Clay Mineral Minerals as a Strategy for Biomolecule Incorporation: Amino Acids Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Amino Acids Incorporation
2.3. Characterizations
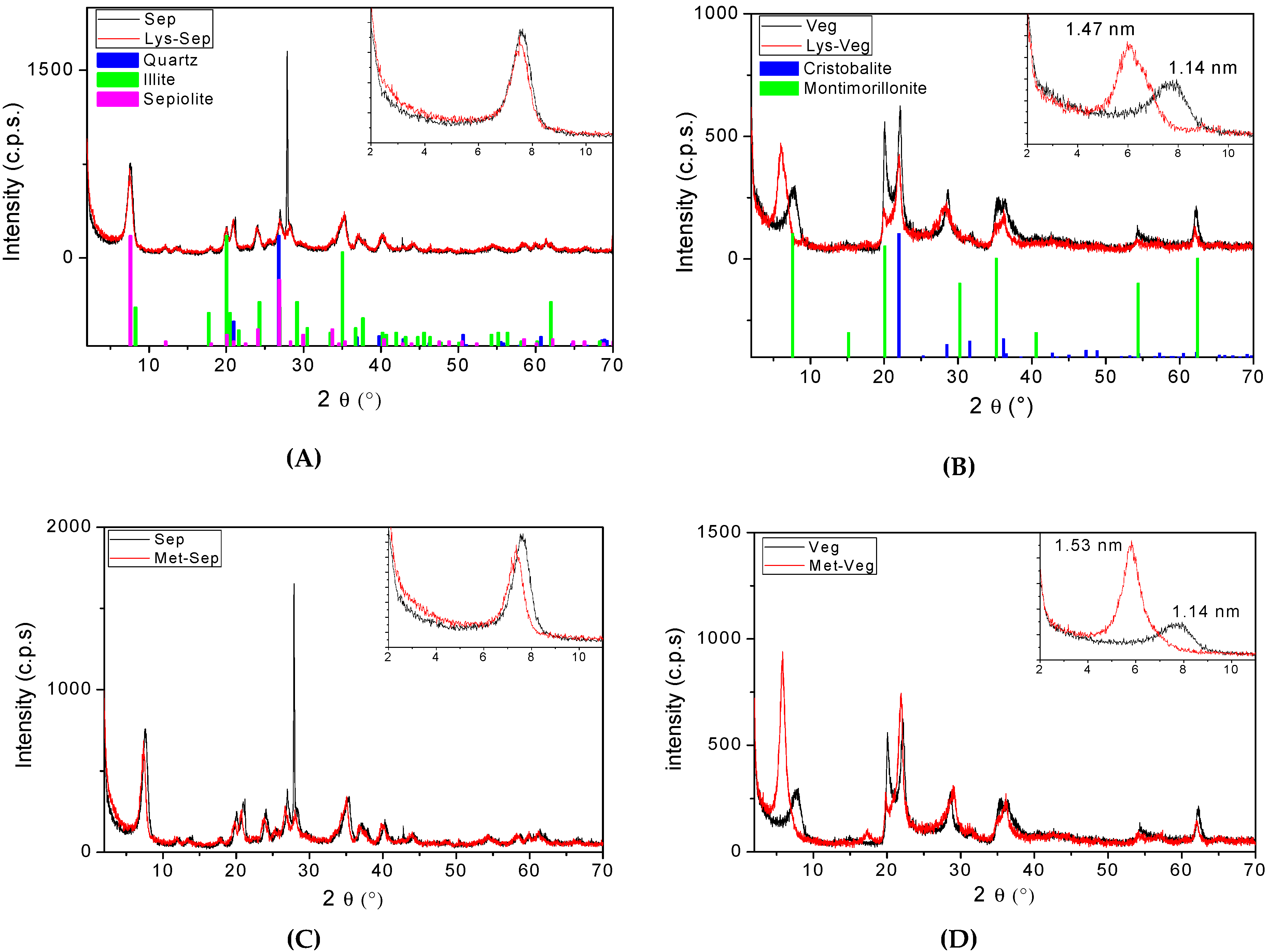
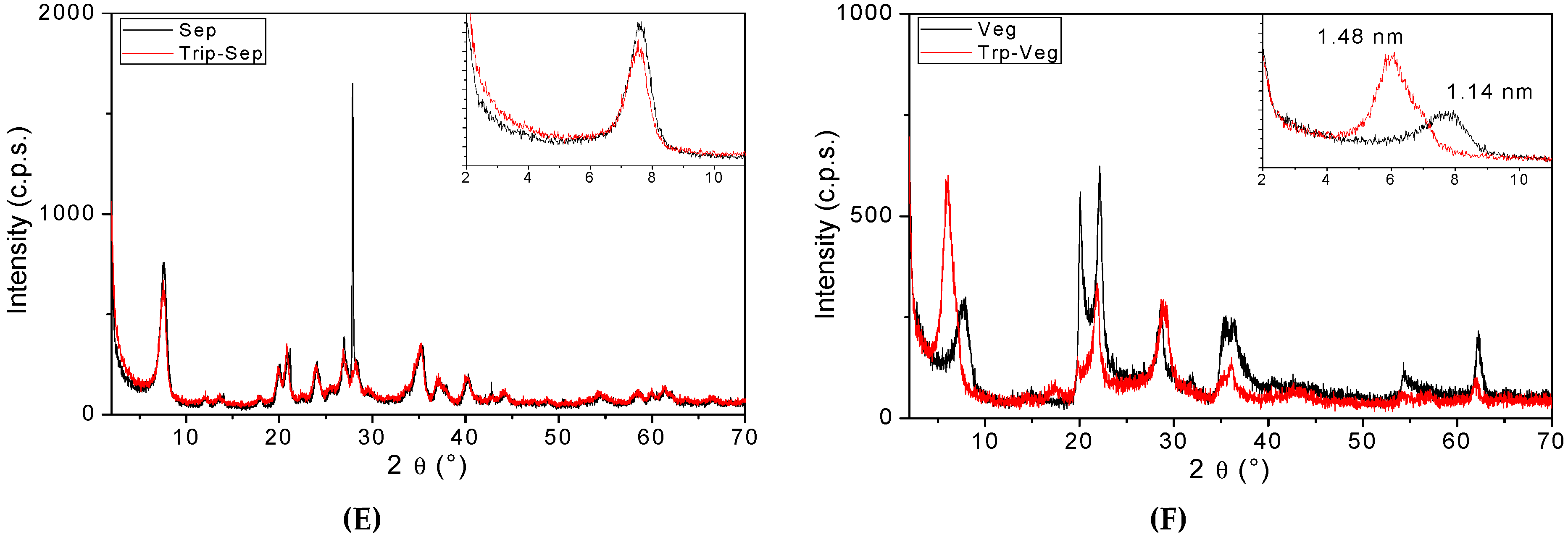
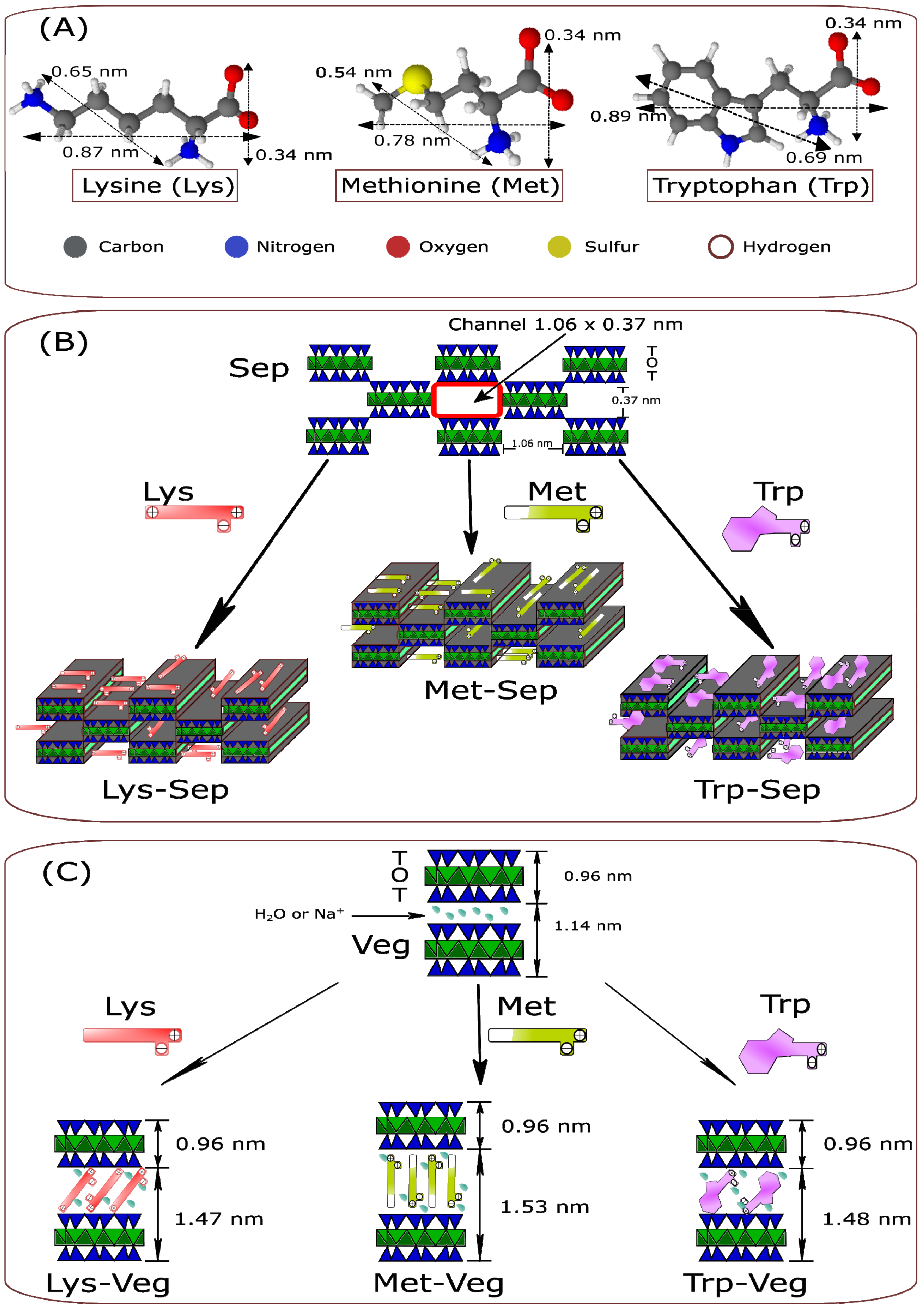
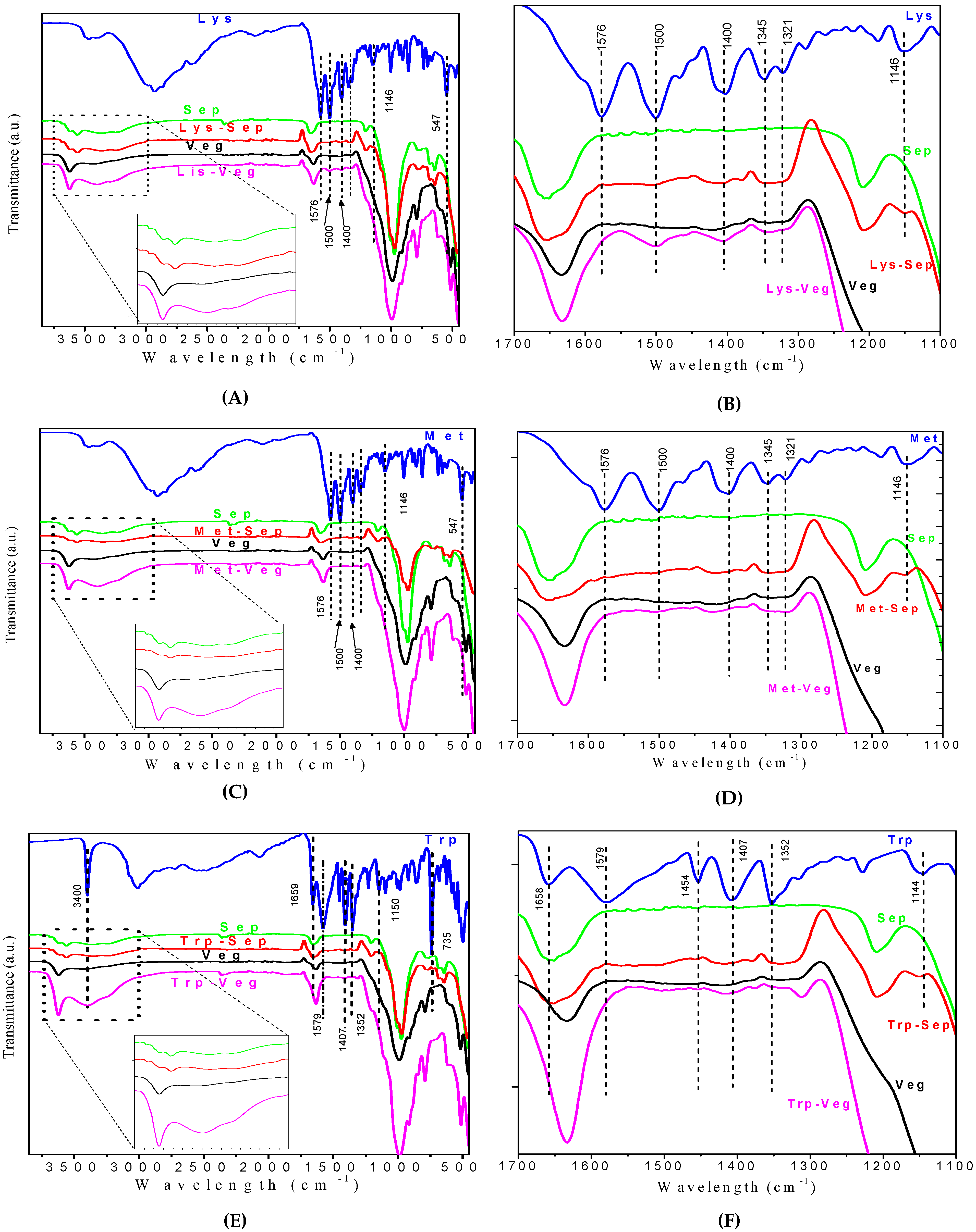
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suida, D. Formulação Por Proteína Ideal e Consequências Técnicas, Econômicas e Ambientais. In Proceedingsofthe Simpósio Internacional de Nutrição Animal: Proteína Ideal, Energia Líquida e Modelagem; Embrapa: Santa Maria, Brazil, 2001; pp. 27–43. [Google Scholar]
- Monteiro, A.N.T.R.; Dourmad, J.Y.; Pozza, P.C. Life Cycle Assessment as a Tool to Evaluate the Impact of Reducing Crude Protein in Pig Diets. Cienc. Rural 2017, 47, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zouaoui, M.; Létourneau-Montminy, M.P.; Guay, F. Effect of Phytase on Amino Acid Digestibility in Pig: A Meta-Analysis. Anim. Feed. Sci. Technol. 2018, 238, 18–28. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Fox, D.G.; Fonseca, M.A.; Cavalcanti, L.F.L. Models ofProteinand Amino AcidRequirements for Cattle. Rev. Bras. Zootec. 2015, 44, 109–132. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, C.; Bhatti, H.S.; Novoa-Garrido, M.; Lind, V.; Roleda, M.Y.; Weisbjerg, M.R. Amino Acid Profiles of Nine Seaweed Species and Their in Situ Degradability in Dairy Cows. Anim. Feed. Sci. Technol. 2018, 241, 210–222. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-47323-1. [Google Scholar]
