Res. Express. 2019, 6, 105062. [Google Scholar] [CrossRef]
- Kim, I.; Sangwan, V.; Jariwala, D.; Wood, J.; Park, S.; Chen, K.; Shi, F.; Ruiz-Zepeda, F.; Ponce, A.; Jose-Yacaman, M.; et al. Influence of Stoichiometry on the Optical and Electrical Properties of Chemical Vapor Deposition Derived MoS2. ACS Nano 2014, 8, 10551–10558. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Hu, L.; Zhu, T.; Zhao, X. The texture related anisotropy of thermoelectric properties in bismuth telluride based polycrystalline alloys. Appl. Phys. Lett. 2011, 99, 124102. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B.; Li, J.; Zhang, H.; Liu, W. Enhanced thermoelectric and mechanical properties in textured n-type Bi2Te3 prepared by spark plasma sintering. Solid State Sci. 2008, 10, 651–658. [Google Scholar] [CrossRef]
- Bao, D.; Chen, J.; Yu, Y.; Liu, W.; Huang, L.; Han, G.; Tang, J.; Zhou, D.; Yang, L.; Chen, Z. Texture-dependent thermoelectric properties of nano-structured Bi2Te3. Chem. Eng. J. 2020, 388, 124295. [Google Scholar] [CrossRef]
- Solomon, G.; Song, E.; Gayner, C.; Martinez, J.A.; Amouyal, Y. Effects of Microstructure and Neodymium Doping on Bi2Te3 Nanostructures: Implications for Thermoelectric Performance. ACS Appl. Nano Mater. 2021, 4, 4419–4431. [Google Scholar] [CrossRef]
- Witting, I.T.; Chasapis, T.C.; Ricci, F.; Peters, M.; Heinz, N.A.; Hautier, G.; Snyder, G.J. The Thermoelectric Properties of Bismuth Telluride. Adv. Electron. Mater. 2019, 5, 1800904. [Google Scholar] [CrossRef]
- Grauer, D.C.; Hor, Y.S.; Williams, A.J.; Cava, R.J. Thermoelectric Properties of the Tetradymite-type Bi2Te2S–Sb2Te2S Solid Solution. Mater. Res. Bull. 2009, 44, 1926–1929. [Google Scholar] [CrossRef]
- Joo, S.-J.; Ryu, B.; Son, J.-H.; Lee, J.E.; Min, B.-K.; Kim, B.-S. Highly Anisotropic Thermoelectric Transport Properties Responsible for Enhanced Thermoelectric Performance in The Hot-deformed Tetradymite Bi2Te2S. J. Alloys Compd. 2019, 783, 448–454. [Google Scholar] [CrossRef]
- Fagerquist, R.L.; Kirby, R.D. Metastable conduction states in Mo2S3: Pulse Conductivity and Thermoelectric Power. Phys. Rev. B 1988, 38, 3973–3985. [Google Scholar] [CrossRef]
- Zhang, G.; Kirk, B.; Jauregui, L.A.; Yang, H.; Xu, X.; Chen, Y.P.; Wu, Y. Rational Synthesis of Ultrathin N-Type Bi2Te3 Nanowires with Enhanced Thermoelectric Properties. Nano Lett. 2012, 12, 56–60. [Google Scholar] [CrossRef]
- Zhang, C.; Ng, H.; Li, Z.; Khor, K.A.; Xiong, Q. Minority Carrier Blocking to Enhance the Thermoelectric Performance of Solution-Processed BixSb2-xTe3 Nanocomposites via a Liquid-Phase Sintering Process. ACS Appl. Mater. Interfaces 2017, 9, 12501–12510. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kuroda, N.; Nakamura, S.; Katsura, Y.; Kanazawa, I.; Kimura, K.; Mori, T. Bonding Heterogeneity in Mixed-Anion-compounds Realizes Ultralow Lattice Thermal Conductivity. J. Mater. Chem. A 2021, 9, 22660–22669. [Google Scholar] [CrossRef]
- Tao, Q.; Meng, F.; Zhang, Z.; Cao, Y.; Tang, Y.; Zhao, J.; Su, X.; Uher, C.; Tang, X. The Origin of Ultra-low Thermal Conductivity of the Bi2Te2S Compound and Boosting the Thermoelectric Performance Via Carrier Engineering. Mater. Today Phys. 2021, 20, 100472. [Google Scholar]


| Bi2Te3/X mol MoS2 Nanocomposite—Hall Effect at 300 K | |||
|---|---|---|---|
| X = 0 | X = 25 | X = 50 | |
| n (cm−3) | 8.94 × 1019 | 4.75 × 1019 | 3.20 × 1019 |
| μe (cm2 V−1s−1) | 10.90 | 51.50 | 29.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bourgès, C.; Rajamathi, R.; Nethravathi, C.; Rajamathi, M.; Mori, T. The Effect of Reactive Electric Field-Assisted Sintering of MoS2/Bi2Te3 Heterostructure on the Phase Integrity of Bi2Te3 Matrix and the Thermoelectric Properties. Materials 2022, 15, 53. https://doi.org/10.3390/ma15010053
Wang Y, Bourgès C, Rajamathi R, Nethravathi C, Rajamathi M, Mori T. The Effect of Reactive Electric Field-Assisted Sintering of MoS2/Bi2Te3 Heterostructure on the Phase Integrity of Bi2Te3 Matrix and the Thermoelectric Properties. Materials. 2022; 15(1):53. https://doi.org/10.3390/ma15010053
Chicago/Turabian StyleWang, Yanan, Cédric Bourgès, Ralph Rajamathi, C. Nethravathi, Michael Rajamathi, and Takao Mori. 2022. "The Effect of Reactive Electric Field-Assisted Sintering of MoS2/Bi2Te3 Heterostructure on the Phase Integrity of Bi2Te3 Matrix and the Thermoelectric Properties" Materials 15, no. 1: 53. https://doi.org/10.3390/ma15010053