- de Carvalho Neto, J.P.; Bezerra, L.R.; da Silva, A.L.; de Moura, J.F.P.; Pereira Filho, J.M.; da Silva Filho, E.C.; Guedes, A.F.; Araújo, M.J.; Edvan, R.L.; Oliveira, R.L. Methionine Microencapsulated with a Carnauba (Copernicia Prunifera) Wax Matrix for Protection from Degradation in the Rumen. Livest. Sci. 2019, 228, 53–60. [Google Scholar] [CrossRef]
- Ambre, A.; Katti, K.S.; Katti, D.R. In Situ Mineralized Hydroxyapatite on Amino Acid Modified Nanoclay minerals as Novel Bone Biomaterials. Mater. Sci. Eng. C 2011, 31, 1017–1029. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Preparation and Characterization of New Organoclay minerals Using Natural Amino Acids and Cloisite Na+. Appl. Clay Miner. Sci. 2011, 51, 353–359. [Google Scholar] [CrossRef]
- Moro, D.; Ulian, G.; Valdrè, G. Amino Acids-Clay mineral Interaction at the Nano-Atomic Scale: The l-Alanine-Chlorite System. Appl. Clay Miner. Sci. 2019, 172, 28–39. [Google Scholar] [CrossRef]
- Silva, F.D.C.; Lima, L.C.B.; Silva-filho, E.C.; Fonseca, M.G.; Lambert, J.; Jaber, M. A Comparative Study of Alanine Adsorption and Condensation to Peptides in Two Clay mineral Minerals. Appl. Clay Miner. Sci. 2020, 192, 105617. [Google Scholar] [CrossRef]
- Kohay, H.; Sarisozen, C.; Sawant, R.; Jhaveri, A.; Torchilin, V.P.; Mishae, Y.G. PEG-PE/clay composite carriers for doxorubicin: Effect of composite structure on release, cell interaction and cytotoxicity. Acta Biomater. 2017, 55, 443–454. [Google Scholar] [CrossRef]
- Cheikh, D.; García-Villén, F.; Majdoub, H.; Zayani, M.B.; Viseras, C. Complex of chitosan pectin and clay as diclofenac carrier. Appl. Clay Sci. 2019, 172, 155–164. [Google Scholar] [CrossRef]
- Sid, D.; Baitiche, M.; Arrar, L.; Djerboua, F.; Bourzami, R.; Alcouffe, P.; Boutahala, M.; Gil, A.; David, L.; Borgne, M.L. Improved biological performance of ketoprofen using novel modified halloysite clay nanotubes. Appl. Clay Sci. 2022, 216, 106341. [Google Scholar] [CrossRef]
- Swain, R.; Nandi, S.; Sahoo, R.N.; Swain, S.S.; Mohapatra, S.; Mallick, S. Bentonite clay incorporated topical film formulation for delivery of trimetazidine: Control of ocular pressure and in vitro-in vivo correlation. J. Drug Deliv. Sci. Technol. 2021; in press. [Google Scholar] [CrossRef]
- Bonini, M.; Gabbani, A.; Del Buffa, S.; Ridi, F.; Baglioni, P.; Bordes, R.; Holmberg, K. Adsorption of Amino Acids and Glutamic Acid-Based Surfactants on Imogolite Clay minerals. Langmuir 2017, 33, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.E.; Huertas, F.J. Adsorption of Glycine on Montmorillonite in Aqueous Solutions. Appl. Clay Miner. Sci. 2013, 80–81, 10–17. [Google Scholar] [CrossRef]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the Simulation of Interlayer Structure. Appl. Clay Miner. Sci. 2019, 169, 40–47. [Google Scholar] [CrossRef]
- Bu, H.; Yuan, P.; Liu, H.; Liu, D.; Qin, Z.; Zhong, X.; Song, H.; Li, Y. Formation of Macromolecules with Peptide Bonds via the Thermal Evolution of Amino Acids in the Presence of Montmorillonite: Insight into Prebiotic Geochemistry on the Early Earth. Chem. Geol. 2019, 510, 72–83. [Google Scholar] [CrossRef]
- Viseras, C.; Lopez-Galindo, A. Pharmaceutical Applications of Some Spanish Clay minerals (Sepiolite, Palygorskite, Bentonite): Some Preformulation Studies. Appl. Clay Miner. Sci. 1999, 14, 69–82. [Google Scholar] [CrossRef]
- Viseras, C.; Cultrone, G.; Cerezo, P.; Aguzzi, C.; Baschini, M.T.; Vallés, J.; López-Galindo, A. Characterisation of Northern Patagonian Bentonites for Pharmaceutical Uses. Appl. Clay Miner. Sci. 2006, 31, 272–281. [Google Scholar] [CrossRef]
- Gamoudi, S.; Srasra, E. Characterization of Tunisian Clay mineral Suitable for Pharmaceutical and Cosmetic Applications. Appl. Clay Miner. Sci. 2017, 146, 162–166. [Google Scholar] [CrossRef]
- Viseras, C.; Aguzzi, C.; Cerezo, P.; Lopez-galindo, A. Uses of Clay mineral Minerals in Semisolid Health Care and Therapeutic Products. Appl. Clay Miner. Sci. 2007, 36, 37–50. [Google Scholar] [CrossRef]
- Silva, F.C.; Lima, L.C.B.; Viseras, C.; Osajima, J.A.; da Silva, J.M.; Oliveira, R.L.; Bezerra, L.R.; Silva-Filho, E.C. Understanding Urea Encapsulation in Different Clay mineral Minerals as a Possible System for Ruminant Nutrition. Molecules 2019, 24, 3525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Bi, H.; Li, H.; Mao, X.; Liang, Y. Synthesis, Characterization, and Sustained Release Property of Fe3O4 @ (Enro Fl Oxacin-Layered Double Hydroxides) Nanocomposite. Mater. Sci. Eng. C 2017, 78, 886–891. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.S.; Li, P.; Nygaard, J.V.; Kassem, M.; Besenbacher, F.; Bünger, C. Fabrication and Characterization of a Rapid Prototyped Tissue Engineering Scaffold with Embedded Multicomponent Matrix for Controlled Drug Release. Int. J. Nanomed. 2012, 7, 4285–4297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, M.A.; Ciuffi, K.J.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; de Faria, E.H. Effect of Chemical Modification of Palygorskite and Sepiolite by 3-Aminopropyltriethoxisilane on Adsorption of Cationic and Anionic Dyes. Appl. Clay Miner. Sci. 2017, 135, 394–404. [Google Scholar] [CrossRef]
- Parbhakar, A.; Cuadros, J.; Sephton, M.A.; Dubbin, W.; Coles, B.J.; Weiss, D. Adsorption of L-Lysine on Montmorillonite. Colloids Surf. A Physicochem. Eng. Asp. 2007, 307, 142–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Wang, F.; Liang, J.; Ran, S.; Sun, J. Phase Transformation and Morphology Evolution of Sepiolite Fibers during Thermal Treatment. Appl. Clay Miner. Sci. 2017, 143, 205–211. [Google Scholar] [CrossRef]
- Luo, W.; Sasaki, K.; Hirajima, T. Influence of the Pre-Dispersion of Montmorillonite on Organic Modification and the Adsorption of Perchlorate and Methyl Red Anions. Appl. Clay Miner. Sci. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Madejová, J.; Jankovič, Ľ.; Slaný, M.; Hronský, V. Conformation Heterogeneity of Alkylammonium Surfactants Self-Assembled on Montmorillonite: Effect of Head-Group Structure and Temperature. Appl. Surf. Sci. 2020, 503, 144125. [Google Scholar] [CrossRef]
- Kitadai, N.; Yokoyama, T.; Nakashima, S. In Situ ATR-IR Investigation of L-Lysine Adsorption on Montmorillonite. J. Colloid Interface Sci. 2009, 338, 395–401. [Google Scholar] [CrossRef]
- Dong, F.; Guo, Y.; Liu, M.; Zhou, L.; Zhou, Q.; Li, H. Spectroscopic Evidence and Molecular Simulation Investigation of the Bonding Interaction between Lysine and Montmorillonite: Implications for the Distribution of Soil Organic Nitrogen. Appl. Clay Miner. Sci. 2018, 159, 3–9. [Google Scholar] [CrossRef]
- Ahmad, R.; Hasan, I. L-Methionine Montmorillonite Encapsulated Guar Gum-g-Polyacrylonitrile Copolymer Hybrid Nanocomposite for Removal of Heavy Metals. Groundw. Sustain. Dev. 2017, 5, 75–84. [Google Scholar] [CrossRef]
- Chu, Y.; Zhu, S.; Xia, M.; Wang, F.; Lei, W. Methionine-Montmorillonite Composite–A Novel Material for Efficient Adsorption of Lead Ions. Adv. Powder Technol. 2019, 31, 708–717. [Google Scholar] [CrossRef]
- Wagner, C.C.; Baran, E.J. Spectroscopic and Magnetic Behaviour of the Copper (II) Complex of L-Tryptophan. Acta Farm. Bonaer. 2004, 23, 339–342. [Google Scholar]
- Faizan, M.; Ahmad, S. Experimental Vibrational Spectroscopy (FTIR and FT-Raman) of D-Tryptophan and Its Anharmonic Theoretical Studies Using Density Functional Theory. J. Mol. Struct. 2018, 1171, 315–322. [Google Scholar] [CrossRef]
- Kalburcu, T.; Tabak, A.; Ozturk, N.; Tuzmen, N.; Akgol, S.; Caglar, B.; Denizli, A. Adsorption of Lysozyme from Aqueous Solutions by a Novel Bentonite–Tyrptophane (Bent–Trp) Microcomposite Affinity Sorbent. J. Mol. Struct. 2015, 1083, 156–162. [Google Scholar] [CrossRef]
- CHEN, Y.; WANG, X.; LUO, S.; BAO, Y. Synthesis of New Tb-Doped Zn-Al LDH/Tryptophan Hybrids and Their Fluorescent Property. J. Rare Earths 2016, 34, 1095–1102. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Li, S.X.; Hu, X.P.; Wang, P.J.; Zeng, J.B.; Wang, X.L.; Wang, Y.Z. Structure, Morphology, and Properties of LDPE/Sepiolite Nanofiber Nanocomposite. Polym. Adv. Technol. 2017, 28, 958–964. [Google Scholar] [CrossRef]
- da Rocha, M.C.; de AraujoBraz, E.M.; Honório, L.M.C.; Trigueiro, P.; Fonseca, M.G.; Silva-Filho, E.C.; Carrasco, S.M.; Polo, M.S.; Iborra, C.V.; Osajima, J.A. Understanding the Effect of UV Light in Systems Containing Clay mineral Minerals and Tetracycline. Appl. Clay Miner. Sci. 2019, 183, 105311. [Google Scholar] [CrossRef]
- Silva, M.M.F.; Oliveira, M.M.; Avelino, M.C.; Fonseca, M.G.; Almeida, R.K.S.; Silva Filho, E.C. Adsorption of an Industrial Anionic Dye by Modified-KSF-Montmorillonite: Evaluation of the Kinetic, Thermodynamic and Equilibrium Data. Chem. Eng. J. 2012, 203, 259–268. [Google Scholar] [CrossRef]
| Amino Acid | pKa Values | 1 IP | ||
|---|---|---|---|---|
| pK1 (-COOH) | pK2 (-NH3+) | pK3 (Grupo R) | ||
| Lysine | 2.18 | 8.95 | 10.53 | 9.74 |
| Methionine | 2.28 | 9.21 | - | 5.74 |
| Tryptophan | 2.38 | 9.39 | - | 5.89 |
| Sample | pH | Sample | pH | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Lys-Sep | 7.41 ± 0.24 | 7.79 ± 0.22 | Lys-Veg | 7.41 ± 0.24 | 8.30 ± 0.18 |
| Met-Sep | 7.23 ± 0.12 | 7.83 ± 0.13 | Met-Veg | 7.23 ± 0.12 | 7.93 ± 0.29 |
| Trp-Sep | 7.10 ± 0.09 | 7.76 ± 0.22 | Trp-Veg | 7.10 ± 0.09 | 8.02 ± 0.11 |
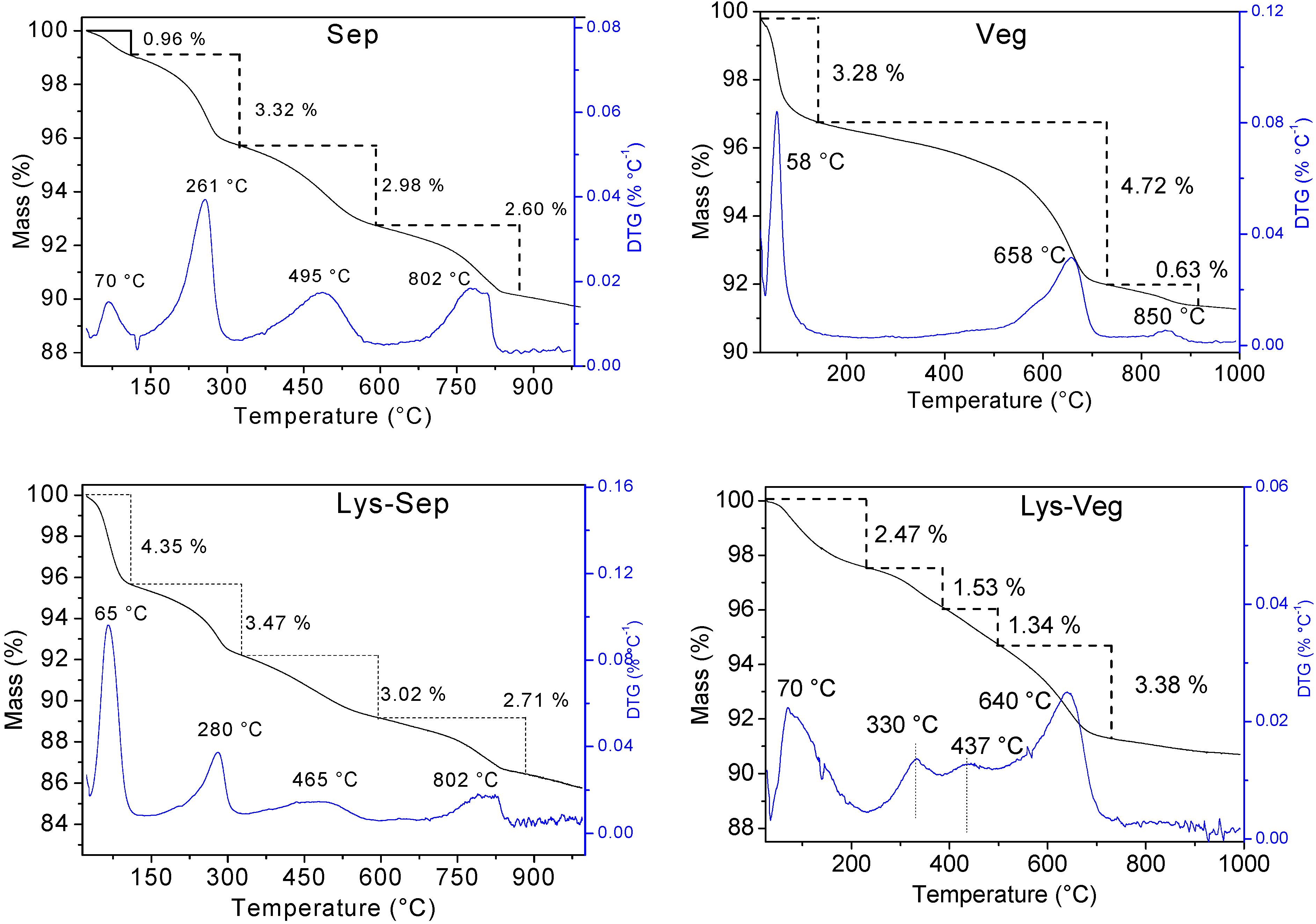
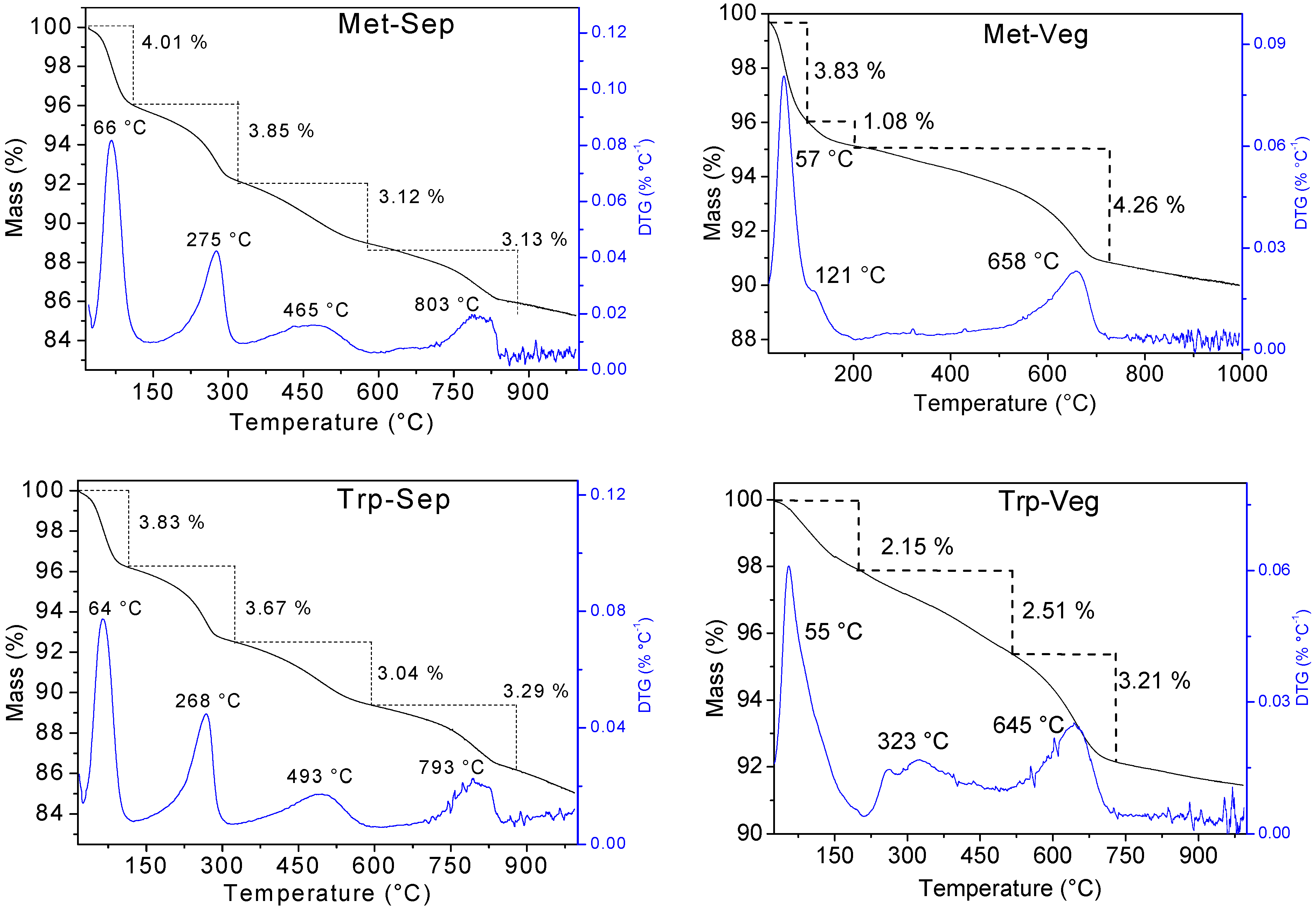
| Sample | Tmax (°C) | Mass Loss (%) | Residue (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | ||
| Sep | 70 | 261 | 495 | 802 | 0.96 | 3.32 | 2.98 | 2.60 | 90.57 |
| Lys-Sep | 65 | 280 | 465 | 802 | 4.35 | 3.47 | 3.02 | 2.71 | 89.67 |
| Met-Sep | 66 | 275 | 465 | 803 | 4.01 | 3.85 | 3.12 | 3.13 | 88.86 |
| Trp-Sep | 64 | 268 | 493 | 793 | 3.83 | 3.67 | 3.04 | 3.29 | 88.44 |
| Veg | 58 | 658 | 850 | - | 3.28 | 4.72 | 0.62 | - | 94.37 |
| Lys-Veg | 70 | 330 | 437 | 640 | 2.47 | 1.53 | 1.34 | 3.38 | 92.95 |
| Met-Veg | 57 | 121 | 658 | - | 3.83 | 1.08 | 4.26 | - | 93.57 |
| Trp-Veg | 55 | 323 | 645 | - | 2.15 | 2.51 | 3.21 | - | 93.34 |
| Sample | (%) | (mmol g−1) | C/N Theoretical | C/N Experimental | ||
|---|---|---|---|---|---|---|
| C | N | C | N | |||
| Sep | 0.09 ± 0.02 | - | 0.07 ± 0.02 | - | - | - |
| Lys-Sep | 0.78 ± 0.03 | 0.24 ± 0.01 | 0.65 ± 0.03 | 0.17 ± 0.01 | 3.00 | 3.82 |
| Met-Sep | 0.67 ± 0.04 | 0.14 ± 0.01 | 0.56 ± 0.04 | 0.11 ± 0.01 | 5.00 | 5.09 |
| Trp-Sep | 1.72 ± 0.09 | 0.39 ± 0.02 | 1.43 ± 0.09 | 0.28 ± 0.02 | 5.50 | 5.15 |
| Veg | 0.08 ± 0.01 | - | 0.06 ± 0.01 | - | - | - |
| Lys-Veg | 1.42 ± 0.07 | 0.53 ± 0.03 | 1.18 ± 0.07 | 0.38 ± 0.03 | 3.00 | 3.10 |
| Met-Veg | 0.45 ± 0.02 | 0.12 ± 0.01 | 0.38 ± 0.02 | 0.08 ± 0.01 | 5.00 | 4.75 |
| Trp-Veg | 1.83 ± 0.09 | 0.40 ± 0.02 | 1.52 ± 0.09 | 0.29 ± 0.02 | 5.50 | 5.24 |
| Sample | Incorporated Amino Acid (%) | Sample | Incorporated Amino Acid (%) | ||
|---|---|---|---|---|---|
| Elemental Analysis | TG/DTG | Elemental Analysis | TG/DTG | ||
| Lys-Sep | 1.58 ± 0.03 | 0.90 | Lys-Veg | 2.88 ± 0.07 | 2.87 |
| Met-Sep | 1.66 ± 0.04 | 1.71 | Met-Veg | 1.12 ± 0.02 | 1.08 |
| Trp-Sep | 2.66 ± 0.09 | 2.13 | Trp-Veg | 2.83 ± 0.09 | 2.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão-Lima, L.C.; Silva, F.C.; Costa, P.V.C.G.; Alves-Júnior, E.A.; Viseras, C.; Osajima, J.A.; Bezerra, L.R.; de Moura, J.F.P.; de A. Silva, A.G.; Fonseca, M.G.; et al. Clay Mineral Minerals as a Strategy for Biomolecule Incorporation: Amino Acids Approach. Materials 2022, 15, 64. https://doi.org/10.3390/ma15010064
Brandão-Lima LC, Silva FC, Costa PVCG, Alves-Júnior EA, Viseras C, Osajima JA, Bezerra LR, de Moura JFP, de A. Silva AG, Fonseca MG, et al. Clay Mineral Minerals as a Strategy for Biomolecule Incorporation: Amino Acids Approach. Materials. 2022; 15(1):64. https://doi.org/10.3390/ma15010064
Chicago/Turabian StyleBrandão-Lima, Luciano C., Fabrícia C. Silva, Paulo V. C. G. Costa, Edgar A. Alves-Júnior, César Viseras, Josy A. Osajima, Leilson R. Bezerra, Jose F. P. de Moura, Aline G. de A. Silva, Maria G. Fonseca, and et al. 2022. "Clay Mineral Minerals as a Strategy for Biomolecule Incorporation: Amino Acids Approach" Materials 15, no. 1: 64. https://doi.org/10.3390/ma15010064







